Introduction
The mother’s diet during gestation and lactation, as well as her nutritional status at the time of conception, provides the macro- and micronutrients required to support the growth and differentiation of neuronal tissues. If the nutrient supply is unable to meet the demands, the offspring’s neurodevelopment will be compromised. Epidemiological evidence from human populations and studies of experimental animals suggest that nutrient deficiencies or imbalances encountered during fetal and infant life influence the behaviour of the offspring.Reference Menting, van de Beek and Mintjens1 A well-documented example is early-life iron deficiency, which in humans increases the risk of the child developing a range of social problems (such as increased anxiety, increased likelihood of depression), poor educational outcomes (such as arithmetic achievement and written expression), as well as interfering with the development of specific cognitive processes such as spatial memory and selective recall.Reference Lozoff, Klein, Nelson, McClish, Manuel and Chacon2–Reference Doom and Georgieff6 Crucially because there are specific changes in the structure of neuronal networks, poor development cannot be reversed by iron supplementation later in childhood or adult life.Reference Beard7–Reference Beard and Connor11 Similar adverse effects are reported for inadequate provision of other nutrients essential for neurodevelopment, such as polyunsaturated fatty acids (PUFAs).Reference Mennitti, Oliveira and Morais12
Deficiencies, especially in micronutrients such as iron, can have wide-ranging effects on the metabolism of both the mother and her child. The developing fetus has a complex metabolic relationship with the mother, depending on maternal metabolism for the synthesis of several key nutrients. This relationship continues into infancy as the mother’s milk continues to provide essential nutrients. In case of iron, a hierarchy of iron supply develops early in gestation, benefitting the fetus to the detriment of the mother.Reference Gambling, Czopek and Andersen13 It is likely that the metabolic consequences of iron deficiency are more profound in the maternal compartment, raising the possibility that the offspring’s brain development is affected indirectly by a limitation in the supply of essential products of maternal metabolism.
Lipid metabolism is particularly sensitive to iron deficiency as iron is an essential cofactor in a number of key enzymes. Since lipids are a major structural component of the fetal brain, accounting for up to 60% of the dry weight,Reference O’Brien and Sampson14 any perturbation of the lipid supply will adversely affect neurodevelopment. For example, studies in pre-term infants suggest that cognitive development is influenced by the availability of long-chain PUFAs (LC-PUFAs), which are essential components of neuronal membranes and the accompanying myelin.Reference Lien, Richard and Hoffman15 These essential lipids are derived from the mother, initially through placental transfer and then through the milk after birth. In pregnant rats fed an iron-deficient diet, there was an increase in plasma triglyceride and a decrease in the fat content of the maternal liver, especially in the later stages of gestation, suggesting substantial changes in lipid metabolism.Reference Hay, McArdle, Hayes, Stevens and Rees16
This study was undertaken to investigate the effects of iron deficiency on the fatty acid composition of the neonatal brain at different stages of development. We also measured the fatty acid profiles in the stomach contents to examine changes in the supply of fatty acids from the dam via the milk. Since early iron supplementation is widely used to ameliorate the effects of iron deficiency in infants,Reference Cusick, Georgieff and Rao17 the effects of repletion on the fatty acid supply were investigated by recuperating some of the iron-deficient dams by feeding iron-sufficient rations once they had given birth to their pups.
Methods
Experimental diets
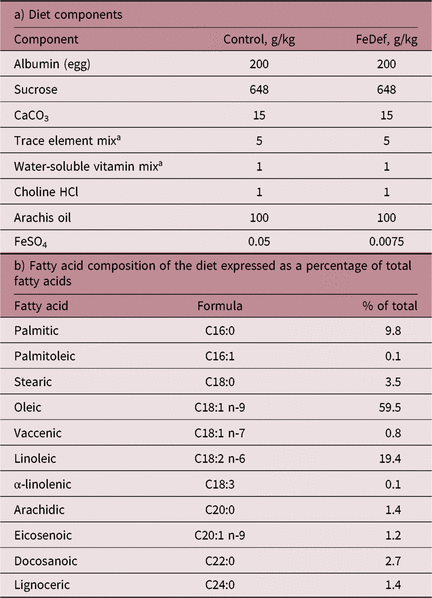
The composition and fatty acid profiles of the semi-synthetic diets are given in Table 1. The control diet contained 50 mg ferrous sulphate per kg diet compared to 7.5 mg ferrous sulphate per kg diet in the iron-deficient diet (FeDef). Ingredients were purchased from Mayjex Ltd (Chalfont-St Peter, UK), BDH Chemicals (Poole, UK) or Sigma (Poole, UK).
Table 1. Composition of the experimental diets

a As described by Williams and Mills.Reference Williams and Mills18
Animals
All experimental procedures were approved by the ethical review committee of the Rowett Research Institute and conducted in accordance with the UK Animals (Scientific Procedures) Act, 1986. Animals used in this study have been described previously.Reference Lenartowicz, Kennedy, Hayes and McArdle19 Briefly, 120 weanling female rats of the Rowett Hooded Lister strain were randomly allocated into two groups as shown in the flow diagram (Fig. 1). Animals were housed in plastic cages on sawdust bedding with an illumination photoperiod of 12L:12D. Conditions of temperature and humidity were constant with food and water provided ad libitum throughout the experiment. The first group of 40 animals (Con) was fed the control diet for the duration of the experiment. Animals in the second experimental group of 80 animals were fed the iron-deficient diet for 4 weeks. Animals in both groups were then individually housed and mated with males of the same strain. Mating was confirmed by detection of a vaginal plug. Control and experimental animals were maintained on their respective diets throughout pregnancy. Viable litters were obtained from 38 animals in the control group and 66 animals in the iron-deficient group.

Fig. 1. Experimental design. Female rats were fed either a control (open bars, 40 animals) or an iron-deficient diet (hatched bars, 80 animals) for 4 weeks prior to mating. On postnatal day 0, half of the dams fed the FeDef diet were fed the control diet to create the recuperated (FeDef-R) group. Dams and their litters were killed on postnatal days 0, 1 3, 7 and 10. The numbers indicate the number of dams suckling a litter of six or more pups used for further study.
At parturition, six dams and their pups from each group were killed and blood and tissue samples taken. The remaining animals in the control group continued to be fed the complete control diet. The remaining animals in the iron-deficient group were divided into two groups, one remaining on the iron-deficient diet (FeDef), while animals in the other group were recuperated by feeding the iron-containing control diet (FeDef-R). Within 24 h of birth, all litters were culled to eight pups, with male pups being kept preferentially. On days 1, 3, 7, 10 and 14 after birth, dams and their litters from each of the three groups were killed. Only those dams suckling a litter of six or more pups were used for further study. Animals were anaesthetised with isoflurane, and maternal blood samples collected by cardiac puncture. The dams were then killed by cervical dislocation. The fetuses or neonates were killed by decapitation. Blood samples were collected from each of the neonates, and pooled to provide a representative litter sample. Pups were dissected; the organs were weighed, immediately frozen in liquid nitrogen and then stored at −70°C for subsequent analysis. The stomach contents were collected and stored at −70°C.
Maternal and neonatal haematocrit was measured by drawing blood into capillary tubes, which were then centrifuged in a high-speed haematocrit centrifuge (Universal 32R, Hettich; Scientific Laboratory Supplies, Coatbridge, UK) and read in a microhaematocrit reader.
Tissue iron content
The total iron content of tissue samples was determined by drying them at 90°C under air until they were a constant weight and then treating them with nitric acid (Ultrapure; Merck, Poole, UK). The total iron content in these samples was determined using a graphite furnace atomic spectrophotometer (Analyst 600; Perkin Elmer, Beaconsfield, UK). Standards and quality controls were included as appropriate.
Lipid extraction
Brain tissue (postnatal days 0, 3 and 10) or stomach contents (postnatal days 3 and 10) from one pup per litter, chosen randomly from litters with at least six surviving pups, was used for lipid analysis. Approximately 100 mg of tissue was homogenised in 3.6 ml chloroform:methanol (118:63 v/v) containing butylated hydroxytoluene (50 µg/ml). Following the addition of 0.4 ml NaCl (0.145 M), the phases were separated and the upper aqueous layer removed. The organic layer was re-extracted with 3 ml chloroform:methanol:NaCl (3:48:47 v/v), and the combined organic phases were evaporated to dryness and stored under N2.
To convert the component fatty acids to their corresponding methyl esters (FAMEs), aliquots of lipid (10–20 mg) were dissolved in 0.5 ml of hexane and 2 ml of methanol:acetyl chloride(10:1 v/v) together with an internal standard (C19:0; Sigma). The samples were heated for 2 h at 100–105°C, cooled, and FAMEs were extracted with hexane. Samples of the FAME mixture (1 µl) were resolved by gas chromatography on a CP-SIL 88 column (50 m × 0.25 mm), using helium as carrier gas (flow rate of 0.8 ml/min at 16 psi). Samples were injected at 250°C using a 15:1 split injection. The temperature gradient was 165°C for 3 min; then increased by 1°C per min to 190°C; then increased at 10°C per min to a final temperature of 230°C. FAMEs were detected by flame ionisation detection, and retention times and peak areas were calculated using the instrument software. Individual fatty acids were identified by comparing the retention times with a reference mixture of methyl esters (Supelco 37 component FAME mix; Sigma). Data were expressed as a percentage of the total fatty acids present in the sample. Fatty acid concentrations were calculated from the relative peak areas compared with the internal standard.
Statistical analysis
Data were analysed by two-way ANOVA (Genstat 17th edition; Lawes Agricultural Trust, Rothamsted Experimental Station, Harpenden, Herts, UK). Where more than one pup per litter was analysed, dam was included as a block factor (random factor). Data were analysed by Fisher’s post hoc unprotected test or by Tukey’s test as indicated.
Results
A total of 38 animals in the control group and 66 animals in the FeDef group gave birth to viable litters. The FeDef pups were approximately 11% lighter than the control pups on postnatal day 1 (control: 5.41 ± 0.09 g (n = 38); FeDef: 4.86 ± 0.05 g (n = 66); p < 0.001). Pups nursed by the FeDef dams also gained less weight and, by postnatal day 10, were approximately 30% smaller (p < 0.001) than the control group (Fig. 2). Animals from the FeDef group, recuperated by feeding the dams the complete diet after birth (FeDef-R), initially remained on the same growth trajectory as the FeDef group but by postnatal day 7 began to recover. On postnatal day 1, the haematocrit of the dams in the FeDef group was less than that of the control group (p < 0.01) and remained so for the duration of lactation (Fig. 3a). In contrast, the animals recuperated by feeding the complete iron-sufficient diet recovered so that their haematocrit was similar to that of the control by postnatal day 7. The haematocrit of the pups (Fig. 3b) was reduced in the FeDef group compared to the control on postnatal day 1 (p < 0.001) and became progressively lower as lactation progressed, whereas the haematocrit of animals in the recuperated FeDef-R group remained similar to the control.

Fig. 2. Pup growth during lactation. Mean pup weights during postnatal growth of animals killed on postnatal day 10. Pups were nursed by dams fed control (closed circles), FeDef (open circles) and FeDef-R (closed triangles) diets. Data are mean ± SEM (n = 6). Superscript letters indicate significant differences (p < 0.05).

Fig. 3. Maternal (upper panel) and neonatal (lower panel) haematocrit (percent packed cell volume) during postnatal life in dams and pups fed control (closed circles), FeDef (open circles) and FeDef-R (closed triangles) diets. Data are mean ± SEM (n = 6). Superscript letters indicate significant differences (p < 0.05).
The iron content of the maternal liver was lower in the FeDef group compared to the control group (p < 0.001) and remained so up to postnatal day 14 (Fig. 4a). The iron content of the maternal liver of the recuperated animals (FeDef-R) began to increase after postnatal day 3, but was still lower than that of the control on postnatal day 10, only reaching similar concentrations to the control by postnatal day 14 (not shown). The iron content of the neonatal livers in the FeDef group was also lower than in the control (p < 0.001; Fig. 4b). In both the control and FeDef groups, there was a substantial fall in iron concentrations in the neonatal liver during the first 10 days of postnatal life, although the change was more pronounced in the FeDef group. Iron levels in the livers of FeDef-R pups remained similar to those in the FeDef group throughout. The concentrations of iron in the pup’s stomach contents (Fig. 4c), which reflect the iron content of the milk, were lower in FeDef pups compared to the controls for much of the lactation period. However, in contrast to the maternal and neonatal liver, iron concentrations in the stomach contents of the recuperated FeDef-R animals rapidly recovered to the control level by postnatal day 3.

Fig. 4. Iron content of maternal and fetal tissues. Iron content (micrograms per gram dry weight) of maternal liver (top panel), fetal liver (middle panel) and milk (bottom panel) during postnatal life in dams and pups fed control (closed circles), FeDef (open circles) and FeDef-R (closed triangles) diets. Data are mean ± SEM (n = 6). Superscript letters indicate significant differences (p < 0.05).
Male pups from each litter killed on postnatal days 3 and 10 were chosen at random for further analysis (Table 2). The weight of the brains of the FeDef group tended (p = 0.058) to be approximately 20% less than the controls and FeDef-R animals by postnatal day 10; however, brain weight as a percentage of the total body weight was not different in any of the three groups.
Table 2. Growth characteristics of pups used for analysis

Weights of pups chosen for analysis together with the weights of their brains and stomach contents. Data are expressed as mean ± standard error. Data were analysed by ANOVA followed by Fisher’s post hoc unprotected test.
a,b,cMean values within a row with unlike superscript letters were significantly different (p < 0.1). ns, p > 0.1.
Fatty acid profiles in stomach contents
The fatty acid composition of the stomach contents of rat pups has been shown to reflect the composition of their dams’ milk.Reference Bowen and Clandinin20 The fatty acid profile of the stomach contents of pups nursed by dams fed the control and FeDef diets was not different on postnatal day 3 apart from a reduction in C20:3 n-3 and a small increase in C22:0 (Table 3). These changes caused by iron deficiency were reversed in the dams recuperated by feeding the control diet after birth (FeDef-R). By postnatal day 10, there were significant differences in the fatty acid profiles of the stomach contents of the pups nursed by the dams fed the FeDef diet compared to the control. The total saturated fatty acids (SFAs; C10:0, C12:0, C14:0, C16:0 and C18:0) had declined from a total of 45.2% in the control to 33.6% of the total in the FeDef group. At the same time, the monounsaturated fatty acids (MUFAs; C16:1 and C18:1) had increased from 41.9% in the control to 50.6% of the total in the FeDef group. There was also an increase in C18:2 n-6 and C20:3 n-6 PUFAs from 7.4% in the control to 9.6% of the total in the FeDef group. While not completely restored, the profile of the FeDef-R group, recuperated by feeding the control diet at parturition, was closer to that of the control group with SFA comprising 39.0%, MUFA 47.3% and PUFA 8.4% of the total. Because of these changes, the unsaturation ratios for fatty acids in both the C16 and C18 series, while not different on postnatal day 3, were increased in both FeDef and FeDef-R groups on postnatal day 10.
Table 3. Fatty acid composition of stomach contents

Fatty acids are expressed as a percentage of total (mol/100 mol) and presented as mean ± standard error. Some minor unidentified peaks (not significantly different from control) are not included. Data were analysed by ANOVA. ns, p > 0.05.
a,b,cMean values within a row with unlike superscript letters were significantly different (p < 0.05) following Tukey post hoc comparisons.
Fatty acid profiles in the brain
The fatty acid profile of the pups’ brains on postnatal day 1 is shown in Table 4. In the pups born to dams fed the FeDef diet, there was a small decrease in the abundance of C18:3 n-3 and a small increase in the abundance of C22:6 n-3 compared to the control. By postnatal day 3 (Table 5), the proportion of C18:2 and C20:3 n-6 fatty acids was increased in the FeDef group compared to the control. The proportions of total SFAs and total MUFAs were unchanged on postnatal days 1 and 3; however, by day 10 of lactation (Table 6), the proportion of total SFA had decreased from 47.8% in the control to 46.9% in the FeDef. This was accompanied by an increase in the total MUFA from 11.5% in the control to 12.3% in the FeDef group. These changes mirrored the change in SFA and MUFA in the stomach contents. By postnatal day 10 there was a decrease in arachidonic acid (ARA; 20:4 n-6) and another unidentified long-chain fatty acid (U49.52) in the FeDef group compared to the control, but no change in docosahexaenoic acid (DHA; 22:6 n-3). The profile of fatty acids in the Fe Def-R group resembled that of the control, again reflecting the changes in milk composition.
Table 4. Fatty acid composition of the brain on postnatal day 0

Unk = unidentified with retention time (Rt) shown in parentheses. Some minor unidentified peaks (not significantly different from control) are omitted. Fatty acids are expressed as a percentage of total (mol/100 mol) and presented as mean ± standard error. Data were analysed by ANOVA. ns, p > 0.05.
Table 5. Fatty acid composition of the neonatal brain on postnatal day 3

Unk = unidentified with retention time (Rt) shown in parentheses. Some minor unidentified peaks (not significantly different from control) are not included. Fatty acids are expressed as a percentage of total (mol/100 mol) and presented as mean ± standard error. Data were analysed by ANOVA. ns, p > 0.05.
a,b,cMean values within a row with unlike superscript letters were significantly different (p < 0.05) following Tukey post hoc comparisons.
Table 6. Fatty acid composition of the neonatal brain on postnatal day 10

Unk = unidentified with retention time (Rt) shown in parentheses. Some minor unidentified peaks (not significantly different from control) are not included. Fatty acids are expressed as a percentage of total (mol/100 mol) and presented as mean ± standard error. Data were analysed by ANOVA. ns, p > 0.05.
a,b,cMean values within a row with unlike superscript letters were significantly different (p < 0.05) following Tukey post hoc comparisons.
Discussion
This study showed that rat maternal iron deficiency changed the fatty acid composition of the neonatal brain, such that by postnatal day 10, the proportion of SFA and n-6 PUFA was reduced and replaced with MUFA. There is a similar change in the fatty acid composition of the dam’s milk, which became more pronounced as the maternal iron status worsened during lactation. One explanation for this close association between the fatty acid profiles of neonatal brain and stomach contents is that a change in the milk composition caused changes in the neonatal brain. This conclusion is supported by the observation that when the dams were fed an iron-sufficient diet at birth, there was a rapid normalisation of the fatty acid composition of lipids in both the dam’s milk and the neonatal brains.
Gestational iron deficiency reduces fetal growth in rats,Reference Gambling, Andersen, Czopek, Wojciak, Krejpcio and McArdle21 with the effects becoming more pronounced as gestation progresses.Reference Woodman, Care and Mansour22 The body weight of the pups tended to be reduced by approximately 14% in the iron-deficient offspring by day 3, and by postnatal day 10, the body weight of the pups was approximately 30% lower in the iron-deficient offspring. In contrast, the absolute weight of the brain in the offspring of the iron-deficient dams was not different from the control at birth or postnatal day 3, and only reduced by approximately 20% on postnatal day 10. This suggests that, in this particular model, total brain growth during gestation and early stages of lactation was not limited by iron deficiency; however, between postnatal days 3 and 10, the brain can no longer be completely protected. This corresponds to the period when myelination is at its peak in the first weeks after birth,Reference Semple, Blomgren, Gimlin, Ferriero and Noble-Haeusslein23 and also when maternal iron deficiency is having its greatest impact on fatty acids in the milk. As the effects of any deficiency in the supply of fatty acids are likely to be greater in those regions of the brain that are actively proliferating, region-specific effects may be underestimated in this analysis of the whole brain.
During the later stages of gestation and during lactation, the dam mobilises fatty acids from adipose tissue to supplement lipids from the diet.Reference Herrera24 The decreased expression of liver-type carnitine palmitoyl transferase-1 (L-CPT-1) in the maternal liver late in gestation, which was observed in our earlier studies of iron deficiency in pregnancy, suggests that iron deficiency results in less lipid being oxidised by the mitochondria.Reference Hay, McArdle, Hayes, Stevens and Rees16 This may be the result of less lipid being mobilised from the maternal adipose stores, and by implication less is available for milk production. This would be consistent with the reported reduction in the total fat content of milk in rats fed iron-deficient rations.Reference O’Connor, Picciano and Sherman25 It is, therefore, likely that there is a greater effect on milk production at the latter stages of lactation when the iron stores of the dam are being increasingly depleted.
There are pronounced changes in the proportion of unsaturated fatty acids in the brain of iron-deficient pups by postnatal day 10. The degree of fatty acid desaturation determines membrane fluidity and function. By postnatal day 10, the ratio of monoenoic-to-saturated fatty acids (16:1/16:0 and 18:1/18:0) has increased in the brain of pups in the FeDef group compared to the control. While not as pronounced as in the brain, the desaturation ratio of C18 fatty acids in the stomach contents also increased from 11.7 in the control to 13.0 in the FeDef group at day 10. It is possible that an increase in the incorporation of MUFA is a compensatory response to a decrease in ARA. This result is in marked contrast to previous studies reporting a reduction in the ratio of monoenoic-to-saturated fatty acids in the brain of rat pups nursed by iron-deficient dams.Reference Rao, Manix and Larkin26,Reference Larkin, Jarratt and Ananda Rao27 Monoenoic fatty acids in milk are produced by the endogenous desaturation of stearic acid within the mammary gland by the iron-dependent enzyme stearoyl-CoA desaturase.Reference Bichi, Toral and Hervás28 The activity of this enzyme is reduced in liver microsomes prepared from rats fed an iron-deficient diet,Reference Rao, Crane and Larkin29 suggesting that a similar decrease in the activity of the enzyme in mammary tissue would reduce the production of monoenoic fatty acids and decrease the desaturation ratio of milk fat. However, this is not the case; the increased ratio suggests either that the enzyme activity is unaffected or that additional factors, such as the dam’s lipid stores, metabolism and diet, compensate for the reduced enzyme activity.
Differences in the diet compositions are also an important factor. The semi-synthetic diet used in this study was prepared with arachis (peanut) oil, whereas other studies have used corn oil as the fat source. While both oils are relatively low in SFAs (15 vs. 17%), arachis oil has more oleic acid (C18:1 n-9; 46% vs. 30%) and less linoleic acid (C18:2 n-6; 32% vs. 55%) compared with corn oil. It is possible that any impairment of the desaturase activity due to iron deficiency was overcome by the provision of additional oleic acid. These findings highlight the potential interactions between micronutrients and the fatty acid composition of the diet, particularly in experiments using semi-synthetic diets such as AIN-93 for rodent studies.
The two principal biologically active LC-PUFAs in the brain – ARA (20:4 n-6) and DHA (22:6 n-3) – are differentially affected by iron deficiency; the proportion of ARA was reduced, whereas DHA was unchanged. Indeed, it is possible that because of the functional importance of DHA in the brain, it will be preserved at the expense of ARA. ARA and DHA are produced from distinct precursors obtained from the maternal diet. The precursor of n-3 PUFAs, alpha-linolenic acid (18:3 n-3), is particularly low in the diet used (Table 1), whereas the precursor of n-6 PUFAs, linoleic acid (18:2 n-6), is plentiful. It is also possible that the activity of delta-5 and delta-6 desaturase enzymes, which are both dependent on iron as a cofactor, could be influenced by deficiency.Reference Cunnane and McAdoo30 It is interesting to note that there was an increase in linoleic acid and dihomo-gamma-linolenic acid as well as a decrease in ARA. These two fatty acids are produced earlier in the pathway of desaturase enzymes that ultimately produce ARA. An increase in these fatty acids would be consistent with a reduction in the activity of the delta-5 desaturase enzyme. As the precursor for DHA is low, the impact of a reduced desaturase activity on the production of DHA may be small. Furthermore, if the desaturase activity is only affected once iron availability drops below a particular threshold, much of the variation between studies may be accounted for by differences in the iron stores of the pregravid animals and the different rates at which these stores are depleted.
This study showed the interaction between maternal iron status and the fatty acid composition of the weanling rat brain. The study also suggests a window of opportunities when these effects can be reversed by iron repletion of the dam’s diet immediately after birth, in line with previous studies suggesting that the provision of additional iron can reverse the detrimental effects of iron deficiency in utero.Reference Beard, Unger, Bianco, Paul, Rundle and Jones31 The interaction between iron status and lipid metabolism, particularly in relation to the effects on milk composition, suggests that a change in the supply of lipids or the provision of additional LC-PUFA either through the lactating mother’s diet or directly to the offspring via milk supplements may be beneficial in addressing the effects of iron deficiency in pregnancy. There is already evidence from animal studies showing that the effects of iron deficiency in rats can be reversed by the provision of cofactors required for the synthesis of phospholipids, such as choline.Reference Tran, Kennedy and Pisansky32,Reference Kennedy, Tran, Kohli, Maertens, Gewirtz and Georgieff33 The interactions between micronutrient status and macronutrient nutrition, especially in pregnancy and lactation, are complex and far-reaching and worthy of additional research.
Acknowledgements
We wish to express our thanks to the staff from Bioresources Unit for animal care.
Financial support
This work was funded as a part of the Scottish Government Rural and Environment Science and Analytical Services (RESAS) Strategic Research Program.
Conflict of interest
None.
Ethical Standards
All experimental procedures were approved by the ethical review committee of the Rowett Research Institute and conducted in accordance with the UK Animals (Scientific Procedures) Act, 1986.