INTRODUCTION
Maize (Zea mays L.) is the most important cereal food crop and is a staple food for more than 300 million people in sub-Saharan Africa (SSA) (FAO, 2008). Maize is widely grown by smallholder farmers in Zimbabwe where average maize yield barely exceeds 0.8 t ha−1 (Kanonge et al., Reference Kanonge, Nezomba, Chikowo, Mtambanengwe and Mapfumo2009). About 70% of Zimbabwe's arable land is characterised by sandy soils derived from granite (Nyamapfene, Reference Nyamapfene1991a). The sandy soils have a low nutrient status, low organic matter content and are generally acidic (FAO, 2006). The combination of inherent low soil fertility, unaffordability of fertilizers, prevalence of soil pests such as nematodes, low and unreliable rainfall, result in low maize productivity in the smallholder farming sector.
Over the years, researchers and development agencies have focused more on addressing soil chemical fertility and the use of improved germplasm as the basis for sustainable intensification (Nezomba et al., Reference Nezomba, Mtambanengwe, Chikowo and Mapfumo2014; Vanlauwe et al., Reference Vanlauwe, Kihara, Chivenge, Pyper, Coe and Six2011). However, soil health transcends several domains including physical and biological aspects. In maize-based farming systems which are dominant in Zimbabwe, nematodes have been reported as one of the major causes of low maize yield (Kagoda et al., Reference Kagoda, Hearns, Adewuyi and Coyne2015; Nhamo, Reference Nhamo2007), and then their sustainable control is needed to increase maize yield. More than 60 species of nematodes are associated with maize across the globe (Coyne et al., Reference Coyne, Nicol and Claudius-Cole2007). Pratylenchus spp. (root lesion nematodes) and Meloidogyne spp. (root-knot nematodes) are species which attack maize and have the potential to cause economic yield losses (Kagoda et al., Reference Kagoda, Derera, Tongoona, Coyne and Talwana2011) by damaging, feeding on maize roots tissues and providing point of entry for root pathogens. In Zimbabwe, Pratylenchus spp. was found to be commonly associated with maize (Nhamo, Reference Nhamo2007). The problem of nematodes in crop production in Zimbabwe is well-recognised in tobacco farming systems and relatively unknown in maize (Muzhandu et al., Reference Muzhandu, Chinheya, Manjeru and Dimbi2013). In tobacco, Meloidogyne spp. is the most common nematode genus which is controlled by four year rotations with a non-susceptible crop as recommended for large-scale tobacco systems (Lipan et al., Reference Leppan, Lecours and Buckles2014). Furthermore, the presence of nematodes in soil is influenced by the crop type, carbon source and soil physical properties (Muzhandu et al., Reference Muzhandu, Chinheya, Manjeru and Dimbi2013).
Conservation agriculture (CA), a practice premised on reduced soil disturbance, provision of permanent soil cover and the use of crop rotations (Kassam and Friedrich, Reference Kassam and Friedrich2012), is a cropping system that mimics natural agro-ecosystems where higher density and diversity of crop damaging and beneficial organisms is high (Nana et al., Reference Nana, Degué, Mkomwa, Da Sansan, Essecofy, Bougoum, Zerbo, Ganou, Andrieu, Douzet, Jat, Sahrawat and Kassam2013). The balance between species is often determined by the rates of crop residue retention and the type of crop rotations practiced. However, despite their observed benefits (Nyagumbo et al., Reference Nyagumbo, Mkuhlani, Pisa, Kamalongo, Dias and Mekuria2016), rotations are hardly implemented under CA for various practical reasons and hence the need to understand the consequences of maize mono-cropping (Rusinamhodzi, Reference Rusinamhodzi, Farooq and Siddique2015). Nematodes are some of the organisms that have been used as ecological bio-indicators reflecting environmental changes (Habig et al., Reference Habig, Hassen, Swart, Farooq and Siddique2014). In addition, soil disturbance through tillage can cause redistribution of the plant parasitic nematode community structure. Thus CA, through reduced soil disturbance, is hypothesised to result in a more stable nematode community structure with long-term effects on crop health and sustainability (Djigal et al., Reference Djigal, Saj, Rabary, Blanchart and Villenave2012; Stirling, Reference Stirling2014). Smallholder farmers practice continuous maize mono-cropping (Makwara, Reference Makwara2010; Mazvimavi and Twomlow, Reference Mazvimavi, Twomlow, Goddard, Zoebisch, Gan, Ellis, Watson and Sombatpaint2008) which potentially increases nematode infestations (Nie et al., Reference Nie, Fahad, Peng, Huang and Cui2014) on nutrient deficient sandy soils found in most of the smallholder farming systems. The current study, therefore, sought to understand CA practice and different nematode management strategies on nematode infestation and maize productivity. The study was carried out on sandy soils in smallholder farming systems in a sub-humid region of Zimbabwe.
MATERIALS AND METHODS
Study site
The study was carried out in Chinhamhora communal area, sub-humid Goromonzi district (31°13ʹE; 17°30ʹS, altitude: 1420 to1560 m) over three consecutive cropping seasons. The experiment was established between November and December of the 2011/2012 season while field measurements on nematodes were only carried out during the 2012/2013 and 2013/2014 cropping seasons. Chinhamhora smallholder farming area lies in Zimbabwe's agro-ecological region IIa, which experiences daily air temperatures ranging between 21 and 31 °C during the cropping season, typically extending from mid-October to end of April and receives 750 to 1000 mm of rainfall per year.
Farming in Chinhamhora is dominated by horticultural production of tomatoes (Solanum lycopersicum) and leafy vegetables such as kale/Covo (Tronchuda portuguesa), Indian Kale (Brassica juncea) and rape (Brassica napus). Soils in the upper slope are well-drained, moderately shallow to deep and are classified as Typic Kandiustalfs according to the USDA taxonomy soil classification (Nyamapfene, Reference Nyamapfene1991b). The shallow soils limited to mid and upper slope positions, are classified as Lithic Ustorthents based on the USDA system (Nyamapfene, Reference Nyamapfene1991b). The soils are generally acidic, with soil pH (CaCl2) ranging from 3.9 to 4.5 (Nyamangara et al., Reference Nyamangara, Mugwira and Mpofu2000). Average farm size per household in Chinhamhora is 1–1.5 ha with the largest proportion being allocated to the staple maize crop in summer (Mutenje et al., Reference Mutenje, Kassie, Gwara and Mujeyi2014). The farming area is characterised by urbanisation, which progressively continues to result in fragmentation of farms. Use of improved hybrid maize varieties among the farmers is high and estimated at 80–90% (Mutenje et al., Reference Mutenje, Kassie, Gwara and Mujeyi2014).
Experimental design and treatments
Ten farmers were initially selected for the purposes of this experiment and prior to setting-up the experiment, soil samples were collected from a depth of 0–15 cm from each of the 10 farms for laboratory analyses of soil characteristics. The ten farms were screened down to six farms with closely matching soil characteristics. Four treatments on plots 15 × 15 m each were imposed on each farm: fenamiphos 40 EC (a commercial synthetic nematicide ethyl 4-methylthio-m-tolyl isopropylphosphoramidite sprayed around each planting station in the soil 7 days before planting at label rate of 25 Lha−1.), lime + fenamiphos 40EC, lime (Calcitic (CaCO3) applied 30 g/planting station which translates to 660 kg ha−1 two weeks before planting) and an untreated control with no lime and no nematicide. Calcitic lime, ground limestone composed of calcium carbonate (CaCO3) was used in the experiment. Each farm was considered a replicate in a completely randomised block design.
From the collected soil samples, soil organic carbon was measured using the modified Walkley Black method (Okalebo et al., Reference Okalebo, Gathua and Woomer2002), soil texture was determined using the hydrometer method (Okalebo et al., Reference Okalebo, Gathua and Woomer2002) while soil pH was measured using Hanna HI 8314 membrane pH meter in a 1:5 soil:0.1 M CaCl2 suspension. The soils were generally characterised as low in soil organic carbon (3.1 ± 0.3 g kg−1), sandy in texture (Clay 9.3 ± 2.8%, Silt 7.3 ± 2.7%, Sand 84 ± 3.4%) and as stated earlier, acidic in pH (CaCl2), ranging from 3.8 to 5.2 giving an average of 4.3 ± 0.5. Exchangeable bases were on average as follows; magnesium (Mg2+) (1.8 ± 0.5 mg L−1), calcium (Ca2+) (5.7 ± 3.5 mg L−1), potassium (K+) (2.4 ± 0.5 mg L−1), sodium (Na+) (1.6 ± 0.3 mg L−1).
At the start of each season in November or early December, maize SC513, a medium maturity variety, was planted in CA basins of 15 cm diameter and 15 cm depth after receiving at least 30 mm rainfall within three consecutive days. The planting basins were spaced at 90 cm inter-row and 50 cm in-row with two plants per station giving an average plant population of 44,000 plants per hectare.
Basal fertilizer (compound D; 7% N, 14% P2O5, 7% K2O) was applied in basins at planting at a rate of 300 kg ha−1. Top dressing with ammonium nitrate (34.5% N) at a rate of 200 kg ha−1 was split applied at four weeks after emergence (WAE) and eight WAE (AGRITEX, Reference Gamu and Mangena2008). Manual weeding was done two to three times per season using hand hoes or when weeds grew to more than 10 cm in height.
Nematode sampling
Sampling for nematodes was done at 6 and 12 WAE, in both soil and maize roots (Coyne et al., Reference Coyne, Nicol and Claudius-Cole2007) in 2012/2013 and 2013/2014 season. The same sampling intervals were maintained in 2013/2014 season except that only maize root samples were collected at six WAE. Composite soil samples were collected from rhizosphere of 10 randomly selected plants from each treatment plot on each farm using an auger. Similarly, root samples were collected by destructive excavation of five randomly selected plants using a hoe and carefully placed in khaki paper bags. All the soil and root samples were taken for extraction within the same day of collection.
Sample processing and nematode extraction from soil and root samples
Samples were processed at Kutsaga Research Station, Harare, Zimbabwe. The samples were stored in a cold room at 10 °C whilst they waited processing. From the composite samples, 200 g soil and 10 g root sub-samples were processed for nematode counts. Nematode density or populations in soil were expressed as numbers/200 g of soil and numbers/10 g of roots (Coyne et al., Reference Coyne, Nicol and Claudius-Cole2007). Nematodes in soil were extracted using the Seinhost two-Erlenmeyer flask technique and the modified Bearmann filter method (Van Bezooijen, Reference Van Bezooijen2006) as it offers relatively high extraction efficiency which can be obtained with simple equipment and small amounts of water. However, the method is labour intensive and, when one set of bottles is used, it is very time consuming. As the method does not require running tap water, it can be carried out outside the laboratory (Van Bezooijen, Reference Van Bezooijen2006). Roots were washed thoroughly in water and were chopped into small pieces of approximately 1 cm length. About 10 g were weighed from each root sample macerated in a blender for 10 seconds in 0.5% NaOCl solution before the sample was poured into a Bearman tray and left for 48 h (Hooper et al., Reference Hooper, Hallman, Subbotin, Luc, Sikora and Bridge2005). The nematodes were identified using a compound microscope and identification was up to the genus level. A nematode identification key was used as a guide for differentiating nematode genus and species.
Yield assessment
Maize biomass and grain yield were measured from each treatment plot per farm and from check plots measuring 5 m long × 2 rows wide randomly placed in each treatment. The width of the two rows was measured three times along the check plot to obtain the average width of the two rows. All freshly harvested maize cobs and biomass from each check plot were weighed using a digital hanging balance after counting the number of plants and cobs. One sub-sample containing 10 maize cobs was collected from each plot and immediately weighed before air drying. Approximately, 500 g of biomass sub-sample was taken from each plot. Maize cobs and biomass sub-samples were then air-dried and re-weighed after two to three weeks. The maize cobs were shelled and the maize grain yield was adjusted to 12.5% moisture content. Percentage yield loss and gain was calculated using equation (1) and (2) respectively,
 $$\begin{eqnarray}
&&{\rm{percentage}}\;\ {\rm{yield}}\;{\rm{loss}} \nonumber\\
&&\quad = \left[ {\frac{{\left( {Y\ \;{\rm{in\ }}\;{\rm{nematicide\ }}\;{\rm{treated}}\ \;{\rm{plot}}\ \left( {\frac{{kg}}{{ha}}} \right) - Y\ \;{\rm{in}}\ \;{\rm{untreated\ }}\;{\rm{plot}}\left( {\frac{{kg}}{{ha}}} \right)} \right)}}{{Y\;{\rm{in}}\;\ {\rm{nematicide}}\;{\rm{treated}}\;{\rm{plots\ }}\left( {\frac{{kg}}{{ha}}} \right)}}} \right]\nonumber\\
&&\qquad \times 100\ \%
\end{eqnarray}$$
$$\begin{eqnarray}
&&{\rm{percentage}}\;\ {\rm{yield}}\;{\rm{loss}} \nonumber\\
&&\quad = \left[ {\frac{{\left( {Y\ \;{\rm{in\ }}\;{\rm{nematicide\ }}\;{\rm{treated}}\ \;{\rm{plot}}\ \left( {\frac{{kg}}{{ha}}} \right) - Y\ \;{\rm{in}}\ \;{\rm{untreated\ }}\;{\rm{plot}}\left( {\frac{{kg}}{{ha}}} \right)} \right)}}{{Y\;{\rm{in}}\;\ {\rm{nematicide}}\;{\rm{treated}}\;{\rm{plots\ }}\left( {\frac{{kg}}{{ha}}} \right)}}} \right]\nonumber\\
&&\qquad \times 100\ \%
\end{eqnarray}$$
where, Y = maize grain yield; A = harvested area; MC = grain moisture content; and GW = weight of grain after cobs air drying and shelling (Kagoda et al., Reference Kagoda, Derera, Tongoona, Coyne and Talwana2011).
 $$\begin{eqnarray}
&&{\rm{percentage}}\;{\rm{yield}}\;{\rm{gain}} \nonumber\\
&&\quad = \left[ {\frac{{Y\;{\rm{in}}\;{\rm{treated}}\;{\rm{plot}}\;\ \left( {\frac{{kg}}{{ha}}} \right) - Y\;\ {\rm{in}}\;{\rm{untreated}}\;{\rm{plot}}\ \left( {\frac{{kg}}{{ha}}} \right)}}{{Y\;{\rm{in}}\;{\rm{untreated}}\;{\rm{plot}}\left( {\frac{{kg}}{{ha}}} \right)}}} \right] \times 100\ \%
\end{eqnarray}$$
$$\begin{eqnarray}
&&{\rm{percentage}}\;{\rm{yield}}\;{\rm{gain}} \nonumber\\
&&\quad = \left[ {\frac{{Y\;{\rm{in}}\;{\rm{treated}}\;{\rm{plot}}\;\ \left( {\frac{{kg}}{{ha}}} \right) - Y\;\ {\rm{in}}\;{\rm{untreated}}\;{\rm{plot}}\ \left( {\frac{{kg}}{{ha}}} \right)}}{{Y\;{\rm{in}}\;{\rm{untreated}}\;{\rm{plot}}\left( {\frac{{kg}}{{ha}}} \right)}}} \right] \times 100\ \%
\end{eqnarray}$$
Data analysis
Nematode density data were transformed using log (x+0.5) to normalize the data. The transformed nematode data from both the soil and root extracts and the maize yield data were subjected to analysis of variance using GENSTAT 14. Where no significant differences were found between the interaction of years and treatments, the data were pooled for a combined analysis over the two years. Correlations and linear regressions between yield and nematode densities were done in Statistics 9. The least significant difference (LSD) at 5% significance level was used for mean separation where there were significant treatment effects.
RESULTS
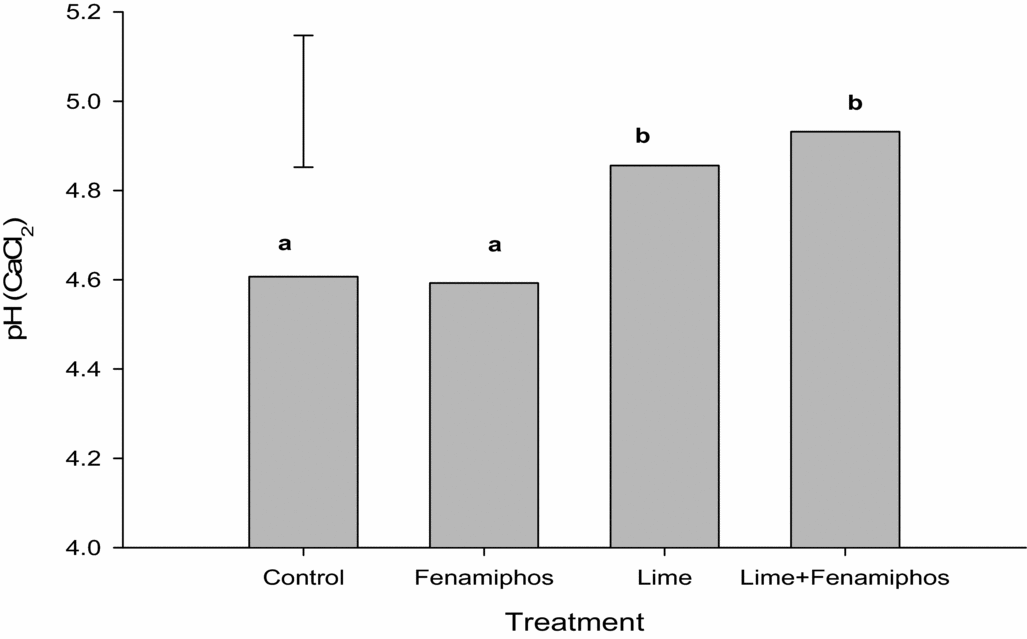
Analyses of the soil samples collected after applying the treatments indicated that soil pH (CaCl2) in the treated plots was significantly increased in the lime and combined lime plus fenamiphos-treated than in the untreated control and sole fenamiphos-treated plots (Figure 1).
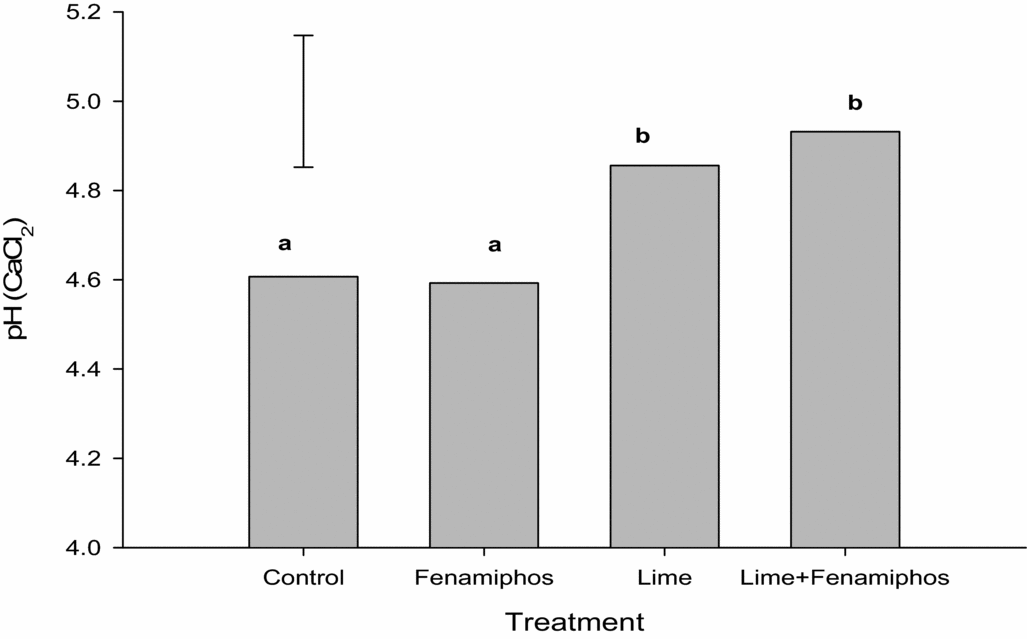
Figure 1. Soil pH response to the application of lime and fenamiphos for seasons 2012/2013 to 2013/2014 at Chinhamhora. Error bar denotes LSD(0.05) = 0.3. Different letters above each bar denote significant differences between treatments at p < 0.05.
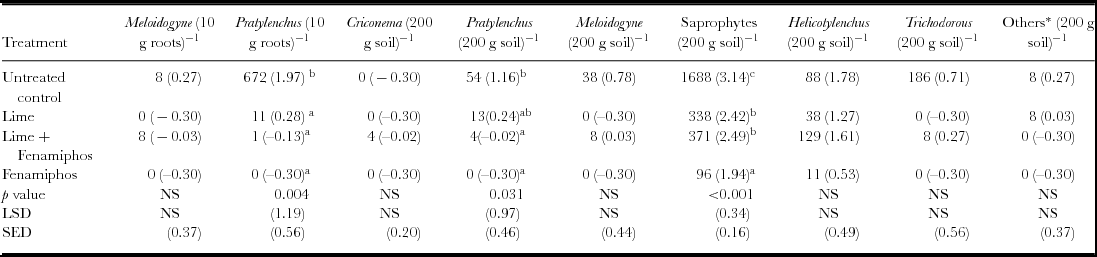
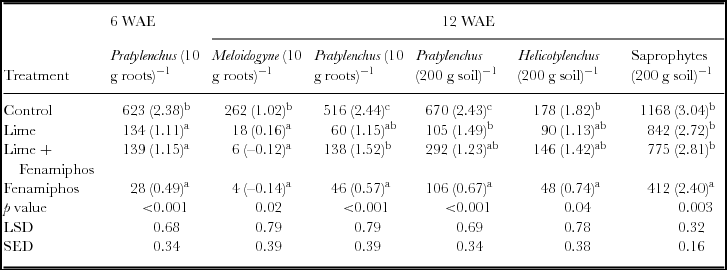
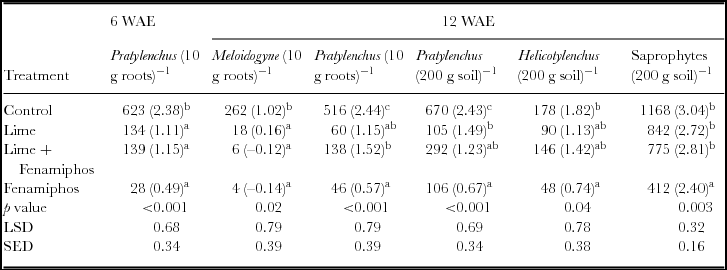
Dominant species at genus level found at the site included Meloidogyne spp., Pratylenchus spp., Criconema spp., Trichodorous spp., Helicotylenchus spp., saprophytes and other less common nematodes such as Tylenchulus spp. and Mononchus spp. (Table 1). Due to their abundance, Pratylenchus spp. further classified to species level, included Pratylenchus zeae, Pratylenchus penetrans and Pratylenchus brachyurus. Meloidogyne spp. identified at the site was Meloidogyne javanica. The beneficial non-plant parasitic saprophytic nematodes were significantly higher in untreated soils at six WAE. Furthermore, Pratylenchus spp. infestation was significantly higher in untreated plots in both maize roots and surrounding soil during the first season of 2012/2013 at six WAE; while fenamiphos-treated plots had significantly lower infestations (Table 1). The nematode densities for the two years were thus significantly higher in untreated plots (Table 2). Consequently, Pratylenchus spp. density was significantly higher in control plots both at 6 and 12 WAE in both maize roots and soil samples. In contrast, the nematodes densities in fenamiphos treated plots were significantly lower than those found in the control even at 12 WAE. Because of its importance as one of the most damaging species to maize (Bridge and Starr, Reference Bridge and Starr2007; Coyne et al., Reference Coyne, Fourie, Moens, Perry and Moens2013), special attention was given to the Pratylenchus spp., in subsequent analyses.
Table 1. Nematode genera at six weeks after emergence (six WAE) in Chinhamhora for the season 2012/2013 (n = 6).

NS = not significant. LSD = least significant difference at 5% level; SED = standard error of differences of means; Numbers in brackets denote transformed means of nematode density as log (x+0.5); Means in the same column followed by the same letter are not significantly different at p < 0.05. *refers to nematodes that were not in abundance such as Tylenchulus spp and Tylenchorynchus spp.
Table 2. Mean nematode infestation in maize roots and soil at 6 and 12 weeks after emergence (6 and 12 WAE) in Chinhamhora (n = 6 replicates) for season 2012/2013 and 2013/2014.
NS = not significant, LSD = least significant difference at 5% significant level; SED = standard error of differences of means Numbers in brackets denote log (x+0.5) transformed means of nematode density as log (x+0.5);
Means in the same column followed by with the same letter are not significantly different at p < 0.05.
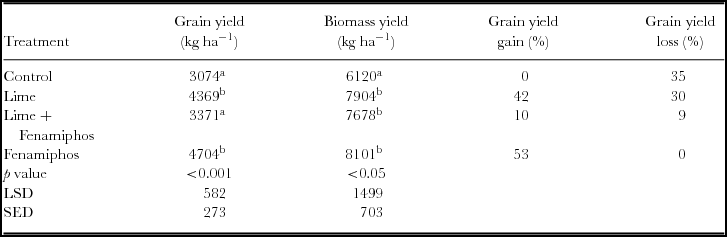
In terms of maize grain yield, the differences in treatments were also found to be significant (Table 3). The high nematode infestation in untreated control plots (no lime and no nematicide) resulted in much lower yield compared to fenamiphos (nematicide) treated plots which had the highest mean yield over the two years. This fenamiphos treatment gave a yield increase of 53% over the untreated control and had the lowest nematode density (Table 3). Relative to the fenamiphos treated plots, the grain yield loss due to nematode damage was equivalent to 35% in the control plots, 30% in lime and fenamiphos plots and lowest in lime treated plots at 9%. Biomass yield was also highest in fenamiphos-treated plots and lowest in the control with significant differences across the treatments over the two years (Table 3). Similarly, the percentage losses in biomass yield followed the same pattern as grain yields though somewhat lower in magnitude.
Table 3. Mean two-year maize grain yield, biomass and grain yield loss in Chinhamhora.

LSD = least significant difference at 5% level; SED = standard error of differences of means; Means in the same column followed by with the same letter are not significantly different at p < 0.05.
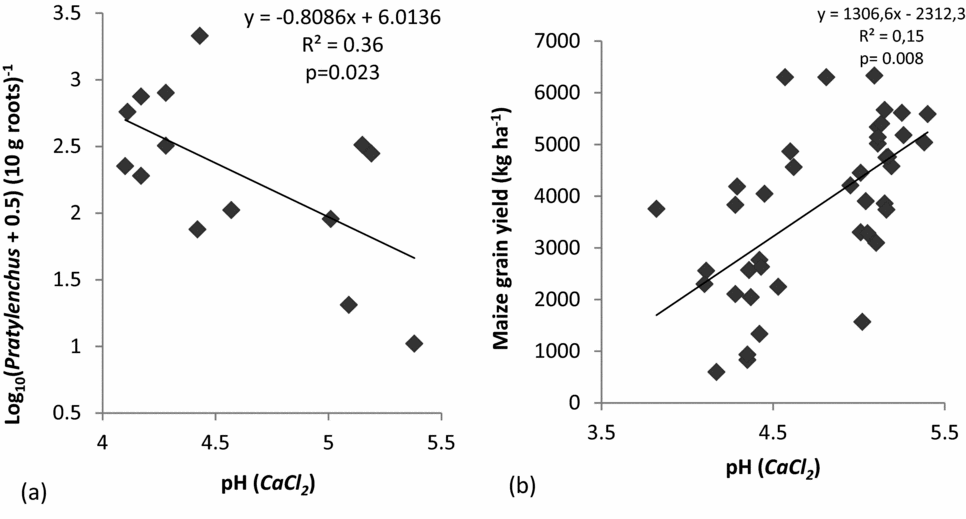
For the untreated controls, regression analysis showed a negative linear relation between Pratylenchus spp. and pH (Figure 2a). This regression between soil pH in untreated controls and log density of Pratylenchus spp. was significant at p = 0.04 (Figure 2a). However, for the fenamiphos-treated system the relationship between pH and Pratylenchus spp. was insignificant. Further regression analyses also showed a strong positive and significant (p = 0.008) linear relationship between maize grain yield and pH (Figure 2b).
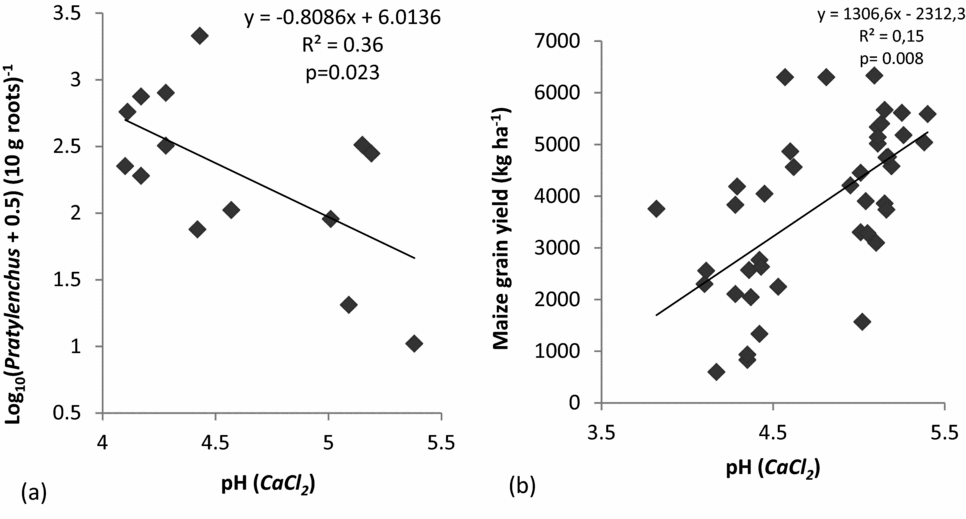
Figure 2. The relationship between Log density of Pratylenchus spp. infestation in maize roots and soil pH in untreated control systems (a), and the relationship between maize grain yield and pH (CaCl2) based on yield results from 2012/2013 (b) in Chinhamhora, Goromonzi district, Zimbabwe. In (a), data are based on samplings at 12 WAE, taken in two seasons (polled data).
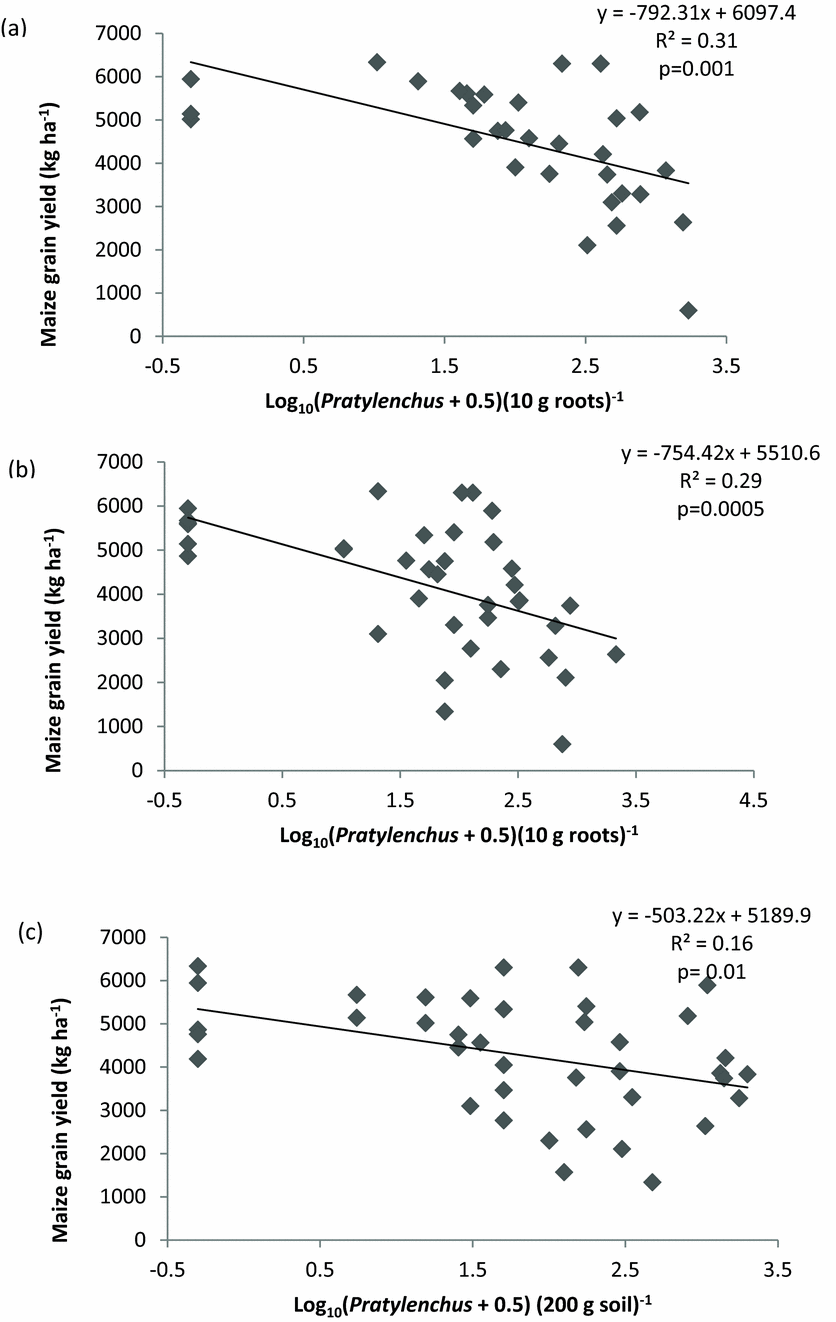
Consequently, the relationship between nematode infestation by Pratylenchus spp. and maize grain yield was negatively correlated (Figure 3). The resulting regression between log density of Pratylenchus spp. and maize yield was thus significant at both 6 WAE (Figure 3a) and 12 WAE (Figure 3b) in maize roots. The same pattern was observed at 12 WAE in soil samples (Figure 3c) using pooled data from both seasons.

Figure 3. The relationship between maize grain yield and log density of Pratylenchus spp. infestation in maize roots at 6 weeks after emergence (a) and at 12 weeks after emergence (b) and in soil at 12 weeks after emergence (c) in Chinhamhora, Goromonzi district, Zimbabwe combined for 2012/2013 and 2013/2014.
DISCUSSION
Application of lime and the nematicide fenamiphos generally reduced the nematode densities in soil. The non-plant parasitic nematodes such as the saprophytes were also reduced in numbers (Tables 1 and 2). The decrease in saprophytes in soil treated with lime and nematicide is a negative effect on soil health since the saprophytes are essential for organic matter decomposition and nutrient cycling (Massawe, Reference Massawe2010). Furthermore, parasitic nematodes such as Helicotylenchus spp., known to contribute positively to soil health, have been reported in agricultural lands but excessively large numbers potentially cause a significant reduction in maize yield (Kandji et al., Reference Kandji, Ogol and Albrecht2002). Thus, a certain balance for Helicotylenchus spp. is required in the soil. Differences in Pratylenchus spp. density in roots at 6 and 12 WAE was attributed to phenological development of the species feeding and reproducing in roots. The Pratylenchus spp. migrate in the roots as they feed, causing physical damage to the root system which depress the plant's ability to take up water and nutrients from the soil. Root openings by nematodes allow pathogens to access the damaged root tissues causing secondary root infections thereby further reducing yield potential. Therefore, regression of Pratylenchus spp. infestation in roots versus grain yield was more significant at 12 weeks (Figure 3b) than at 6 weeks (Figure 3a) because of increases in Pratylenchus spp. population with time (Massawe, Reference Massawe2010).
Grain yield in fenamiphos-treated plots (Table 3) that had the lowest nematode infestation (Table 2) was higher (+53%) when compared to the untreated control. Clearly, this indicates that nematodes are associated with yield losses in maize (Thompson et al., Reference Thompson, Stirling and Bell2008). This also confirms other research findings suggesting that nematodes reduce maize yield (Kagoda et al., Reference Kagoda, Derera, Tongoona, Coyne and Talwana2011). Grain yield in lime treated plots was higher by 42% when compared to the control (Table 3). The mean grain yield obtained in the current study is above the average yield (0.8 t ha−1) attainable by smallholder farmers in this region of Zimbabwe (Marongwe et al., Reference Marongwe, Nyagumbo, Kwazira, Kassam and Friedrich2012). In the lime-treated plots, nematode densities were significantly lower than in control plots, indicating that lime indirectly induces changes in the microbial community affecting soil nematode communities and food webs (Wang et al., Reference Wang, Zhao, Fu, Zhao, Wan and Zhou2015); thus soil pH is important in management of nematodes. By applying fenamiphos, a yield loss of 35% due to nematode infestation is avoided while a yield loss of 30% is avoided by application of lime. However, combining lime and fenamiphos had no significant reduction in grain yield loss from that of the untreated control compared to using lime alone, thereby suggesting that combining the two treatments does not give any extra benefit in controlling nematodes. Reactivity of both lime and fenamiphos is reduced when used in combination and this explains the compromised yield (Singh et al., Reference Singh, Walker, Morgan and Wright2003). As fenamiphos degrades faster when lime is added, the combination of the two treatments should be avoided (Singh et al., Reference Singh, Walker, Morgan and Wright2003). Although smallholder farmers usually and mostly rely on grain yield as a measure of productivity and a major reason for adoption of a technology, biomass yield is also of critical importance in CA; especially if livestock is an integral component of the farming system (Valbuena et al., Reference Valbuena, Erenstein, Tui, Abdoulaye, Claessens, Duncan, Gerard, Rufino, Teufel, van Rooyen and van Wijk2012).
In the current study, biomass yield in the untreated control plots was also significantly lower than the treated plots and biomass yield patterns were similar to grain yield. Biomass yield was highest in fenamiphos followed by lime and a combination of lime and fenamiphos. The results therefore suggest that nematode infestations equally affect both maize grain and biomass yield. Since CA requires retention and a buildup of organic material to give at least 30% soil cover by the time of planting (Kassam and Friedrich, Reference Kassam and Friedrich2012), mono-cropping by smallholder farmers and failure to control nematodes could result in a continual decline of biomass yield; resulting in major implications on CA performance. The negative response of yield to nematodes in the untreated control system reflects on the current management practices by smallholder farmers in Chinhamhora and other similar areas of Zimbabwe. The commonly practised maize mono-cropping promotes multiplication of nematodes in soil and increases the risk of nematode damage (Eche et al., Reference Eche, Iwuafor, Amapu and Bruns2013). Yet, if farmers were to practice rotations as recommended for CA with non-host leguminous crops, then the buildup of nematodes would also be controlled. Current rotations mainly involve horticultural crops (Mutenje et al., Reference Mutenje, Kassie, Gwara and Mujeyi2014) such as tomatoes which also favour proliferation of nematodes. Future studies thus need to further explore the nematode status on farms where rotations are being practised (Eche et al., Reference Eche, Iwuafor, Amapu and Bruns2013) in order to evaluate the contribution of rotations to nematode control in this farming system.
Although application of lime may have resulted in improved nutrient availability in the soil since the soil was very acidic, the low nematode numbers in the infestation assessments suggest that the yield benefit obtained from application of lime was also due to its effectiveness in reducing the nematode population (Wang et al., Reference Wang, Zhao, Fu, Zhao, Wan and Zhou2015). The results of the study show a reduction in the nematode population on application of lime (Tables 1 and 2) and generally with increase in pH (Figure 2a). Thus, yield response in fenamiphos-treated systems where pH remained low (Table 3) was similar to lime-treated systems, indicating that the yield increase response observed for lime is not only due to nutrient availability benefits related to pH management but rather to the control of Pratylenchus spp. activity. Fenamiphos effectively reduces the nematode population irrespective of pH levels in soil as found in this study, therefore nematode attack to plants is reduced as confirmed by the lack of a significant correlation between Pratylenchus spp. and pH in the fenamiphos-treated plots. This deactivation of Pratylenchus spp. through fenamiphos results in increased root growth for water and nutrient uptake hence the pronounced significant yield gain even when pH conditions were sub-optimal for maize. Furthermore, the nematode population declined as pH increased in untreated control plots thus providing further evidence for the influence of pH on nematode density (Figure 2a). Thus, liming is effective in altering and creating an unfavourable environment (Wang et al., Reference Wang, Zhao, Fu, Zhao, Wan and Zhou2015) for the nematodes to thrive and consequently increasing yield.
The current study demonstrated that nematodes are contributing significantly to yield losses in maize cropping systems on sandy soils in smallholder systems. Low pH also results in yield losses due to aluminium toxicity to crops (Nyamangara et al., Reference Nyamangara, Mugwira and Mpofu2000) thereby also resulting in yield losses. Therefore, lime could serve a dual purpose in such nematode-infested systems. The results also suggest that since both lime and fenamiphos are effective in nematode control when applied separately, yet the use of lime would be environmentally sustainable and a more preferred option for wider application since it also has positive benefits with regards to nutrient availability in the system. The lime requirements for this soil averaged 660 kg ha−1 and costs USD$ 6 per 50 kg bag, which equates to about USD$ 120 ha−1 inclusive of transport to smallholder communities around Harare located within 50 km radius. The bulkiness of lime impacts negatively on transport costs and ranks lower than other inputs thus hindering its use by smallholder famers (Musharo and Nyamangara, Reference Musharo, Nyamangara, Bationo, Waswa, Okeyo, Maina and Kihara2011). Unlike chemical fertilizers and other agricultural inputs, lime is not readily available from local agro-dealers shops that are within the farmers reach. In comparison, fenamiphos 40EC costs USD$ 24 a litre but only 1.7 litre of undiluted fenamiphos 40EC is required per hectare. A farmer thus needs approximately USD$ 60 ha−1 including transport if using the nematicide which is a much lower cost than if they have to use lime. Fenamiphos, therefore is much more attractive option cost wise than lime. However, lime is more sustainable than fenamiphos and lime is easier to handle than a hazardous nematicide. Lime may however have the set back that it reacts slowly with the soil and in situations where the nematode problem has become pandemic; the use of lime may not bring about immediate results unless the lime is applied prior to the start of the season. This means the choice of a nematode control method also depends on timing; let alone cost. The use of fenamiphos is limited due to its persistence in the environment and its effect on non-target organisms. Continuous use of fenamiphos reduces its efficacy against targeted nematodes due to its biodegradability hence; it is recommended to change to other commercially available nematicides after every three years (Hugo et al., Reference Hugo, Mouton and Malan2014).
CONCLUSION
The study showed that Pratylenchus spp. reduces maize grain yield. The use of fenamiphos, results in significantly lower nematode infestations and consequently higher maize grain yield. These findings suggest that failure to control nematodes results in maize grain yield loss of at least 35%. Then, maize grain yield can improve by over 1.5 t ha−1 when a nematicide is applied. The study also demonstrated that lime is as effective as the nematicide in Pratylenchus spp. control and other nematodes and can result in maize grain yields improving by 1 t ha−1. Yield gain due to the use of lime for nematode control amounted to 42% compared to the untreated control. The study therefore highlights the need for nematode control, especially the Pratylenchus spp. in maize produced on granitic sandy soils since yield loss can amount to more than 30%. Farmers may be recommended to use lime also as a nematode control strategy in sandy soils of Zimbabwe under maize production because of its extra positive established effects on pH and nutrient availability. However, in situations where quick results are required to control the nematodes, the use of a nematicide could be more appropriate and the usual precautions in handling pesticides are then needed. Further, long-term studies are required to understand nematode dynamics and management in smallholder CA systems, especially in granitic sands.
Acknowledgements
The authors would like to acknowledge the International Maize and Wheat Improvement Centre (CIMMYT) for funding this study and the extra logistical support facilitated through the ACIAR-funded project ‘Integrating crop and livestock production for improved food security and livelihoods in rural Zimbabwe (ZimCLIFS)’. We extend our gratitude to Kutsaga Research Station for the technical assistance in the nematology laboratory. We also want to acknowledge the farmers in Chinhamhora for hosting the trials. Last but not least we thank the Agricultural Technical and Extension Services (AGRITEX) extension staff especially Mr. Evans Magama, for his role in supervising and monitoring the trials.