Introduction
Under a paper title beginning with ‘Apprehending novel biodiversity’, Gordon (Reference Gordon2014) described 15 new genera from the north-central to southernmost extension of the largely submerged geological continent of Zealandia as far as the seamounts near the Hjort Trench at 59°S. Zealandia ranges from north of New Caledonia to features associated with the southern margin of the subantarctic Campbell Plateau (Mortimer & Campbell, Reference Mortimer and Campbell2014) and includes a highly diverse described and known-undescribed bryofauna of 1266 species (current to April 2016) (Gordon et al., Reference Gordon, Bock, Souto-Derungs and Reverter-Gil2019). Although Zealandia is very much smaller than the other continents including Australia (only 4.92 million km2 total crustal area for Zealandia vs 14.16 million km2 for Greater Australia (Sahul) (Mortimer & Campbell, Reference Mortimer and Campbell2014)), the known bryofauna of the latter realm presently comprises not many more species (1307), and both regions have more described and known-undescribed species than Europe (945) (current to 2016) (Gordon et al., Reference Gordon, Bock, Souto-Derungs and Reverter-Gil2019).
Over and above varied historic and present taxonomic effort in the three continental regions is the contribution of seascape evolution to biodiversity. Zealandia is globally exceptional in its disproportionate area of seafloor (94%) relative to land (6%). Aotearoa/New Zealand, the major geographic and political component of Zealandia, spans 30 degrees of latitude, has a legally ‘extended continental shelf’ of potentially 1.7 million km2 and one of the largest Exclusive Economic Zones in the world, of which about half the area is shallower than 2000 m. New Zealand's subtropical to subantarctic latitudinal spread is accompanied by a fractal coastline (estimated 15,000‒18,000 km) of inlets, headlands, spits, bays, harbours, fiords, sounds and estuaries, and the strikingly varied seafloor relief includes shelves, slopes, plateaus, seamounts, canyons, abyssal plains and two trenches, one exceeding 10 km deep (Gordon et al., Reference Gordon, Beaumont, MacDiarmid, Robertson and Ahyong2010). All this geological diversity is mirrored by the marine biodiversity, estimated at ~17,966 species (current to October 2013) (Gordon, Reference Gordon and Dymond2013).
The collection of the National Institute of Water and Atmospheric Research (NIWA), Wellington has many undescribed bryozoan species from throughout the New Zealand EEZ and southern Zealandia, representing many habitats and almost all seascape areas other than the abyssal sea floor and trench depths beyond 4500 m (Gordon, Reference Gordon and Ross1987). The present paper describes 16 new Zealandian species in 14 genera (13 new) and 11 families, three of them new. Accordingly, the paper is titled with reference to the earlier one in this two-part series, ‘Apprehending novel biodiversity’, appended by ‘redux’, a word that means brought back, resurgent or revived. This title has a dual application because one of the new species represents the first Recent representative of the otherwise Eocene‒Miocene genus Vincularia (Defrance, Reference Defrance1829) and family Vinculariidae (Busk, Reference Busk1852b). Further, one new genus, Elementella gen. nov., exhibits the simplest known skeletal morphology of any living cheilostome, resembling a Jurassic species, and three other species, suggestive of a relict Cenozoic fauna, show skeletal morphologies transitional or intermediate between different family-level taxa.
Materials and methods
Examined specimens were collected during numerous cruises carried out by NIWA and its predecessor, the New Zealand Oceanographic Institute (NZOI), between 1961 and 2015. Until 2007, all vessel-collected biological material, including bryozoans, was preserved in seawater‒formalin before later transfer to 70% isopropanol or ethanol. After 2007, all such material was immediately preserved in 99% ethanol unless specific protocols dictated otherwise. Station locations are shown in Figure 1 and complete station data are given in Appendix 1. Registered examined specimens are lodged in the NIWA Invertebrate Collection, prefixed with NIWA registration numbers.

Fig. 1. Map of station distributions for the new species of Zealandian Bryozoa described herein, based on specimens in the NIWA Invertebrate Collection, Wellington. Bathymetry (light grey) at 1000 m depth intervals.
Specimens prepared for scanning electron microscopy (SEM) were bleached in sodium hypochlorite (NaClO) solution to reveal details of the skeleton. Some specimens were metal-coated in gold-palladium or left uncoated and photographed using a Hitachi TM3000 Tabletop SEM.
Measurements of morphological characters were made of scanned images using FiJi (ImageJ) software (Schindelin et al., Reference Schindelin, Arganda-Carreras, Frise, Kaynig, Longair, Pietzsch, Preibisch, Rueden, Saalfeld, Schmid, Tinevez, White, Hartenstein, Eliceira, Tomcak and Cardona2012). Metrics are given in μm as the range, followed by the mean and standard deviation (bracketed) and number of measurements. The key characters have the following abbreviations:

SYSTEMATICS
Class MYOLAEMATA Schwaha, Ostrovsky & Wanninger, Reference Schwaha, Grischenko and Melnik2020a
Subclass GYMNOLAEMATA Allman, Reference Allman1856
Order CHEILOSTOMATA Busk, Reference Busk and MacGillivray1852a
Suborder INCERTAE SEDIS
Superfamily INCERTAE SEDIS
Family ELEMENTELLIDAE fam. nov.
Type Genus
Elementella gen. nov.
Diagnosis
Colony encrusting, uniserial, sparsely branching. Autozooids simple, pyriform to clavate or parallel-sided with short non-filiform cauda. Cystid gymnocystal, smooth; cryptocyst absent. Opesial area and membranous frontal wall very extensive, with terminal single-layered flap-like operculum that has an inner marginal scleritized rim. No spines, avicularia or ooecia. Basal pore-chambers present, shape variable. Kenozooids present where space limited for full autozooid development. Inferred ancestrula identical to other zooids, bipolar, budding daughter zooids mid-distally and mid-proximally.
Remarks
A new family and genus are introduced here for two deep-sea bryozoan species that have the simplest skeletal morphology of any known living cheilostomes. The large opesia is covered by a membranous frontal wall with a flap-like operculum that has an inner marginal sclerite and lateral flanges. There are no spines or tubercles, heterozooids and reproductive structures are apparently lacking, and the autozooidal cystid has no trace of a cryptocyst. The two species essentially have the appearance of simple runner-like ctenostomes that have calcified their lateral walls.
In existing classifications, Elementella gen. nov. would conventionally be classified in Electridae Stach, Reference Stach1937, a family that currently includes the earliest and morphologically simplest cheilostomes. To do so appears problematic, however, for two main reasons. First, the type genus of Electridae has a well-developed pitted or/and porous gymnocyst that is bordered by a narrow cryptocyst and typically several conical non-articulated spines that are mostly cuticular – unlike Elementella, which lacks a cryptocyst and spines. Species of Electra Lamouroux, Reference Lamouroux1816 also have basal pore-chambers (in zooids near the ancestrula) as well as smaller round mural pore-chambers with multiporous pore-plates (within lateral walls of multiserial zooids) (Prenant & Bobin, Reference Prenant and Bobin1966; Silén, Reference Silén1987), whereas uniserial Elementella has so far been found only with somewhat irregular tiny basal pore-chambers. Overall, then, the two genera differ in significant aspects of skeletal morphology. Second, molecular sequencing has shown that some other genera morphologically closer to Electra than Elementella are not as closely related as hitherto thought (Taylor & Waeschenbach, Reference Taylor and Waeschenbach2015). For example, two species of Einhornia Nikulina, Reference Nikulina2007, both previously included in Electra, are in a separate clade from one that comprises Electra in one branch and Aetea Lamouroux, Reference Lamouroux1812 and Membranipora de Blainville, Reference de Blainville1830 in another. Further, the putative electrid genus Conopeum Gray, Reference Gray1848 is basalmost in the same molecular tree and clearly not an electrid. A similar result for Conopeum had already been obtained by Nikulina & Schäfer (Reference Nikulina and Schäfer2008) (see also Gordon et al., Reference Gordon, Sutherland, Perez, Waeschenbach, Taylor and Di Martino2020). For these reasons, Elementella is not here included in Electridae.
Are there other nominal electrid genera that are morphologically closer to Elementella gen. nov.? Ironically, the species that comes closest is also the earliest – Pyriporopsis pohowskyi Taylor, Reference Taylor1994 from the late Jurassic (Oxfordian/Kimmeridgian: 157.1‒152.1 mya) of Yemen. This species is one of two attributed to Pyriporopsis Pohowsky, Reference Pohowsky and Larwood1973, the type species of which is Pyriporopsis portlandensis Pohowsky, Reference Pohowsky and Larwood1973, also from the late Jurassic (Tithonian: 152.1‒145.6 mya), but separated in time potentially by 6.5‒11.5 my. Both species have the same uniserial to loosely pluriserial colony form, with pyriform to oval or parallel-sided zooids having a mean length of 630 μm and 550 μm, respectively, for the two species. Although the two species are very similar overall, Taylor (Reference Taylor1994) observed two key differences – there is no trace of a cryptocyst or closure plates in P. pohowskyi, even in well-preserved zooids, whereas P. portlandensis has a distinctly striate (non-pustulose) cryptocyst and closure plates. These differences may be significant at genus level and more information on cheilostome diversity in the late Jurassic is desirable. Of the two species of Pyriporopsis, Elementella is morphologically closer to P. pohowskyi. Both Pyriporopsis species are here provisionally included in the Elementellidae. The status of Pyripora d'Orbigny, Reference Orbigny1852 in this context is contingent upon better knowledge of the Miocene type species Pyripora pyriformis (Michelin, Reference Michelin1848). The Cretaceous‒Eocene genus Herpetopora Lang, Reference Lang1914 differs from Elementella in having more-discrete, less variably shaped autozooids in which the cauda is narrower, becoming filiform in runner-like branches. Herpetopora zooids also lack basal pore-chambers. Taylor (Reference Taylor1988) describes Herpetopora as lacking a ‘non-pustulose cryptocyst’ (in contradistinction to Pyripora) but close examination of pl. 43, figure 5 in Taylor (Reference Taylor1988) shows that there is at least a very narrow cryptocyst-like opesial margin that is textured, quite unlike the thin, non-textured opesial margin in Elementella.
The ancestrula of Elementella is bipolar, i.e. budding of daughter zooids is both distopetal and proxipetal in the terminology of Nikulina (Reference Nikulina2002), an ancestral character state shared, inter alia, by Pyriporopsis, Conopeum, Herpetopora, Pyripora and Electra (all Electridae s.l.), as well as Aetea (Aeteidae), Eucratea (Eucrateidae) and Scruparia (Scrupariidae) (see Silén, Reference Silén1987, p. 32; Taylor, Reference Taylor1988; Nikulina, Reference Nikulina2001, p. 510; Nikulina, Reference Nikulina2002, p. S383). The ancestrula of P. portlandensis is very small and appears kenozooidal, though Taylor (Reference Taylor1986) thought this unlikely, suggesting that fine micritic sediment may have concealed any trace of a closure plate. The ancestrula of P. pohowskyi is unknown.
Thus, the sum of the characters exhibited by Elementella gen. nov., especially proxipetal budding from the ancestrula, suggests that the genus probably belongs in suborder Membraniporina. On the other hand, the possibility that it is a highly derived form that only superficially resembles primitive malacostegans cannot be ruled out. Inter alia, nothing is known about the mode of reproduction. Do the two new species of Elementella described here produce small eggs that develop into planktotrophic larvae in a deep-water environment? Evidence from a 5 km-deep ctenostome, Haywardozoon pacificum Grischenko, Gordon & Melnik, Reference Grischenko, Gordon and Melnik2018, shows that is possible (Schwaha et al., Reference Schwaha, Ostrovsky and Wanninger2020b). Or do ovicells or some other form of brooding occur in Elementella gen. nov. for which evidence is presently lacking? A putative new calloporid genus (Niwapora gen. nov.) is described below that somewhat resembles Elementella at the zooidal level but is multiserial and has vestigial ooecia.
Genus Elementella gen. nov.
Type Species
Elementella simplex sp. nov.
Etymology
Latin elementum, first principle, rudiment, plus diminutive suffix -ella, alluding to the very basic structure of the zooids.
Material Examined
Holotype: NIWA 22501, Station KAH0204/28, southern Three Kings Ridge, New Zealand, 490‒515 m depth.
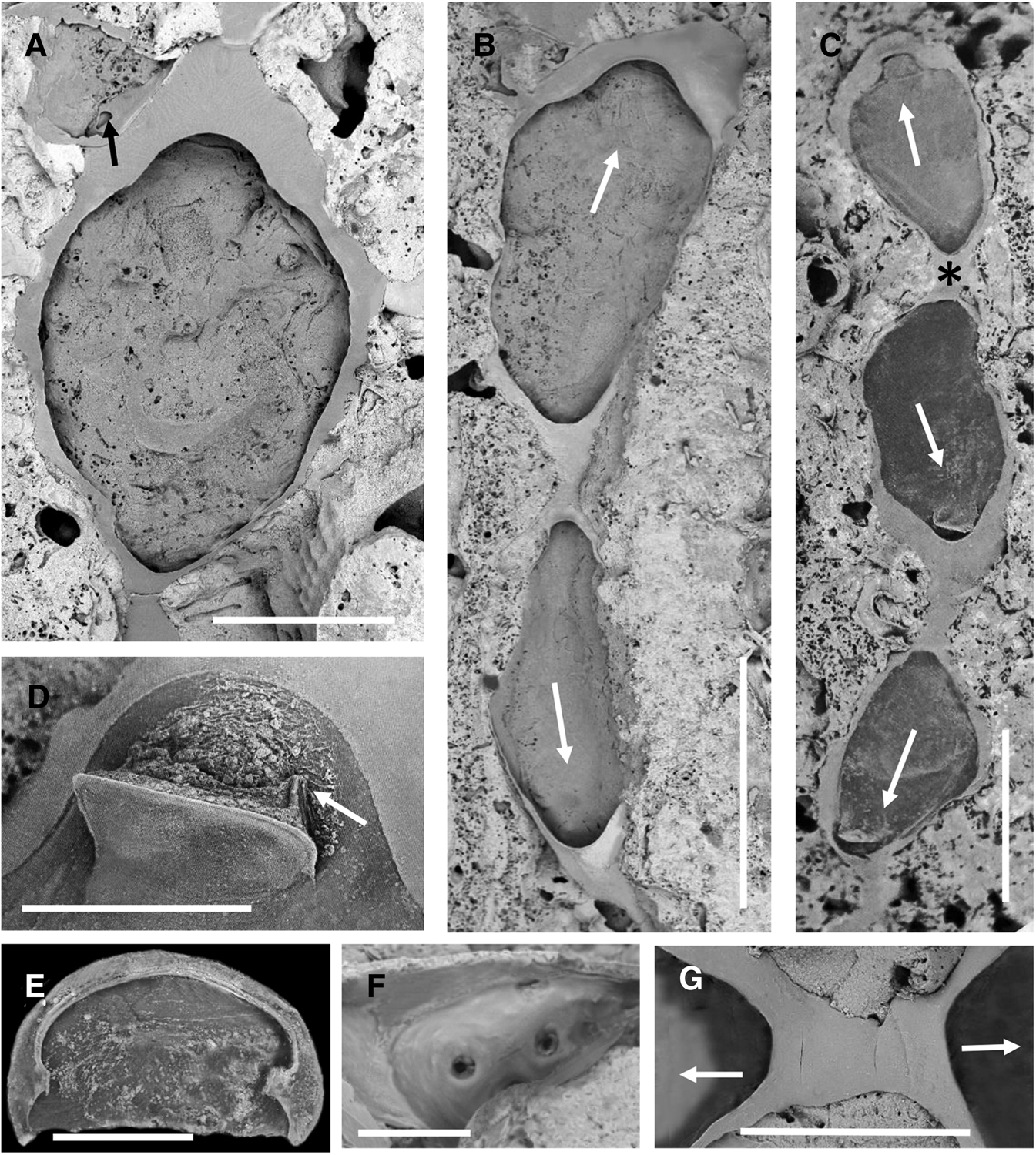
Fig. 2. Elementella simplex gen. et sp. nov., NIWA 22501, holotype, Station KAH0204/28, Three Kings Ridge: (A) autozooidal cystid; at top left is an aborted zooid (kenozooidal cystid) from an adjacent zooid that has established communication via a pore-chamber (opening arrowed). (B) bipolar pair, one of which (identity uncertain) is an ancestrula; arrows indicate growth direction. (C) linear group of three operculate zooids, the top two constituting a bipolar pair, one of which (identity uncertain) is an ancestrula; growth directions are arrowed; asterisk shows area magnified in G. (D) open operculum, with right-hand flange arrowed. (E) inner face of an operculum. (F) interior view of a biporous basal pore-chamber. (G) close-up of asterisked area of bipolar zooidal pair in C; there is no indication of fracture or repair. Scale bars: A, 0.5 mm; B, C, 1 mm; D, 200 μm; E, 100 μm; F, 30 μm; G, 400 μm.
Paratype: NIWA 95665, Station G3, northern Norfolk Ridge, 710 m depth.
Etymology
Latin simplex, simple, alluding to the simple morphology of the colony and autozooids.
Description
Colony encrusting, uniserial, sparsely branching. Maximum length seen ~19 mm.
Autozooids fragile, very simply constructed, variable in shape but generally pyriform to clavate or parallel-sided with short non-filiform cauda (ZL 1382‒1906 (1500 ± 207), N = 21; ZW 627‒1041 (829 ± 89), N = 21). Cystid entirely gymnocystal (Figure 1A), thin, smooth, with no trace of cryptocyst along its inner margin; in frontal view gymnocyst comprising a transversely broad arcuate area distal and lateral of the orifice, very narrow steep-sided walls laterally, and frontolateral caudal portion. Basal wall apparently uncalcified except for thin margin adjacent to lateral walls. Relative to length and width, autozooids very low in profile (height). Opesial area and membranous frontal wall very extensive (OpsL 817‒1473 (1114 ± 186), N = 21; OpsW 537‒1369 (712 ± 182), N = 21).
Operculum (Figure 1D, E) terminal in frontal membrane, almost semicircular, in dry state appearing thin, single-layered, with slightly thicker outer marginal band and weakly sclerotized rim on inner margin, the sides of which are extended downwards (inwards) as short vertical flanges (OpcL 140‒192 (170 ± 15), N = 21; OpcW 201‒309 (252 ± 34), N = 21). No spines.
Each autozooid with 1–2 small (~64 μm) basal pore-chambers in angle of each lateral wall with substratum; pore-chambers (Figure 1F) with 2‒4 pores. Lateral budding of new autozooids generally mid-distal, sometimes on each side distolaterally. Occasionally, if two zooid rows occur in close proximity and space is limited, a small operculate autozooid or non-operculate kenozooid may be interpolated between adjacent pore-chambers.
Avicularia and ooecia not encountered. No information on reproductive mode.
Inferred ancestrula identical to other zooids, budding daughter zooids mid-distally and midproximally (Figure 1B, C, G) (AnL 1611‒1649 (1634 ± 20) N = 3; AnW 766‒1041 (889 ± 140), N = 3).
Remarks
Apart from the exceptionally simple characters of the genus, Elementella simplex gen. et sp. nov. is also notable for its very large zooid size – up to almost 2 mm long. The depth of the zooid body cavity (i.e. zooid height as seen in profile) is very low relative to zooid length and the zooids are highly adaptable to surface irregularities in the substratum (rock and dead scleractinian coral), with the basal wall moulding itself into hollows and over bumps, regardless of the presumed disposition of the polypide; one zooid in the paratype even has a 90° bend midlength, which evidently had not affected its function in life.
The interpolation of a small operculate autozooid or non-operculate kenozooid between adjacent pore-chambers when space is limited was also observed in Herpetopora (Taylor, Reference Taylor1988).
A second species, Elementella secunda sp. nov., described below, differs in having shorter autozooids (mean 899 μm) that are proportionally narrower (mean 295 μm) with a relatively longer cauda; the substantially smaller operculum (and orifice) in E. secunda implies a significantly smaller tentacle crown, thereby supporting the distinction of two species. A third morphology, represented by a specimen (NIWA 3710) from Station KAH0204/15, southern Three Kings Ridge, 470‒480 m, is somewhat intermediate between the two new species in autozooid size and proportions. Zooids have a mean length of 1037 μm and mean width of 499 μm. This intermediate form could potentially represent a third species but the zooids are all somewhat damaged and more material is needed for a fuller assessment.
Elementella simplex sp. nov. is so far known only from the northern Norfolk Ridge to southern Three Kings Ridge at 490‒710 m depth.
Elementella secunda sp. nov.
(Figure 3A‒C)
Material Examined
Holotype: NIWA 95666, Station Z11008, Norfolk Ridge, 710 m depth.

Fig. 3. Elementella secunda sp. nov., NIWA 95666, holotype, Station Z11008, Norfolk Ridge: (A) two operculate clavate autozooids. (B) enlargement of more-distal zooid in A. (C) an operculate autozooid that has encountered another zooid, producing an aborted zooid (kenozooid) at the point of contact. Scale bars: A, C, 500 μm; B, 300 μm.
Etymology
Latin secundus, second, being the second described species of the genus.
Description
Colony encrusting, uniserial, very sparsely branching; material fragmented, longest uniserial portion 6 mm.
Autozooids fragile, clavate, with proximally tapering caudal portion (Figure 3A‒C) (ZL 759‒1088 (899 ± 132), N = 6; ZW 268‒321 (295 ± 22), N = 6).
Cystid entirely gymnocystal, thin, smooth, with no trace of cryptocyst along its inner margin; in frontal view gymnocyst comprising lateral walls and frontolateral caudal portion. Basal wall very thin, apparently mostly cuticular. Opesial area and membranous frontal wall very extensive (OpsL 184‒517 (399 ± 115), N = 6; OpsW 174‒219 (199 ± 18), N = 6). Operculum terminal in frontal membrane, almost semicircular, in dry state appearing thin, single-layered, with slightly thicker outer marginal band and weakly sclerotized rim on inner margin (OpcL 53‒62 (59 ± 5), N = 3; OpcW 76‒82 (79 ± 3), N = 3). No spines.
Budding loci mid-distal and distolateral or mid-lateral; no evidence of proximolateral budding sites in the limited material. One kenozooid seen (Figure 3C), presumably connecting with a distolateral pore-chamber in an encroaching autozooid, obstructing further growth.
No avicularia or ooecia. No information on reproductive mode. Ancestrula not seen.
Remarks
Elementella secunda sp. nov. is most readily distinguished from E. simplex sp. nov. by its much smaller zooids that are clavate with a tapering cauda and by the absence of a moderately broad gymnocystal area distal of the orifice. Both species have extremely simple morphology, including the absence of a cryptocyst.
As mentioned above, a third morphology was seen that is intermediate in dimensions between E. simplex sp. nov. and E. secundus sp. nov. Like E. simplex it has a moderate area of gymnocyst distal to the orifice but the proximal caudate portion is proportionally broader. Elementella secunda sp. nov. encrusted deep-sea rock.
The species is so far known only from the type locality on Norfolk Ridge, at 710 m depth.
Suborder FLUSTRINA Smitt, Reference Smitt1868
Superfamily CALLOPOROIDEA Norman, Reference Norman1903
Family CALLOPORIDAE Norman, Reference Norman1903
Genus Niwapora gen. nov.
Type Species
Niwapora grandis sp. nov.
Etymology
From NIWA, acronym for the National Institute of Water & Atmospheric Research, Wellington, plus Latin pora, the feminized form of porus, hole, a common suffix in bryozoan generic names.
Diagnosis
Colony encrusting, multiserial, contiguous. Autozooids with very large opesia bordered by narrow gymnocyst and vestigial cryptocyst with single series of tiny tubercles. No spines or avicularia. Ovicell immersed, cleithral. Ooecium vestigial, derived from distal zooid, comprising transversely narrow flattened outfold. Sparse uniporous mural septula present. Ancestrula like autozooids.
Remarks
Niwapora gen. nov. is monotypic for a species that occurs on the Cavalli Seamount off north-eastern North Island. It is reminiscent of Aplousina Canu & Bassler, Reference Canu and Bassler1927 in its simple autozooidal morphology; the type species, Aplousina gigantea from the western Atlantic, likewise has a vestigial cryptocyst with a single series of beading, and the gymnocyst is so reduced as to be vestigial to virtually absent. The ooecium differs, however, in its larger size, appearing as a moderately conspicuous bulge that extends under the distal zooidal margin into the proximal end of the daughter autozooidal cavity (Winston, Reference Winston1982, Reference Winston2010). In Niwapora, the reduced ooecium appears as a frontally inclined cap with a proximofrontal crest. It replaces the mid-distal area of gymnocystal wall, such that the underside of the ooecium can be seen from below.
It is moot whether or not Niwapora is a calloporid. It differs markedly from the type genus and species of the family, which is characterized by stout periopesial spines, adventitious avicularia, well-developed ooecia with a frontal skeletal exposure of endooecium, and basal pore-chambers. Calloporidae is morphologically heterogeneous, with ~81 living and fossil genera, and awaits comprehensive molecular sequencing of a range of genera that are putatively included in it. Calloporidae, as presently conceived, exhibits a varied range of expression of gymnocyst, cryptocyst, spines, avicularia, ooecia and pore-chambers, in various permutations and combinations. Presently, there is no other family in which Niwapora may be included.
Could Elementella gen. nov. and Niwapora gen. nov. be related? Niwapora grandis sp. nov. has such a microscopic cryptocyst that, if it were to disappear altogether, infertile zooids would resemble those of Elementella spp., which are otherwise caudate because of uniseriality. Elementella gen. nov. lacks ovicells, but it is possible these have not been found yet or it is an internal brooder. Mediating against a possible relationship between the two genera are differences in ancestrular budding (unipolar in Niwapora, bipolar in Elementella) and interzooidal communications (uniporous mural septula in Niwapora, multiporous basal pore-chambers in Elementella).
Niwapora grandis sp. nov.
(Figure 4A‒H)
Material Examined
Holotype: NIWA 95667, Station KAH0204/8, Cavalli Seamount, 610‒640 m depth.

Fig. 4. Niwapora grandis gen. et sp. nov., NIWA 95667, Station KAH0204/8, Cavalli Seamount: (A) part of colony, showing self-overgrowth; some zooids ovicellate. (B) distal ends of non-ovicellate (left) and ovicellate (right) zooids. (C) distal view of an ovicellate zooid at the bifurcation of a zooid row; uniporous septula arrowed. (D, E) frontal and left-lateral views of the same ooecium; uniporous septula arrowed. (F) frontal view of parts of adjacent autozooidal lateral walls showing minute cryptocystal beading. (G, H) frontal and right-lateral views of the same ooecium. Scale bars: A, 1 mm; B, C, 500 μm; D, E, G, H, 300 μm; F, 50 μm.
Etymology
Latin grandis, large, alluding to the large autozooid size.
Description
Colony encrusting, multiserial, initially unilamellar, but capable of self-overgrowth; sole colony 17 mm long, 9 mm wide.
Autozooids large, mostly more or less roundly subhexagonal in outline (Figure 4A), relatively thin-walled (ZL 935‒1373 (1082 ± 110), N = 23; ZW 679‒1015 (791 ± 92), N = 23). Opesia very extensive, occupying 92% of zooid length on average (OpsL 783‒1029 (988 ± 100), N = 15; OpsW 609‒898 (716 ± 81), N = 15). Gymnocyst very narrow, periopesial, slightly wider distolaterally and proximolaterally, a little elevated as distal oral rim. Cryptocyst periopesial, vestigial, comprising single series of microscopic tubercles along inner margin of gymnocyst (Figure 4F).
Operculum not seen. No spines or avicularia.
Ovicell immersed, cleithral. Ooecium (Figure 4B‒E, G, H) much reduced, wider than long, comprising transversely narrow hollow cap that is inclined frontalwards with proximofrontal crest; ectooecial surface smooth with thin transverse striae on each side. Developmentally derived from distal zooid via long slit, occupying/replacing mid-distal area of gymnocystal wall, such that underside of ooecium can be seen from below (OoL 77‒136 (101 ± 17), N = 11; OoW 305‒422 (359 ± 38), N = 11).
Sparse uniporous mural septula (Figure 4C, E) present; typically two on each lateral wall; only one in each transverse wall (pore-plate diameter 47‒66 (56), N = 7; pore diameter 8‒12 (11), N = 5). No distinction in size of pore-plate or pore between transverse and lateral walls.
Sole ancestrula like autozooids, except proximal margin more truncate (AnL 934, AnW 697). Budding of paired daughter zooids distopetal.
Remarks
The sole specimen encrusted a piece of rock collected during a rock-dredge tow from 640 to 610 m depth on the Cavalli seamount.
Genus Quasicallopora gen. nov.
Type Species
Quasicallopora bathyalis sp. nov.
Etymology
Latin quasi, appearing as if, simulating, plus Callopora, a related genus.
Diagnosis
Colony encrusting, unilaminar, multiserial. Some autozooids partly disjunct. Opesia extensive, frontally visible gymnocyst greatly reduced, cryptocyst periopesial, narrow throughout, with weak longitudinal lineations not pustules. Operculum proportionally large, terminal in membranous frontal wall. Spines articulated, sparse, long, not periopesial. No avicularia. Ovicell terminal, cleithral. Ooecium a kenozooid budded from maternal autozooid. Ectooecium almost completely membranous, endooecium with hexagonal reticulation; closure cleithral. Biporous mural septula present. Ancestrula like autozooids.
Remarks
Quasicallopora gen. nov. resembles the type and other species of Callopora Gray, Reference Gray1848 in general appearance and in having ooecia with an extensive endooecial surface, but differs in that the ooecia are terminal and kenozooidal, avicularia are lacking and there are multiporous mural septula instead of basal pore-chambers.
Callopora is also strictly a northern-hemisphere genus. Three austral species (one fossil) have been attributed to Callopora but these attributions are here considered erroneous. Callopora precocialis Gordon, Reference Gordon1984, described from the Kermadec Ridge, is now included in Judyella gen. nov. (see below). South African Callopora jamesi O'Donoghue & de Watteville, Reference O'Donoghue and de Watteville1944 needs further study but it appears not to belong to Callopora – it lacks avicularia and its cryptocyst is negligible. Further, if ‘Crassimarginatella? sp.’ of Florence et al. (Reference Florence, Hayward and Gibbons2007) is conspecific with C. jamesi, then the smooth skeletal surface of the ooecium in this species is endooecium, which is normally characteristically granular when not restricted to a small tabulate area. Palaeogene Amphiblestrum moniliferum Maplestone, Reference Maplestone1901 from Australia, included in Callopora on the Bryozoa Home Page (Bock, Reference Bock2016), likewise needs further study; ooecia were not described and the only avicularium was interpreted by Maplestone as vicarious.
Alderina Norman, Reference Norman1903 resembles Quasicallopora gen. nov. in lacking avicularia but it differs in also lacking lateral spines while possessing an ectooecial tabula and basal pore-chambers. Allantocallopora d'Hondt & Schopf, 1985 is a monotypic deep-sea genus, but differs from Quasicallopora gen. nov. in being uniserial, with only distal spines, a weakly carinate ooecium and a laterally flared opesial margin. Species of Copidozoum Harmer, Reference Harmer1926 can have a large exposure of endooecium but the ovicell is acleithral and the genus is characterized by the presence of large interzooidal or subvicarious avicularia with a tapering rostrum.
Quasicallopora bathyalis sp. nov.
(Figure 5A‒C)
Material Examined
Holotype: NIWA 144896, Station TAN1501/CARAVEL FF4, head of Bounty Trough, eastern South Island, New Zealand, 1126 m depth.

Fig. 5. Quasicallopora bathyalis gen. et sp. nov.: (A) NIWA 146071, Station TAN1301/CARAVEL FF2, Bounty Trough, ovicellate colony on tube of arenaceous foraminiferan Rhabdammina major. (B), NIWA 22534, Station E416, Bounty Trough, ancestrula (lower left) and daughter zooids. (C) close-up of lower-right ovicellate zooid in A. Quitocallopora aviculata gen. et sp. nov.: NIWA 95628, paratype, TAN0413/171, Bay of Plenty: (D) aviculiferous ovicellate colony; note the very long filiform spines. (E) NIWA 23244, TAN0413/171, Bay of Plenty, ooecia at two stages of development; note the ectooecium in the zooid at right. Scale bars: A, 2 mm; B, C, 500 μm; D, 1 mm; W, 300 μm.
Paratypes: NIWA 146071, Station TAN1301/CARAVEL FF2, head of Bounty Trough near Karitane Canyon, eastern South Island, New Zealand, 1117 m depth; NIWA 146100, Station TAN1501/ANADARKO REF 6, head of Bounty Trough, eastern South Island, New Zealand 1024 m depth.
Other material: NIWA 22534, 95592, Station E416, 45.3500°S 171.9500°E, head of Bounty Trough, eastern South Island, New Zealand, 1225 m depth; NIWA 95593, Station E417, head of Bounty Trough, eastern South Island, New Zealand, 860 m depth; NIWA 95591, Station S148, SW Chatham Rise, New Zealand, 859 m.
Etymology
From latinized Greek, bathys, deep, alluding to the occurrence of the species at bathyal depths.
Description
Colony encrusting, unilaminar, multiserial, small comprising spots (Figure 5A) with maximum diameter 4 mm.
Autozooids elongate-oval (ZL 621‒955 (789 ± 88), N = 17; ZW 442‒655 (536 ± 60), N = 17), separated by deep interzooidal furrows, many slightly disjunct at corners, connected by very short broad tubes. Opesia extensive, occupying 74% or more of zooid length on average (OpsL 510‒652 (584 ± 60), N = 5; OpsW 349‒430 (393 ± 31), N = 5). Frontally visible gymnocyst greatly reduced, confined to small area between each pair of distolateral spines, steeply sloping proximal side walls and frontal surface of connecting tubes. Cryptocyst periopesial, narrow, of more or less equal width throughout, surface smooth or with weak, variable longitudinally wavy lineations, not pustulose as such.
Operculum proportionally large, terminal in membranous frontal wall, broadly semicircular (OpcL 115‒135 (127 ± 10), N = 3; OpcW 210‒263 (237 ± 27), N = 3).
Spines articulated, sparse, but conspicuous for their length; not periopesial but confined to distal half to two-thirds of zooid length; comprising two distolateral pairs and 1‒2 lateral pairs, or one of the most proximolateral pair missing; elevated vertically or slightly inclined distad if distolateral or outwards if lateral; length 355‒665 μm. No avicularia.
Ovicell (Figure 5C) well-developed, terminal, cleithral, not touching substratum when viewed in profile (OoL 189‒220 (203 ± 13), N = 5; OoW 274‒323 (291 ± 19), N = 5). Ectooecium almost completely membranous apart from narrow proximofrontal strip; endooecium with generally hexagonal reticulation. Closure cleithral.
Two widely separated mural septula on each lateral wall; typically biporous, rarely uniporous; a pair of side-by-side biporous septula on the distal transverse wall (biporous pore-plate diameter 45‒60 (54), N = 5; uniporous pore-plate diameter 25‒38 (32), N = 5; pore diameter 4).
Ancestrula (Figure 5B) like autozooids but slightly smaller (AnL 510‒583 (547 ± 51), AnW 387‒437 (412 ± 35), N = 2). One mid-distal and two distolateral daughter zooids. Proximal communications established with later zooids proximolaterally and proximally.
Remarks
Colonies of Quasicallopora bathyalis gen. et sp. nov. were found solely on tubes of the arenaceous deep-sea foraminiferan Rhabdammina major de Folin. These occurred at the sediment surface at depths of 859‒1225 m in the head of the Bounty Trough south and south-west of Banks Peninsula, South Island. Other undescribed cheilostome bryozoans occur on the same substratum, including a species of Chaperiopsis and one of Fenestrulina.
Genus Quitocallopora gen. nov.
Type Species
Quitocallopora aviculata sp. nov.
Etymology
Latin quitus, enabled, strong, alluding to the presence of robust interzooidal avicularia in the type species, plus Callopora, a related genus.
Diagnosis
Colony encrusting, unilaminar, multiserial. Autozooids with large opesia. Cryptocyst periopesial, narrow to moderate, of almost equal width throughout, granular. Gymnocyst scarcely visible laterally, more evident proximally and proximolaterally. Articulated spines in distal third of zooid or periopesial. Avicularia, if present, interzooidal with sloping gymnocystal walls; rostral part and opesial parts almost equivalent in size, each semi-circular; rostrum with proportionately large foramen; pivot bar complete; opesia transversely crescentic bordered by granular crescentic cryptocyst. Ovicell hyperstomial. Ooecium formed from roof of distal zooidal/kenozooidal bud. Ectooecium membranous except for basal peripheral part, endooecium calcified, with granular-tubercular surface. Basal pore-chambers present. Ancestrula tatiform, with periopesial spines and granular cryptocyst.
Remarks
Quitocallopora gen. nov. is established here for two deep-sea New Zealand species that form spot-type colonies. The genus appears closest to Crassimarginatella Canu, Reference Canu1900 in its affinities. Gordon (Reference Gordon2014) and Min et al. (Reference Min, Seo, Grischenko, Lee and Gordon2017) have discussed the range of morphological variation in Crassimarginatella, especially that expressed by ooecia and avicularia (see also Harmelin, Reference Harmelin1973), concluding that, while splitting the genus appeared to have merit, more information was needed. The type species, Crassimarginatella crassimarginata (Hincks, Reference Hincks1880a), has, inter alia, avicularia with a stout pivot bar, basal pore-chambers and a tatiform ancestrula with spines, all features shared with the type species of Quitocallopora gen. nov. The ovicell in Quitocallopora, however, justifies recognition of a new genus; unlike C. crassimarginata, which has a cleithral ovicell and a smooth, wholly ectooecial surface (Chimenz Gusso et al., Reference Chimenz Gusso, Nicoletti and Bondanese2014, figure 33c, d), Quitocallopora has a non-cleithral ovicell and ectooecial calcification is restricted to a narrow band. The occurrence of some ooecia at the edge of the colony in the two new species described here shows them to be formed from the roof of the distal basal pore-chamber, which is, in fact, a zooidal bud with a membranous terminal part that can potentially grow into an autozooid. Its basal wall in the present material does not always rest on the substratum. Quitocallopora gen. nov. also differs from Corbulella Gordon, Reference Gordon1984 in having non-cleithral ovicells and a complete pivot bar in the avicularia.
Quitocallopora aviculata sp. nov.
(Figures 5D, E; 6A‒D)
Material Examined
Holotype: NIWA 23244, Station TAN0413/171, outer Bay of Plenty, North Island, New Zealand, 310‒410 m depth.

Fig. 6. Quitocallopora aviculata gen. et sp. nov.: (A) NIWA 95628, paratype, TAN0413/171, Bay of Plenty, operculate ovicellate zooid from colony in Figure 5D. (B) NIWA 23244, TAN0413/171, Bay of Plenty, fully developed ooecium flanked by avicularia. (C), same, distal view of large uniporous pore-chamber. (D) NIWA 95628, avicularium with mandible in place. Quitocallopora pusilla sp. nov., NIWA 95618, Station TAN0205/19, Kermadec Ridge: (E) ancestrulate ovicellate spot colony. (F) ovicellate zooid in E. (G) close-up of ancestrula in E. Scale bars: A, C, F, G, 200 μm; B, 300 μm; D, 100 μm; E, 500 μm.
Paratypes: NIWA 95628, 95629, same data as holotype.
Other material: NIWA 95630, same data as holotype.
Etymology
Alluding to the presence of avicularia.
Description
Colony encrusting, unilaminar, multiserial, small, comprising spots up to 3.7 mm diameter. Autozooids in quincunx, or one of the six surrounding zooids is an avicularium, or there are seven surrounding zooids, one an avicularium.
Autozooids mostly longer than wide, roundly subhexagonal to oval or pyriform, with deep interzooidal furrows between adjacent lateral walls (ZL 413‒627 (487 ± 60), N = 20; ZW 331‒447 (392 ± 39), N = 20). Opesia relatively large, bordered by conspicuously granular cryptocyst, this moderately wide, of almost equal width throughout, a little narrower distally; granules larger towards rim (OpsL 220‒323 (272 ± 28), N = 20; OpsW 187‒314 (245 ± 37), N = 20). Gymnocyst scarcely visible laterally, more evident proximally and proximolaterally. Typically two pairs of widely separated spines in distal third of zooid; usually a midproximal spine or this displaced to one side by an ooecium. Some spines can be exceedingly long – up to 2.8 mm (Figure 5D). Opercular flap (Figure 6A) relatively large, the proximolateral corners turned slightly outwards (OpcL 100‒126 (113 ± 10), N = 7; OpcW 162‒202 (175 ± 14), N = 7).
Avicularia interzooidal (Figure 6B), conspicuous, reasonably numerous. Cystid roundly subquadrate to subrectangular with sloping gymnocystal walls (AvL 219‒319 (260 ± 31), N = 9; AvW 159‒273 (210 ± 41), N = 9). Rostral part equal in size or slightly smaller than opesial part, semi-circular, with proportionately large foramen surrounded by narrow palatal margin and, on proximal side, a complete pivot bar with tiny tubercle(s) simulating midproximal ligula. Proximal part of avicularium semicircular, with small transversely crescentic opesia bordered by broadly crescentic cryptocyst with conspicuous granulation.
Ovicell hyperstomial, often terminal, acleithral [OoL 157–206 (181 ± 17), n = 9; OoW 189–218 (201 ± 11), n = 9]. Ooecium (Figures 5E, 6A, B) formed from the roof of the large, distal basal pore-chamber. Most of frontal skeletal surface is endooecium with coarse granules, many tabulate; ectooecium mostly membranous, its calcareous part comprising a very narrow band around periphery and proximofrontal rim. Opening flanked by distalmost pair of spines.
Interzooidal communications via relatively large and shallow lateral and distal pore-chambers (Figure 6C), each with a single small pore in the middle.
Ancestrula tatiform, with seven periopesial spines and granular cryptocyst encircling opesia.
Remarks
Quitocallopora aviculata gen. et sp. nov. occurred on rocky substrata. The largest (paratype, NIWA 95629) has 31 zooids (12 with ooecia) and five avicularia. Zooidal basal walls are generally fully adherent to the substratum, but can produce short props – outpocketings of the basal wall – where zooids cross cavities or depressions. All eight colonies in the collection have some zooids that are produced by intramural reparative budding. New buds standing proud of the colony surface begin a new layer of zooids that overgrows the underlying layer. Ancestrular cystids bud autozooids, and both ancestrulae and autozooids can produce up to two stacked zooids frontally.
The species is so far known only from the type locality in the outer Bay of Plenty, North Island, at 310‒410 m depth.
Quitocallopora pusilla sp. nov.
(Figure 6E‒G)
Callopora precocialis Gordon, Reference Gordon1984: 26 (part).
Material Examined
Holotype: NIWA 1161, Station K795, mid-Kermadec Ridge, New Zealand, 350 m depth.
Paratypes: NIWA 22962, 95618, Station TAN0205/19, mid-Kermadec Ridge, New Zealand, 420‒471 m depth.
Etymology
Latin pusillus, very little, alluding to the small spot-like colonies.
Description
Colony encrusting, unilaminar, multiserial, small, comprising spots up to 1.4 mm diameter; largest colony with nine zooids including ancestrula.
Autozooids longer than wide, subpyriform, with interzooidal furrows between adjacent lateral walls (ZL 315‒583 (450 ± 84), N = 11; ZW 252‒389 (321 ± 48), N = 11). Opesia relatively large, bordered by narrow granular cryptocyst, of almost equal width throughout, a little narrower and non-granular distally (OpsL 157‒335 (256 ± 53), N = 11; OpsW 174‒288 (223 ± 40), N = 11). Gymnocyst very narrow laterally, moderately developed proximally. Eleven or 12 well-developed periopesial spines (Figure 6E), sparser proximally, with proportionately stout bases and a high articulation point; acicular beyond this point. Spines straight, near-vertical to inclined slightly outwards distally and proximally, arching a little over opesia proximally; longest spine only 317 μm. Opercular flap transversely D-shaped, almost parallel-sided, the proximolateral corners turned slightly outwards (OpcL 71‒87 (79 ± 11), N = 2; OpsW 106‒109 (108 ± 2), N = 2).
No avicularia seen. Ovicell hyperstomial, terminal, apparently acleithral. Ooecium (Figure 6F) formed from a distal kenozooid visible in lateral profile but not from above. Most of frontal skeletal surface a granular-tubercular endooecium; calcareous part of ectooecium comprising very narrow proximofrontal rim. Opening flanked by distalmost pair of spines (OoL 180‒204 (190 ± 12), N = 3; OoW 165‒182 (174 ± 9), N = 3).
Interzooidal communications via relatively large and shallow lateral pore-chambers, each with a single small pore in the middle. Ancestrula tatiform (Figure 6G), with 10–11 periopesial spines and granular cryptocyst encircling opesia (AnL 240‒262 (251 ± 16), N = 2; AnW 205‒256 (231 ± 37), N = 2).
Remarks
Quitocallopora pusilla sp. nov. occurred on small rocks and dead scleractinian coral. The two largest of three colonies have nine zooids, one of them with two ooecia. Zooidal basal walls are generally fully adherent to the substratum, but, as in Q. aviculata sp. nov., can produce short props – outpocketings of the basal wall – where zooids cross cavities or depressions.
Quitocallopora pusilla sp. nov. differs most obviously from Q. aviculata sp. nov. in having a smaller colony size and smaller mean zooid size as well as lacking avicularia and possessing a larger number of periopesial spines. As in Q. aviculata, there is regeneration of zooids in autozooidal cystids.
The species is so far known only from the mid-Kermadec Ridge at 350‒471 m depth.
Genus Judyella gen. nov.
Type Species
Judyella corona sp. nov.
Etymology
Honorific for Dr Judith E. Winston in recognition of her sterling contributions to bryozoology.
Diagnosis
Colony encrusting, unilaminar, uniserial to multiserial. Autozooids with moderately large subcircular to oval opesia. Cryptocyst periopesial, narrow, of almost equal width throughout, granular. Gymnocyst visible laterally, especially if uniserial, well developed proximally. Articulated spines numerous, upright, periopesial, equally spaced apart. No avicularia. Ovicell hyperstomial, terminal, acleithral. Ooecium formed from distal kenozooid; ectooecium fully calcified with no frontal suture, furrow or fenestra. Interzooidal communications somewhat intermediate in size and position between mural and basal pore-chambers, uniporous. Ancestrula tatiform, with periopesial spines and granular cryptocyst.
Remarks
Judyella gen. nov. is established here for three New Zealand species that form shortly ramifying or small spot-like colonies. They appear most closely related to Pyriporoides Hayward & Thorpe, Reference Hayward and Thorpe1989 and Olisthella Gordon & Taylor, Reference Gordon and Taylor2017, but differ in having an evenly rounded opesia (i.e. not constricted or parallel-sided), a uniformly narrow cryptocyst (i.e. no shelf) and a wholly smooth ectooecium (i.e. no median suture, furrow or fenestra). All three genera have in common an ooecium that develops concurrently with a heterozooid, typically a kenozooid (rarely also an avicularium in the case of Pyriporoides). Following earlier work on uniserial calloporids (Rosso & Taylor, Reference Rosso and Taylor2002), Gordon & Taylor (Reference Gordon and Taylor2017) used cladistic analysis to identify an apparent clade within or derived from Calloporidae that included Pyriporoides and Olisthella (inter alia). If molecular sequencing were to support this morphology-based clade, Judyella gen. nov. would almost certainly belong to it.
Judyella corona sp. nov.
(Figure 7A‒E)
Material Examined
Holotype: NIWA 3714, Station KAH0204/32, Cavalli Seamounts, NE of North Island New Zealand, 780‒810 m depth.

Fig. 7. Judyella corona gen. et sp. nov., NIWA 3714, holotype, Station KAH0204/32, Cavalli Seamount: (A) autozooids and ovicellate zooids; one broken autozooid with reparative kenozooid (kz). NIWA 95607, paratype Station KAH0204/47, Cavalli Seamount: (B) ancestrula and daughter zooid. (C) NIWA 3714, autozooids. (D) autozooids and ovicellate zooid.(E), close-up of lower-left ovicellate zooid in A; notice the kenozooid beneath the ooecium; uniporous septulum arrowed. (F) Judyella precocialis (Gordon, Reference Gordon1984), NIWA 1160, holotype, Station K795, Kermadec Ridge: lateral view of ooecium formed by distal kenozooid. Scale bars: A, 1 mm; B, E, 300 μm; C, D, 500 μm; F, 200 μm.
Paratypes: NIWA 95607, 95608, same data as holotype.
Other material: NIWA 3715, 95609, Station KAH0204/47, Cavalli Seamounts, NE of North Island New Zealand, 792‒880 m.
Etymology
Latin corona, crown, alluding to the impressive corona of periopesial spines.
Description
Colony encrusting, uniserial, ramifying (Figure 7A), dichotomously branching, sometimes in small dense aggregations with self-overgrowth; linear colonies up to 9 mm long.
Autozooids more or less roundly subtriangular to pyriform (Figure 7C), widest in distal third (ZL 527‒642 (596 ± 30), N = 15; ZW 390‒527 (454 ± 40), N = 15). Opesia subcircular, surrounded by steep narrow cryptocyst with strong granulation except mid-distally where rim is thin and smooth with lamina descending towards basal wall (OpsL 159‒258 (203 ± 26), N = 15; OpsW 141‒221 (180 ± 17), N = 15). Cryptocyst surrounded by compact corona of 15–19 (mean 17) basally jointed periopesial spines, these vertical and slightly claviform in distal half of corona, more curved toward opesia and tapering towards spine tip in proximal half (Figure 7A, C, D). Gymnocyst comprising broad sloping sides of autozooidal cystid. No avicularia.
Ovicell hyperstomial, terminal. Ooecium (Figure 7A, E) formed from distal kenozooid that is concealed from frontal view, with smooth completely calcified ectooecium. Cystid of maternal zooid roundly subtriangular, ‘broad-shouldered’ distally, being the widest part of the zooid (OoL 191‒223 (210 ± 27), N + 2; OoW 199‒214 (206 ± 10), N = 2). Opesia of ovicellate zooids as in autozooids, with corona of 15 articulated periopesial spines interrupted by ooecium; each proximolateral corner of ooecium flanked by a pair of spines.
Interzooidal communications (Figure 7E) via pore-chambers that are intermediate between small mural and larger basal pore-chambers in size and position, each uniporous internally. Autozooids and ovicellate zooids budded at diverging angle from distolateral pair of pore-chambers; another pair of pore-chambers, not budding autozooids, present mid-laterally. In ovicellate zooids, pore-chambers present in distolateral shoulders, not budding further autozooids, but in one instance a small kenozooid budded from one such pore-chamber and curved around to distal side of ooecium. In another instance, where an autozooid had encountered another autozooid in different branch, a tubular kenozooid passed from a mid-distal position to a lateral pore-chamber of the adjacent zooid. A triangular kenozooid (Figure 7A) budded in one broken autozooidal cystid connecting caudae of two daughter zooids.
Ancestrula (Figure 7B) like autozooids but with only 13 spines (AnL 380, AnW 232, N = 1).
Remarks
Judyella corona sp. nov. is notable for the dense corona of periopesial spines and the triangular, ‘broad-shouldered’ form of ovicellate zooids. It differs from the following species, Judyella concordia sp. nov., in having pyriform zooids and in lacking a spinose process from the ooecial kenozooid.
Callopora precocialis Gordon, Reference Gordon1984, is also included in the genus, as Judyella precocialis comb. nov. It differs mainly from Callopora in that the ectooecium is wholly calcified (Figure 7F). In noting the ooecial kenozooid (overlooked by Gordon (Reference Gordon1984)), Gordon et al. (Reference Gordon2009, p. 297) included the species in Pyriporoides, but, as noted above, in this genus the opesia is constricted, the cryptocyst has a proximal shelf and the ooecium has a median suture of fissure. Scanning electron microscopy of the paratype specimen of C. precocialis has revealed that it belongs to Quasicallopora pusilla sp. nov. (described above).
Judyella concordia sp. nov.
(Figure 8A‒C)
Material Examined
Holotype: NIWA 3709, Station KAH0204/30, Cavalli Seamounts, NE of North Island New Zealand, 800‒825 m depth.

Fig. 8. Judyella concordia sp. nov., NIWA 3709, Station KAH0204/30, Cavalli Seamount: (A) ovicellate spot colony lacking ancestrula. (B) close-up of distal ovicellate zooid in A. (C) close-up of proximal ovicellate in A; note the normal disposition of the distal kenozooid spinose projection. Scale bars: A, 500 μm; B, C, 200 μm.
Etymology
Alluding to the spinous process of the ooecial kenozooid, which resembles the ‘droop-nose’ of the Anglo-French supersonic passenger aircraft known as Concorde, retired in 2003.
Description
Sole colony (Figure 8A) incomplete, encrusting, tiny, comprising four intact zooids (three ovicellate) and tiny remains of two others; size 0.94 mm maximum dimension.
Autozooids broadly suboval to roundly subhexagonal, widest just proximal of midlength (ZL 248‒377 (328 ± 57), N = 4; ZW 166‒296 (217 ± 57), N = 4). Regeneration of autozooid seen in one older cystid. Opesia also broadly suboval, surrounded by narrow cryptocyst with strong granulation on short steep rim that flattens to smooth narrow shelf around entire opesia (OpsL 147‒182 (168 ± 17), N = 4; OpsW 100‒125 (114 ± 12), N = 4). Cryptocyst surrounded by ~18 spine bases (no whole spines remaining), most around opesial rim, a few lower on the gymnocystal wall. Gymnocyst comprising broad sloping sides of autozooidal cystid, especially proximally where it can be extended. No avicularia.
Ooecium (Figure 8B, C) with smooth ectooecial calcification, formed by distal kenozooid that is almost wholly concealed. A beak-like spine (Figure 8C) emerges distally below kenozooidal opesial opening and angles frontally over distal midline of ooecium (OoL 128‒147 (139 ± 10), N = 3; OoW 130‒132 (131 ± 1), N = 3). Cystid of ovicellate zooid as in autozooids, with 16–20 periopesial spine bases.
Interzooidal communications via small basal pore-chambers, distributed distally and laterally.
Ancestrula not seen.
Remarks
Judyella concordia sp. nov. is notable for the spinose frontal extension of the ooecial kenozooid. The sole colony occurs on a rock taken from 800‒825 m on the Cavalli Seamounts.
Family ANTROPORIDAE Vigneaux, Reference Vigneaux1949
Genus Ellisantropora gen. nov.
Type Species
Retevirgula aggregata Gordon, Reference Gordon1984.
Etymology
A hybrid of the bryozoan genus names Ellisina and Antropora, alluding to some characters of both that are expressed in the new genus.
Diagnosis
Colony encrusting, unilaminar, multiserial, contiguous. Autozooids with elongate-oval opesia surrounded by very narrow granular cryptocyst of equal width throughout; gymnocyst narrow, confined to sloping lateral walls, a little larger proximally or proximolaterally. No spines. Avicularia comprising a small distolateral pair, interzooidal, with minute pivots, no pivot bar. Ovicell hyperstomial, ectooecium wholly calcified and carinate with thin median-longitudinal suture line; closure subcleithral. Interzooidal communications via numerous small uniporous basal pore chambers. Ancestrula like later zooids but with periopesial spines.
Remarks
A new genus is needed to accommodate two species whose affinities have been problematic. One is the nominated type species, Retevirgula aggregata Gordon, Reference Gordon1984, which, when described, was noted as lacking two key characters of the type species of Retevirgula Brown, Reference Brown1948, viz connecting tubes between zooids and avicularian pivot bars. There is also no heterozooid associated with the ooecium. It is here renamed Ellisantropora aggregata comb. nov. The second species is Ellisantropora tilbrooki sp. nov. Tilbrook (Reference Tilbrook1998, p. 36) noted that Harmer (Reference Harmer1926) had included three morphologies under the name ‘Antropora marginella (Hincks, Reference Hincks1884)’, only one of which accorded with Hincks's species (itself a junior synonym of Antropora minor (Hincks, Reference Hincks1880b) according to Tilbrook (Reference Tilbrook1998)). One of the morphologies, NHMUK 1928.9.13.18 from Torres Strait, Queensland, was identified by Tilbrook (Reference Tilbrook1998) as Retevirgula aggregata. In the event, it differs from E. aggregata comb. nov. most obviously in having far fewer avicularia and slightly shorter zooids. It was fully described by Tilbrook (Reference Tilbrook1998, p. 44) in his review of Antropora.
The family attribution of Ellisantropora is less straightforward. As indicated by the hybrid name, choices are Ellisinidae and Antroporidae, as well as Calloporidae. All genera currently included in Ellisinidae (see Cook et al., Reference Cook, Bock, Hayward, Gordon, Cook, Bock, Gordon and Weaver2018) have an ovicell associated with a heterozooid, either an avicularium (as in Ellisina Norman, Reference Norman1903, Retevirgula Brown, Reference Brown1948 and Lamourouxia Hondt d’ & Gordon, Reference Hondt, Gordon and Crosnier1999), a kenozooid (as in Kenoaplousina López Gappa & Liuzzi, Reference López Gappa and Liuzzi2013) or both, as in some species of Ellisina (see Hayward & Ryland, Reference Hayward and Ryland1998, figure 56C, D) and Retevirgula (see Gordon, Reference Gordon1986, pl. 4B; but this genus probably needs splitting). If we solely consider the type species of the genera that typify families, Ellisantropora gen. nov. is morphologically closest to Ellisinidae, exemplified collectively by the large, longitudinally oval opesia bordered by a very narrow granular cryptocyst of even width, narrow gymnocyst, basal pore-chambers, interzooidal avicularia lacking pivot-bars and cleithral ovicells that have a wholly calcified ectooecium. Antropora granulifera (Hincks, Reference Hincks1880a), typifying Antroporidae, shares some of these characters but has an extensive cryptocystal shelf and endozooidal ovicells with small and indistinct ooecia. Callopora lineata (Linnaeus, Reference Linnaeus1767), typifying Calloporidae, also shares some characters, but has a proportionally larger gymnocyst bearing an adventitious avicularium and the ooecium of the acleithral ovicell has a large exposure of endooecium.
Ellisantropora gen. nov. also resembles the antroporid genus Akatopora Davis, Reference Davis1934. Both the Eocene type species, Akatopora clausentina Davis, Reference Davis1934 and Recent Akatopora circumsaepta (Uttley, Reference Uttley1951) have paired heterozooids that when autozooids are regularly in quincunx, each appears to have a complement of six, as in E. aggregata. The heterozooids comprise kenozooids and avicularia, the latter having a semicircular mandible in the Recent species. When bleached, some avicularia can resemble kenozooids owing to the absence of mandibular pivots. The main additional differences between the two genera are the lesser exposure of gymnocyst (vestigial in A. circumsaepta) and non-hyperstomial ovicells in Akatopora, as well as intramural frontal budding and multilamellar growth in the Recent species of this genus. Interestingly, a molecular-genetic study of New Zealand cheilostomes has shown that Ellisantropora aggregata (as Retevirgula aggregata) and Akatopora circumsaepta group with total support in a monophyletic clade distant from a species of Ellisina (Orr et al., Reference Orr, Di Martino, Gordon, Ramsfjell, Mello, Smith and Liow2021). For this reason, Ellisantropora, which lacks the ellisinid character of an ooecial heterozooid, having instead its ooecium formed by the distal autozooid, is provisionally included in Antroporidae. Inasmuch as Antropora has yet to be sequenced, this association has yet to be confirmed.
Ellisantropora aggregata (Gordon, Reference Gordon1984)
(Figure 9A‒G)
Retevirgula aggregata Gordon, Reference Gordon1984, p. 27, pl. 2D; Gordon, Reference Gordon1986, p. 30.

Fig. 9. Ellisantropora aggregata (Gordon, Reference Gordon1984): NIWA 120104, Station B455, NW South Island: (A) part of colony showing disposition of interzooidal avicularia. (B) carinate ooecium. NIWA 120103, NW South Island: (C) tatiform ancestrula. (D) NIWA 120104, developing zooid at colony margin showing numerous basal pore-chambers. (E) NIWA 120103, interzooidal avicularia with mandibles in open (left) and closed (right) positions. (F) NIWA 120104, interior view of lateral wall showing recessed uniporous septula. (G) same, interzooidal avicularium with basal pore-chambers. Scale bars: A, 500 μm; B, 200 μm; C, E, F, 100 μm; D, 300 μm; G, 50 μm.
‘Retevirgula’ aggregata: Gordon et al., Reference Gordon, Taylor, Bigey and Gordon2009, p. 289.
Material Examined
Holotype: NIWA 1266, Station K855, Curtis Island, Kermadec Ridge, New Zealand, 115‒125 m depth.
Paratype: NIWA 1267, same data as holotype.
Other material: NIWA 120103, 120104, Station B455, north-west South Island, New Zealand, 54 m.
Redescription
Colony encrusting, unilaminar, multiserial, contiguous, up to 6 mm maximum dimension.
Autozooids more or less elongate-oval to roundly subhexagonal (ZL 365‒702 (523 ± 105), N = 15; ZW 322‒489 (371 ± 45), N = 15). Elongate-oval opesia (Figure 9A) surrounded by very narrow and steep granular cryptocyst of equal width throughout (OpsL 247‒523 (388 ± 83), N = 15; OpsW 247‒406 (297 ± 43), N = 15). Operculum semicircular, terminal in membranous frontal wall. Gymnocyst narrow, smooth, confined to sloping lateral walls, a little larger proximally or proximolaterally. No spines.
Avicularia (Figure 9A, B, D, E, G) small, interzooidal, comprising a small distolateral pair; hence, when autozooids are in regular quincunx, each appears to be surrounded by six avicularia – a distolateral pair, lateral pair, proximolateral pair – but this pattern can vary at the bifurcation of zooid rows (AvL 112‒194 (139 ± 23), N = 15; AvW 61‒137 (79 ± 18), N = 15). A few accessory identical or smaller such avicularia budded adventitiously from interzooidal avicularia (Figure 9A). Rostral part of avicularium a little smaller than postmandibular part, semicircular, elevated frontalwards, the proximolateral corners projecting as tiny pointed cusps; no pivot-bar. Avicularian opesia bordered by steep crescentic cryptocyst with granular outer margin, with minute pivots. Rarely an interzooidal space is available that is occupied by either a kenozooid, larger-than-usual avicularium or very small autozooid.
Ovicell (Figure 9B) hyperstomial, appearing subcleithral in dried material. Ectooecium wholly calcified and carinate with thin median-longitudinal suture line; proximal corners of ooecium flanking operculum; ovicell closure subcleithral (OoL 201‒265 (239 ± 33), N = 3; OoW 251‒297 (271 ± 24), N = 3).
Interzooidal communications via numerous small uniporous basal pore chambers; these laterally narrow, each internal opening recessed and flanked by irregular buttresses (Figure 9F). Interzooidal avicularia also with small basal pore-chambers (Figure 9G).
Ancestrula (Figure 9C) like later zooids but with 11 periopesial spines (AnL 236‒251 (243 ± 10), N = 2; AnW 209‒220 (215 ± 8), N = 2).
Remarks
Mean zooid length and width in Ellisantropora aggregata comb. nov. are 523 μm and 371 μm, respectively, compared with 410 μm and 300 μm in Ellisantropora tilbrooki sp. nov. from Torres Strait (Tilbrook, Reference Tilbrook1998). The size difference could be a consequence of higher temperatures in Torres Strait, but E. tilbrooki sp. nov. also has far fewer avicularia. In New Zealand, E. aggregata occurs on molluscan shell fragments from 54‒490 m depth from north of Raoul Island (Kermadec Ridge) to north-west South Island. No information is given in Harmer (Reference Harmer1926) or Tilbrook (Reference Tilbrook1998) concerning substratum or depth for E. tilbrooki sp. nov.
Family ELLISINIDAE Vigneaux, Reference Vigneaux1949
Genus Rhizellisina gen. nov.
Type Species
Rhizellisina rhizoidea sp. nov.
Etymology
Greek, rhiza, root, plus Ellisina, a bryozoan genus, alluding to the presence of rootlets (rhizoids).
Diagnosis
Colony small, unilaminar, multiserial, supported on soft sediment by slender rootlets that issue from basal pore-chambers in autozooids and avicularia. Sparse articulated spines bordering distal half of opesia. Avicularia interzooidal, with mandibular pivots only. Ovicell hyperstomial, cleithral. Ooecium associated with avicularian cystid, which forms its floor and crowns its summit; closure subcleithral, ectooecium wholly calcified, smooth. Basal pore-chambers present, with uniporous septula on interior walls. Ancestrula like later zooids.
Remarks
A new genus is required for a deep-sea species resembling Ellisina but having very small colonies of 16 autozooids or fewer that are planar and unilaminar but elevated horizontally above a very-fine sediment surface by slender rootlets. Articulated spines, borne on the gymnocyst, are also present. These are lacking in the type and other species of Ellisina Norman, Reference Norman1903.
Rhizellisina rhizoidea sp. nov.
(Figure 10A‒F)
Material Examined
Holotype: NIWA 95622, Station S151, Bounty Trough, eastern South Island, New Zealand, 1586 m depth.

Fig. 10. Rhizellisina rhizoidea gen. et sp. nov.: (A) NIWA 95627, Station E416, Bounty Trough: operculate ovicellate colony with some rootlets evident. (B) same, bleached fragment with ovicellate zooid, avicularia and spine bases. (C) NIWA 95624, Station S150, Bounty Trough: ovicellate zooid with periopesial spines. (D, E), same, showing cuticular window on geniculations where rootlets issue from avicularia. (F), NIWA 95627, close-up of ooecial avicularium. Scale bars: A, 1 mm; B, C, 300 μm; D, 200 μm; E, F, 100 μm.
Paratypes: NIWA 96523, same data as holotype; NIWA 95624, Station S150, Bounty Trough, 1640 m; NIWA 95625, Station S152, Bounty Trough, 1676 m; NIWA 95626, Station S153, Bounty Trough, 1386 m.
Other material: NIWA 95627, Station E416, head of Bounty Trough, eastern South Island, New Zealand, 1225 m.
Description
Colony small, planar, unilaminar, multiserial, supported horizontally above soft sediment by slender rootlets (Figure 10A). Delicate, suboval to slightly flabellate in shape. Largest colony with 16 feeding zooids (including ancestrula), diameter 2.3 mm.
Autozooids mostly rounded elongate-subhexagonal (ZL 475‒593 (516 ± 47), N = 18; ZW 328‒433 (349 ± 29), N = 18). Opesia large, elongate-oval, sometimes a little acute proximally, bordered by very narrow steeply descending granular cryptocyst of equal width throughout (OpsL 393‒443 (413 ± 19), N = 7; OpsW 245‒319 (267 ± 26)). Operculum terminal in membranous frontal wall, semicircular. Gymnocyst smooth, very narrow, or a little more visible in proximal half of zooid; bearing bases of 4‒9 slender articulated spines (Figure 10B, C), mostly in distal half of zooid though occasionally a spine may occur in mid-proximal position.
Avicularia interzooidal (Figure 10B), cystid smooth, base four-sided to triangular; frontal face orientated transversely, rostrum inclined obliquely frontalwards, triangular with virtually no palate, tip slightly rounded; mandibular pivots stout; post-mandibular cryptocyst relatively broad, steep, granular, opesial part of avicularian foramen much smaller than rostral part (AvL 73‒250 (176 ± 71), N = 12; AvW 124‒207 (176 ± 71), N = 12).
Ovicell hyperstomial, cleithral. Ooecium formed by avicularium (Figure 10B, C, F), the cystid of which constitutes its floor and projects transversely on distal summit of ooecium; ectooecium wholly calcified, smooth. Each proximolateral corner flanked by a spine (OoL 130‒168 (143 ± 13), N = 10; OoW 170‒229 (213 ± 17), N = 10).
Basal pore-chambers small, two on each lateral wall of autozooid, each opening to interior via a uniporous septulum; distal transverse wall with 1‒2 uniporous septula (~16 μm diameter) communicating with distal autozooid or avicularium, but having 2‒3 such septula (~22 μm diameter) when communicating with ooecial avicularium.
Rootlets each issuing from a basal pore-chamber, typically pertaining to an avicularium, but some autozooids may have a rootlet. One partly broken isolated zooid (not an ancestrula) with one lateral rootlet plus three associated avicularia, each with 1‒2 rootlets. These rootlets geniculate at point of attachment with parent autozooid/heterozooid, each with a cuticular window on the ‘knee’ (Figure 10D, E).
Ancestrula like later zooids, usually with six spines and a distal avicularium. Daughter zooids can be budded on each side distolaterally and laterally or these positions occupied by one or more avicularia (AnL 396; AnW 254; N = 1).
Remarks
The bottom sediment at the stations where Rhizellisina rhizoidea gen. et sp. nov. occurred comprised a mix of fine terrigenous and planktonic particles. Colonies were presumably elevated above the sediment surface (rather than lying sideways) by the cluster of rhizoids, which are up to 3.7 mm long in some colonies. Some 20‒38% of zooids in the largest colonies were ovicelled.
The species is so far known only from a relatively small area of the Bounty Trough, off eastern South Island, New Zealand, at depths of 1225‒1676 m.
Family VINCULARIIDAE Busk, Reference Busk1852b
Genus Vincularia Defrance, Reference Defrance1829
Type Species
Vincularia fragilis Defrance, Reference Defrance1829.
Remarks
The family Vinculariidae and genus Vincularia, long regarded as extinct, are included in this account in order to validate their existence in the Recent marine biota, accompanied by a full description and illustrations. Previously, the existence of Vincularia had been noted in the New Zealand-region bryofauna only in a list of taxa (Gordon et al., Reference Gordon, Taylor, Bigey and Gordon2009, Reference Gordon, Bock, Souto-Derungs and Reverter-Gil2019).
Cheetham (Reference Cheetham1966) clarified the status of Vincularia, affirming that it was known with certainty only from the Eocene of England and Europe (Cheetham, Reference Cheetham1966). Subsequently, additional species were recognized in the Eocene and Oligocene of the USA and Oligocene of France (Cheetham, Reference Cheetham and Larwood1973). Several species were described from the early to late Miocene of Indonesia (Di Martino & Taylor, Reference Di Martino and Taylor2014) and an undescribed putative species of Vincularia has been recognized from the early to middle Miocene of the Dominican Republic (https://nmita.rsmas.miami.edu/database/bryozoa/systemat/vincusp.htm). Di Martino & Taylor (Reference Di Martino and Taylor2014) also referred an Indian Miocene species to Vincularia and noted the existence of an undescribed Miocene species from Tanzania.
Busk (Reference Busk1852b, p. 2; Reference Busk1854, p. 95, pl. 65, figure 2) was the first to name the family Vinculariidae (as Vinculariadae), validly basing it on monotypic Vincularia Defrance, Reference Defrance1829 and including some other fossil and living genera in the family. Judging from his sole illustrated species attributed to Vincularia, which appears to be a species of Ogivalia Jullien, Reference Jullien1882, Busk's understanding of the genus did not conform to the type species of Vincularia, which is hardly surprising, given the inadequacy of Defrance's (Reference Defrance1829) description and illustrations. Canu (Reference Canu1907), who noted that Defrance's type material was housed at the University of Caen, gave the first comprehensive description of V. fragilis based on additional new material, highlighting the asymmetrical nature of the abfrontal polymorphs. Because they were ‘divergent’, Canu called them ‘zoécies D’, i.e. D-zooids. Bizarrely, he unnecessarily introduced a new genus, Heterocella, for four French Eocene species with D-zooids, nominating V. fragilis as type species. Cheetham (Reference Cheetham1966) was uncertain of the avicularian status of the D-zooids, whereas Di Martino & Taylor (Reference Di Martino and Taylor2014) referred to them as ‘avicularian autozooids’, in contradistinction to the very small adventitious avicularia that are also typical. The large asymmetrical avicularian polymorphs are absolutely characteristic of the genus and family and occur in Vincularia regia sp. nov., a living species restricted to deep water on the Three Kings Ridge north of New Zealand.
Vincularia regia sp. nov.
(Figure 11A‒D)
Vincularia sp. Gordon et al., Reference Gordon, Taylor, Bigey and Gordon2009, p. 290; Gordon et al., Reference Gordon, Bock, Souto-Derungs and Reverter-Gil2019, p. 16.

Fig. 11. Vincularia regia sp. nov.: NIWA 146101, Station U581, Three Kings Ridge: (A) frontal side of a stem showing autozooids. (B) abfrontal side of a stem showing avicularia. (C) partly lateral view of a stem showing bifurcation of an avicularian zooid row into a longitudinal series of autozooids to the left and an avicularian series to the right (cystids damaged). NIWA 146137, Station U595, Three Kings Ridge: (D) proximal end of a stem showing abfrontal surface with abrupt change in avicularian dimensions. Scale bars: A‒D, 2 mm.
Material Examined
Holotype: NIWA 146137, Station U595, Three Kings Ridge north of Tui Seamount, New Zealand, 1474 m.
Paratype: NIWA 146136, same data as holotype.
Other material: NIWA 146101, Station U581, Three Kings Ridge, New Zealand, 1170 m; NIWA 146135, Station U582, Three Kings Ridge, New Zealand, 790 m.
Etymology
Latin rex, regis, king, alluding to the Three Kings Ridge.
Description
Colony fragments erect, quadri- to multiserial, to 10 mm long and 1.3 mm wide.
Stems basally quadriserial, comprising a pair of autozooids and a pair of avicularian morphs, expanding progressively into 5‒6 longitudinal autozooidal series frontally (Figure 11A) while the abfrontal surface comprises increasingly larger avicularia in just the two series (Figure 11B, D). Increase in number of longitudinal autozooidal series achieved by simultaneous distal bifurcation (Figure 11C) of both avicularia of an alternating pair into a distal daughter avicularium and a laterodistal autozooid precursor of transitional morphology; these latter, one on each side, in turn bud a daughter autozooid that is proximal-most in its longitudinal series. Autozooids elongate-subhexagonal, symmetrical, with raised cryptocystal margin steeply surrounding an elongate-oval opesia (Figure 11C); proximal third of autozooid a concave lightly granular cryptocystal shelf (ZL 893‒1064 (992 ± 63), N = 14; ZW 433‒532 (485 ± 30), N = 14; OpsL. 618‒716 (670 ± 34), N = 14; OpsW 202‒293 (248 ± 30), N = 14). Distal zooidal wall sloping obliquely inwards toward basal wall, with a conspicuous pair of occlusor muscle scars and, below them, a pair of distobasal communication pores. No gymnocyst. No oral spines.
Large vicarious avicularia abfrontal only (Figure 11 B‒D), slightly alternating, in two longitudinal series except where bifurcation of a new longitudinal series laterally interpolates a third avicularium. Cystids asymmetrical, curving outwards towards margin of stem on each side, with raised rims. Cryptocyst granular, shelf-like proximally, moderately broad laterally, tapering abruptly distally. No differentiation into separate rostral or opesial parts and no trace of condyles. Opesia elongate-oval to round, bounded distolaterally by cystid rim (AnL 857‒1652 (1219 ± 227) N = 14, AvW 560‒1137 (812 ± 213) N = 14). Mandibles not seen. Small adventitious structures, interpreted to be avicularia also present, 1‒2 at proximal corner(s) of large avicularian cystids. No condyles or pivot bar.
Ooecia not seen. Ancestrula not seen.
Remarks
All available stems are unbranched, slightly eroded and lacking cuticular parts and were clearly transported. Regrettably, this means that there is no information on mode of attachment, maximum colony size, articulation or the nature of the avicularian polymorphs (do they have polypides?) and their mandibles. The new Indonesian Miocene species described by Di Martino & Taylor (Reference Di Martino and Taylor2014) had large avicularian morphs that were relatively little differentiated from autozooids and it is possible, perhaps even highly likely, that they had polypides. The triangular nature of the small adventitious polymorphs in these species, exquisitely preserved, confirm that they were avicularia without pivots, in contradistinction to rootlet pores, which were present in one species and round. In contrast, Cheetham (Reference Cheetham and Larwood1973) illustrated the small adventitious avicularia in Vincularia fragilis with distinct pivot bars. Whereas the Miocene species illustrated by Di Martino & Taylor (Reference Di Martino and Taylor2014) had large avicularian morphs that, like V. fragilis, were relatively little differentiated, these morphs in V. regia sp. nov. are rather more like those in Eocene Vincularia monstruosa (Canu, Reference Canu1907), which were more-expanded and asymmetrical; furthermore, they became distally transformed into female zooids with vestigial ooecia, which is not the case in V. regia sp. nov.
Ordinary autozooids in V. regia sp. nov. strongly resemble those in Bryopastor Gordon, Reference Gordon1982 (family Bryopastoridae) and the two families may have diverged from a common stem. That stem may include Quadricellariidae, species of which have quadriserial branches. Interestingly, Cheetham (Reference Cheetham1966) included both Nellia Busk, Reference Busk1852b (currently in Quadricellariidae) and Vincularia in the same family (Farciminariidae). While species of Farciminaria Busk, Reference Busk1852b are quadriserial, they lack a cryptocyst, have conspicuous spines, and the kenozooidal ooecium is large and conspicuous with a spinose endooecium and wholly membranous ectooecium, so Farciminariidae appears wholly unrelated to Vincularia. ‘Vincularia’ anceps Brown, Reference Brown1952 from the early Miocene of New Zealand does not belong to Vincularia, as noted by Brown – it appears to be a bryopastorid, possibly related to Bryopastor or Pseudothyracella Labracherie, Reference Labracherie1975.
The three stations on the Three Kings Ridge are geographically isolated and in deep water and the chances of obtaining fresh material are presently remote. Nevertheless the finding of a living example of Vincularia in the New Zealand region highlights the possibility that there might be other, yet-undiscovered, species elsewhere in the deep-sea. Given the widespread distribution of the genus in the Miocene, it is not improbable.
Family Incertae sedis
Genus Radixenia gen. nov.
Type Species
Radixenia radians sp. nov.
Etymology
From Latin radius, ray, spoke, plus Greek xenos, stranger, alluding to the radial arrangement of the zooids in the colony of a strange new bryozoan.
Diagnosis
Colony small, encrusting, unilaminar, comprising clavate autozooids attached to and radiating outwards from ancestrula, with some budding beyond these. Cystid with gymnocystal surface, especially proximally. Opesia elongate-oval, bordered by granular-tubercular cryptocyst that may be a little broader mid-proximally. Basal wall interior nodular, with a pair of large opercular occlusor-muscle scars. No spines. Avicularia interzooidal with open-channelled rostrum and minute pivots. Ovicells not seen. Basal pore-chambers present. Ancestrula kenozooidal.
Remarks
A new genus is required for an unusual encrusting cheilostome that has multiple zooids (nine in life) produced from the ancestrula. These radiate outwards all around the ancestrula, budding additional autozooids and interzooidal avicularia between them. A circlet of multiple daughter zooids appears to be unique in Cheilostomata but is known in Ctenostomata. Silén (Reference Silén1942, figure 38) illustrated multiple stolon segments (kenozooids) attached to the non-feeding ancestrula of a vesiculariid. In particular, Silén (Reference Silén1942, Reference Silén1944) described Labiostomella gisleni Silén, Reference Silén1941 as having a small (100 μm diameter), flattened kenozooidal ancestrula with 10‒15 tubular autozooids arising from it, each autozooid comprising the basis of an erect, bifurcating branch. When originally described, the species was interpreted as a cheilostome, but Silén (Reference Silén1944, pp. 7, 8) admitted that he had been mistaken in stating that the species was calcified.
Some cheilostomes produce an ancestrular complex, with 2‒6 zooidal components depending on the species (e.g. Cook, Reference Cook and Larwood1973, Reference Cook1985; Cheetham et al., Reference Cheetham, Sanner and Jackson2007; Ferretti et al., Reference Ferretti, Magnino and Balduzzi2007), but this differs from what is seen in Radixenia radians gen. et sp. nov., in which the ancestrula must be formed first, there surely not being enough nutrient reserves to support the simultaneous formation of nine daughter zooids. Silén (Reference Silén1942) interpreted the multiple autozooidal buds in the ancestrula of L. gisleni as analogous to, and originating from, spines, such that the ancestral cheilostome should have had an erect colony formed by means of frontal budding, an interpretation that is not presently supported by the fossil record (Taylor, Reference Taylor1990).
Although multiple zooidal budding from the ancestrula in R. radians sp. nov. would seem to be a plesiomorphic feature, the interzooidal avicularia, almost certainly setiform in life, are a fairly derived feature. The autozooids, with large oval opesiae, in conjunction with the heterozooids, recall those in such families as Heliodomidae Vigneaux (Reference Vigneaux1949) and Biselenariidae Håkansson & Zágoršek (Reference Håkansson and Zágoršek2020). In these families, however, which are morphologically very close, ancestrulae resemble autozooids. In Heliodomidae, ancestrulae directly bud two autozooids and two avicularia (Hayward & Cook, Reference Hayward and Cook1979); in Biselenariidae, ancestrulae are cormidial and always associated with an avicularium of unique morphology when metamorphosed. A larger avicularium is budded laterally from the ancestrula, and the two avicularia each bud a concentric spiral of autozooids, all of which have a distolateral avicularium. Heliodomids are ovicellate, whereas ovicells have not been seen in Biselenariidae. Inasmuch as Radixenia gen. nov. has a kenozooidal ancestrula, multiple ancestrular budding, caudate autozooids, no spiral growth and no ovicells seen, inclusion in these two families is precluded. Establishment of a new family to accommodate Radixenia would be premature based on one incomplete colony so the genus remains incertae sedis.
Radixenia radians sp. nov.
(Figure 12A‒C)
Material Examined
Holotype: NIWA 127657, Station G3, northern Norfolk Ridge, 710 m.

Fig. 12. Radixenia radians gen. et sp. nov., NIWA 127657, Station G3, Norfolk Ridge: (A) sole ancestrulate specimen, with clavate autozooids and interzooidal avicularia (all damaged). (B) kenozooidal ancestrula with proximal ends of five daughter autozooids and communication pores where four others had been connected. (C) best-preserved autozooid, showing window in basal wall and two large opercular occlusor-muscle scars; note fractures in thin distolateral wall; a pore-chamber opening is seen at lower right (arrow). Scale bars: A, 1 mm; B, 200 μm; C, 300 μm.
Etymology
Latin radians, radiating, alluding to the radial arrangement of zooids.
Description
Colony small, encrusting, unilaminar; partly broken, but when intact would have comprised nine clavate autozooids attached to and radiating outwards from ancestrula (Figure 12A). Some additional zooids budded between the most proximal pair, conferring asymmetry in budding and overall suboval shape to colony. Maximum diameter 2.12 mm.
Autozooids elongate, narrowing proximally. Cystid (Figure 12C) mostly with gymnocystal surface, narrowest distally and along sloping lateral walls, extensive in caudate part of zooid; proximally very narrow and tapering toward point of attachment with ancestrula or, if budding from another autozooid, truncate proximally (ZL 723‒1063 (940 ± 124), N = 7; ZW 341‒395 (372 ± 20), N = 7). Opesia elongate-oval, bordered by inward sloping granular-tubercular cryptocyst that may be a little broader mid-proximally; surface smoother in arcuate mid-distal portion (OpsL 322‒478 (412 ± 48), N = 7; OpsW 185‒246 (215 ± 19), N = 7). Basal wall with fairly large elongate-oval to pyriform window; interior surface of basal wall unusually nodular, with a pair of large opercular occlusor-muscle scars. No spines.
Avicularia (Figure 12A) inserted in distolateral angles between adjacent autozooids; each more or less teardrop-shaped, with distal margin of opesia damaged, but one example showing an apparent open channel distally, flanked by an indication of minute pivots for a probable setiform mandible (AvL238‒341 (305 ± 39), N = 5; AvW 176‒229 (201 ± 20), N = 5).
No ooecia seen.
Interzooidal communications apparently via basal pore-chambers judging by external openings in distolateral gymnocyst.
Ancestrula kenozooidal (Figure 12B), somewhat shield-shaped in outline, i.e. mostly rounded but with concave distal margin; relatively large communications pores showing where autozooids have become detached. Frontal surface gymnocystal, smooth (AnL 300, AnW 249, N = 1).
Remarks
The sole colony encrusts a small piece of thin shell that broke, revealing the relationship between the ancestrula and autozooids. The colony was dead and partly eroded, with no cuticular structures remaining. Some partly broken lateral walls indicate that they are triangular in cross section and hollow, with basal pore-chambers delimited within the hollow space. If this interpretation is correct, then it is a very curious arrangement. The genus Villicharixa Gordon, Reference Gordon1989, nominally an electrid, is known to have a continuous hollow space running within the lateral walls, but there is no delimitation of pore-chambers within that space. Taylor (Reference Taylor2020) recently described separated hollow spaces in lateral walls in Wawalia Dzik, Reference Dzik1975, also nominally an electrid.
One side of the ancestrula of Radixenia radians gen. et sp. nov. has a concave facet, indicating that the daughter zooid produced from the communication pore in that facet may have been larger than the others, or that the holotype specimen conserves only the distalmost part of a larger colony.
Superfamily MICROPOROIDEA
Family GRANOMURIDAE fam. nov.
Type Genus
Granomurus gen. nov.
Diagnosis
Colony encrusting, unilamellar, multiserial. Autozooids with extensive convex, granular cryptocyst underlying the membranous frontal wall; imperforate or with a few marginal pores. No frontal gymnocyst. Opesia small; operculum occupying half opesial length. Articulated spines tiny, ephemeral and lateral to opesia or short, permanent and distal. No avicularia. Ovicell hyperstomial, acleithral. Ooecium escharelliform, formed by distal autozooid and having fully calcified endooecium and continuity of wholly membranous ectooecium with membranous frontal wall. Basal pore-chambers well developed. Ancestrula not seen.
Remarks
Granomuridae is established for two genera – Granomurus gen. nov. and Reussinella Gordon, Reference Gordon2009. Both conform to the family diagnosis above; in Granomurus the opesia is wider than long with a raised distolateral rim and there are only tiny ephemeral spine bases lateral to the opesia and no frontal cryptocystal pores; in Reussinella the opesia is longer than wide with a proportionately larger operculum and no surrounding rim, there are two short oral spines distally, and the cryptocyst has sparse perforations near the proximolateral margin. Whereas Granomurus is neozelanic, Reussinella is Arctic-Boreal. The ooecium is described according to the terminology of Ostrovsky (Reference Ostrovsky2013).
When Gordon (Reference Gordon2009) established Reussinella he noted the challenge of family attribution, pointing out that it did not wholly conform to the Calloporidae, Aspidostomatidae or Microporidae while provisionally including it in the latter. In the strict sense, as based on the type genus, species of Microporidae should have a combined opesia-orifice. Circumscription of Microporoidea, and which families may be included, is more problematic. It is almost certain that the superfamily as presently conceived comprises several lineages having calloporoidean ancestry, thus comprising a polyphyletic assemblage. Until these relationships are resolved in space and time, the scope of Microporoidea is uncertain; at present, taxa that exhibit an extensive cryptocyst, small opesia and little or no gymnocyst, including Granomuridae, may provisionally be included in the superfamily. Granomuridae differs from Aspidostomatidae and Microporidae in lacking a combined opesia-orifice and avicularia; it differs further from Aspidostomatidae in having basal pore-chambers and oral spines (ephemeral or well developed).
Genus Granomurus gen. nov.
Type Species
Granomurus convexus sp. nov.
Etymology
Latin granum, seed, granosus, seedy, grainy, and murus, wall, alluding to the granular surface of the zooidal frontal cryptocyst.
Diagnosis
As for the family diagnosis, with a wider-than-long opesia in which the opercular sclerite occupies the distal part, a raised opesial margin, tiny spine bases lateral to opesia, and no cryptocystal pores.
Remarks
The genus is presently monotypic.
Granomurus convexus sp. nov.
(Figure 13A‒C)
cf. Aspidostoma sp. Gordon, Reference Gordon1986, p. 74, pl. 28F.

Fig. 13. Granomurus convexus gen. et sp. nov., NIWA 27763, holotype, Station TAN0604/116, Chatham Rise: (A) ovicellate and non-ovicellate zooids. (B) autozooidal opesia and adjacent basal pore-chambers; notice the very tiny spine bases, one either side of the opesia on the zooidal margin. (C) close-up of ooecium and opesia. Scale bars: A, 1 mm; B, C, 300 μm.
Material Examined
Holotype: NIWA 27763, Station TAN0604/116, north slope, central Chatham Rise, 950–1045 m.
Paratype: NIWA 95594, same data as holotype.
Other material: NIWA 146151, Station S45, SE Campbell Plateau, 1262 m. Unregistered, Station E796, Fiordland coast, 226–251 m.
Etymology
Latin convexus, convex, alluding to the convex frontal cryptocyst.
Description
Colony encrusting, unilaminar, multiserial, contiguous, up to 7 mm maximum diameter.
Autozooids (Figure 13A) subhexagonal (ZL 741‒979 (891 ± 71), N = 17; ZW 558‒905 (722 ± 97), N = 17). Opesia small (Figure 13B), c. one quarter zooid length, wider than long, with inner distal and proximal margins subparallel; outer distal and lateral margins elevated, slightly flared with granular rim; narrow skeletal surface surrounding opesia smooth (OpsL 175‒248 (201 ± 22), N = 17; OpsW 243‒286 (266 ± 16), N = 17). Cryptocyst convex, densely granular, with no pores or opesiules. Operculum semicircular, occupying half opesial length. No frontal gymnocyst. Spine bases very tiny, one either side of opesia on zooidal margin.
No avicularia.
Ovicell (Figure 13C) hyperstomial; ooecium formed by distal autozooid; skeletal surface endooecial, continuous with cryptocyst of distal zooid and equally granular; ectooecium wholly cuticular, continuous with membranous frontal wall (OoL 270‒308 (292 ± 11), N = 8; OoW 307‒351 (330 ± 14), N = 8). Acleithral; opening high-arched, subvertical or inclined a little distad.
Interzooidal communications via basal pore chambers; these fairly widely open, with cuticular surface.
Ancestrula not seen.
Remarks
Gordon (Reference Gordon1986) described a small infertile colony of this species from the Fiordland coast and noted its similarity not only to Aspidostoma Hincks, Reference Hincks1881 but also to Reussinella arctica (Osburn, Reference Osburn1950) (as Euritina). More material has confirmed its relationship to Reussinella, but not to Aspidostoma, and expanded knowledge of its geographic and depth range, from the north slope of the Chatham Rise to the southern Campbell Plateau at depths of 226‒1262 m. The genus and species are presently endemic to New Zealand.
Superfamily BUGULOIDEA Gray, Reference Gray1848
Family BUGULIDAE Gray, Reference Gray1848
Genus Carolanna gen. nov.
Type Species
Carolanna schackae sp. nov.
Etymology
Honorific for Dr Carolann Schack, a bryozoologist of exceptional talent.
Diagnosis
Colony erect, supported by rootlets, uniserial, with branch bifurcations at intervals. Autozooids elongate, arranged in chains, each zooid slightly overlapping daughter zooid distally, separated by joint. Opesia occupying entire frontal surface, bordered by non-articulated spines. No avicularia. Ovicellate zooid wider distally than autozooid. Ovicell hyperstomial, terminal, cleithral, closed by large dimorphic operculum; ooecium kenozooidal, budded from maternal zooid; endooecial calcification smooth, ectooecium cuticular. Ovicellate zooid with only 6 pairs of spines, the distal pair stoutest, flanking ooecium. Ancestrula erect, proximally attached.
Remarks
Carolanna gen. nov. is one of relatively few genera of Bugulidae that are strictly uniserial. Of these, only Bugulella Verrill, Reference Verrill1879 and Falsibugulella Liu, Reference Liu1984 have periopesial spines. Bugulella differs from Carolanna in having clavate zooids and bird-head avicularia; Falsibugulella differs in having only a distal opesia, a different mode of budding daughter zooids, and rootlets issuing from a distobasal (not proximolateral) position on the zooid. In the New Zealand region, Luguba Gordon, Reference Gordon1984 is similar in having well-developed spines around an elongate opesia and a broad-based ooecium, but differs in being biserial and unjointed and in having bird-head avicularia. South-west Atlantic Xenoflustra Moyano, Reference Moyano2011 lacks avicularia and has similar spinose zooids and broad-based ooecium, but colonies are semiflustrine with multiserial branches.
Carolanna gen. nov. may also be compared with species of Beaniidae inasmuch as zooids in a majority of beaniid species can have many lateral spines. But no beaniid produces erect, dichotomously branching colonies and their ooecia are never as large and conspicuous as in C. schackae sp. nov., which also lacks the grappling-hook rhizoidal attachments that are characteristic of all beaniids.
Carolanna schackae sp. nov.
(Figure 14A‒F)
Luguba n. sp. Gordon et al., Reference Gordon, Taylor, Bigey and Gordon2009, p. 289.

Fig. 14. Carolanna schackae gen. et sp. nov.: NIWA 132787, Station KAH1706/108, Spirits Bay: (A) bifurcating uniserial branches with rootlet bundles. (B) branch bifurcation. (C) lateral view of autozooid showing joints where connected to neighbours. (D) lateral view of ovicellate zooid. (E) frontal view of ooecium, with creases in collapsed ectooecium. NIWA 146134, Station KAH1706/108, Spirits Bay: (F) ancestrula, flanked by rootlet from daughter zooid. Scale bars: 2 mm; B, 500 μm; C, D, F, 300 μm; E, 200 μm.
Material Examined
Holotype: NIWA 132787, Station KAH1706/108, off Spirits Bay, North Island, New Zealand, 54 m.
Paratypes: NIWA132784, Station Z9687, 48 m; NIWA 132786, Station Z7901, 76 m; both off Spirits Bay, North Island, New Zealand.
Other material: NIWA 132782, Station Z9671, 55 m; NIWA 132783, Station Z9680, 46 m; NIWA 132785, Station Z9692, 52 m; NIWA 132788, Station KAH1706/108, off Spirits Bay, North Island, New Zealand, 54 m.
Etymology
Eponymous for Dr Carolann Schack.
Description
Colony erect, uniserial, with branch bifurcations at intervals (Figure 14A); maximum height 31 mm.
Autozooids elongate (Figure 14B), widest in distal half, narrowest proximally, arranged in chains of at least five zooids before bifurcating. Each zooid slightly overlapping its daughter zooid distally, separated from its neighbour by a distobasal gap in calcification that functions as a joint (Figure 14C) (ZL 527‒602 (557 ± 22), N = 18; ZW 179‒219 (196 ± 18), N = 6). All skeletal surfaces gymnocystal. Opesia extensive, occupying virtually entire frontal surface. Operculum comprising discrete flap in distal end of membranous frontal wall. Opesia bordered by 6‒9 non-articulated spines; the distalmost pair points distad, all others arch across frontal wall, mostly without interdigitating; each spine with distal pore.
No avicularia.
Ovicell hyperstomial (Figure 14D, E), terminal, with helmet-like kenozooidal ooecium budded from maternal autozooid, as wide as long or wider and deeper-bodied in distal half than in autozooids. Endooecial calcification smooth, ectooecium almost wholly cuticular. Cleithral, closed by dimorphic operculum that is wider than in autozooids (OoL 175‒212 (199 ± 14), N = 5; OoW 231‒248 (239 ± 9), N = 3). Ovicellate zooid with only 6 pairs of spines, the distal pair stoutest, flanking ooecium.
A rootlet pore in the proximolateral corner of many zooids; rootlets descending the sides of zooid branches, forming a tangled proximal cord toward the base of the colony.
Ancestrula erect (Figure 14F), attached to substratum directly, budding single daughter zooid from distobasal pore, supportive rootlets developing from the daughter zooid (AnL 579, AnW 168, N = 1).
Remarks
Colonies are slender and delicate. Fewer zooid widths were measured than zooid lengths because zooid lateral margins tended to inroll when preserved in ethanol or prepared for SEM, thereby causing zooid narrowing. The ooecium is similar to that in the genus Bryocalyx Cook & Bock, Reference Cook and Bock2000, and in both genera can be considered as the sum of a flattened distal kenozooid producing a helmet-like ooecial outfold (cf. Ostrovsky et al. Reference Ostrovsky, Nielsen, Vávra and Yagunova2009). Carolanna schackae gen. et sp. nov., monotypic for the genus, is so far known only from the outer reaches of Spirits Bay, Three Kings Shelf, northern New Zealand, where colonies attach to small shell fragments at depths of 46‒76 m.
Superfamily incertae sedis
Family BORIOPLEBIDAE fam. nov.
Type Genus
Borioplebs gen. nov.
Diagnosis
Colony squat, comprising short cylindrical branches from oligoserial basal zooids. Autozooids thick-walled, lageniform, with elevated funnel-like peristomial rim in which opesia-orifice is set at lower level. Wholly cryptocystal; no cryptocystal ridges. Opesia-orifice roundly transversely D-shaped. No oral spines, condyles, avicularia or ooecia. Recessed uniporous mural septula. Ancestrula like autozooids.
Remarks
This monogeneric family is difficult to place. Although external morphology did not suggest that Borioplebs gen. nov. might be ascophoran in nature, an internal examination was made of the cryptocystal wall. Not only are areolar-septular pores lacking externally and internally, there is no indication of an umbonuloid shield. The zooids alone are somewhat suggestive of Cellariidae, especially the genus Syringotrema Harmer, Reference Harmer1926, a genus ranging from the Oligocene to the present day. No known cellariid has an encrusting phase, however; instead, colonies are erect and anchored by rootlets. The form of the colony in the sole species of Borioplebs (see below) is reminiscent of that seen in Inversaria von Hagenow, Reference von Hagenow1851 (Onychocellidae), a genus of perhaps 10 species, all Cretaceous except for one from the latest Paleocene of New Zealand (Taylor et al., Reference Taylor, Martha and Gordon2018). No species of Inversaria, however, has peristomial orifices like that in Borioplebs. Borioplebs has erect cylindrical stems from an encrusting base, and despite the absence of cryptocyst ridges, nevertheless constitutes somewhat of a conceptual morphological intermediate between Onychocellidae and Cellariidae. Had it been discovered in the Cretaceous, its morphology would be suggestive of just such a relationship. In the absence of fossil antecedents, ovicells and molecular-sequence data, however, one can only hypothesize on its relationships. Another related family may be Aspidostomatidae, which also has strongly cryptocystal walls, but orifices are never subtubular and there are adventitious avicularia and ooecia. Interestingly, Hayward (Reference Hayward1995) included the Aspidostomatidae (which can exhibit cryptocyst ridges flanking a sunken area) in Cellarioidea, so there is precedence for including encrusting taxa in the superfamily.
Genus Borioplebs gen. nov.
Type Species
Borioplebs norfolkensis sp. nov.
Etymology
Latin boreas, north, and plebs, folk, alluding to the provenance of the sole included species near Norfolk (‘north folk’) Island on the Norfolk Ridge. Gender masculine.
Material Examined
Holotype: NIWA98172, Station G3, northern Norfolk Ridge, 710 m.

Fig. 15. Borioplebs norfolkensis gen. et sp. nov.: (A) NIWA 146138, Station G3, Norfolk Ridge: largest colony fragment, attached to substratum; ancestrula arrowed. (B) NIWA 98172, holotype, Station G3: bifurcation branch. (C) NIWA 146138, lateral view of autozooids and peristomes seen at lower right in A; duplicated peristomes indicated intramural buds. (D) NIWA 98172, part of branch shown in B. (E) NIWA 146138, showing ancestrula (an) and adjacent zooids, peristomes eroded. Scale bars: A, 3 mm; B, 2 mm; C, 1 mm; D, E, 500 μm.
Paratype: NIWA 146138, same data as holotype.
Other material: NIWA 98173, same data as holotype.
Etymology
Alluding to the eponymous Norfolk Ridge.
Description
Colony comprising robustly encrusting oligoserial zooid rows shortly ramifying on substratum, giving rise to a few briefly bifurcating, relatively short, erect cylindrical stems (Figure 15A, B). Maximum breadth of encrusting portion 6.2 mm, maximum height of erect branch/stem 4.9 mm, maximum stem diameter 1.4 mm.
Autozooids (Figure B‒D) squatly lageniform and typically overlapping, with subventricose cryptocystal wall rising to elevated funnel-like peristomial rim in which opesia-orifice is set at lower level. Cryptocyst minutely and densely granular. Peristome more or less circular (Figure 15C, D) with short vertical wall and discrete granular rim in youngest zooids, its outer side thickening all around and becoming more oblique as frontal cryptocyst thickens. No gymnocyst evident except where frontal cuticularized walls of adjacent zooids attach along interzooidal boundaries; here, an extremely thin smooth strip of calcification is evident in SEM photos (ZL 565‒988 (774 ± 142), N = 8; ZW 379‒527 (454 ± 51), N = 6).
Inner side of peristomial funnel in older zooids steeper proximal to opesia-orifice, broader and shallower distally, granular (PoL 263‒345 (297 ± 25), N = 11; PoW 261‒404 (331 ± 38), N = 11). Opesia-orifice (Figure 15D) subrounded, with straight proximal rim, its sides descending deeply interiorly below rim (OL 133‒189 (155 ± 19), N = 9; OW 159‒197 (186 ± 14), N = 6). No oral spines. Operculum not clearly in evidence; operculum-like structure in a post-ancestrular zooid of same shape as opesia-orifice. No condyles. Reparative budding evident in a few zooids in which a duplicate peristomial rim is set within the original peristome.
No avicularia or ooecia seen. Interzooidal communications via recessed uniporous mural septula.
Ancestrula (Figure 15E) like later autozooids but with smaller dimensions (AnL 504, AnW 266, N = 1). The sole ancestrular area seen shows one distal and one left-distolateral daughter zooids; additionally, a shorter daughter zooid issues from the left proximolateral side, curving in a proximal direction. The two left-produced daughter zooids abut another zooid that is directed laterally leftwards.
Remarks
The colony form – a relatively small encrusting base from which arise short cylindrical stems – is not common among cheilostomes. The lack of ooecia suggests that the sole colony was infertile rather than having internal brooding. Owing to the general overlap of proximal parts of autozooids, especially in erect stems, relatively few lengths and widths could be measured.
Discovery of more material of this morphologically disparate taxon is highly desirable for molecular sequencing and ascertaining its phylogenetic affinities. So far the species is known only from a single locality (NIWA Station G3) on the northern Norfolk Ridge at 710 m depth – the same locality as Elementella simplex gen. et sp. nov. and Radixenia radians gen. et sp. nov.
Superfamily CRIBRILINOIDEA Hincks, Reference Hincks1879
Family CRIBRILINIDAE Hincks, Reference Hincks1879
Genus Seabournea gen. nov.
Type Species
Seabournea rusti sp. nov.
Etymology
Honorific for New Zealand paleontologist Dr Seabourne Rust.
Diagnosis
Colony encrusting, unilaminar, multiserial. Costal field occupying most or all of frontal surface. Narrow granular cryptocyst present around opesia. Numerous costae, entire or bifurcating, non-pinnate, with slit-like intercostal lacunae. No lumen pores. Distal arch of autozooid with conspicuous bowl-like ‘apertural plate’. Oral spines present. Avicularia adventitious, lacking pivot bar. Ovicell subimmersed, ooecium, with conspicuous transverse ectooecial tabula. Uniporous mural septula present. Ancestrula not seen.
Remarks
A new genus is created for an unusual spinocystal cheilostome that has a skeletal character normally associated with Arachnopusia (Arachnopusiidae), viz an ‘apertural plate’. Hayward (Reference Hayward1995) reported it in many species, described as occurring under the operculum. In Seabournea gen. nov., in which it is exceptionally large, it appears in a frontal position in the distal transverse wall of the autozooid, flanked by the bases of the oral spines. Since the present material lacks any cuticular structures, its relationship to the operculum in life is unknown. Its function is unknown but it would be an ideal place to house a large gland. Inasmuch as it is larger in ovicellate zooids it is possible it may be complementary to protecting embryos (perhaps through biochemical deterrence to micropredation).
Seabournea gen. nov. may not be a cribrilinid; the ooecium is very reminiscent of that in the calloporid genus Corbulella Gordon, Reference Gordon1984 and the presence of a narrow granular cryptocyst bordering the opesia is unexpected in a cribrimorph (though admittedly not normally described in the literature even if present; a very narrow cryptocyst seems evident in Larwood's (Reference Larwood, Nielsen and Larwood1985) figures of some ‘myagromorph’ cribrilinids). If Seabournea is a calloporid it would be analogous to the cribrimorph look-alike Membraniporella Smitt, 1873, which differs in having a wholly endooecial frontal skeletal surface in the ooecium and in lacking an ‘apertural plate’.
Seabournea rusti sp. nov.
(Figure 16A‒D)
Material Examined
Holotype: NIWA 146139, Station TAN1503/116, Iceberg Seamount, Andes Seamount complex, south-eastern Chatham Rise, 497‒590 m.

Fig. 16. Seabournea rusti gen. et sp. nov.: NIWA 146139, Station TAN1503/116, Chatham Rise: (A) ovicellate zooid with elongate ‘apertural plate’, parts of autozooids and adventitious avicularia. (B) autozooid, showing four oral-spine bases and ‘apertural plate’. (C) avicularium (at left) with unbroken rostrum, and bowl-like ‘apertural plates’ of two autozooids. (D) close-up of autozooidal ‘apertural plate, oral-spine bases, broken costal-spine bases (at right) and uniporous septula. Scale bars: A, C, 500 μm; B, 400 μm; D, 200 μm.
Etymology
Eponymous for Dr Seabourne Rust.
Description
Colony encrusting, unilaminar, pluri‒multiserial, maximum length 10 mm.
Autozooids (Figure 16A, B) elongate-oval to roundly subhexagonal (ZL 627‒1052 (886 ± 158) N = 7; ZW 462‒600 (533 ± 43), N = 9). Costal field occupying most or all of frontal surface. Narrow granular cryptocyst present around opesia. Numerous costae, 17‒24, these entire or bifurcating, non-pinnate, with slit-like intercostal lacunae. Generally, the suboral costal, simple, parallel-sided; about two-thirds of succeeding costae bifurcating like a tuning fork, with opposing tips fusing in midline as a raised ridge; remaining costae in proximal third of spinocyst tapering convergently to midline. No lumen pores. Distal arch of autozooid with conspicuous bowl-like ‘apertural plate’ (Figure 16B‒D) set between oral-spine bases. Two pairs of oral spines.
Avicularia adventitious, borne proximally or proximolaterally on concealed gymnocyst; elevated, rostrum elongate-triangular (Figure 16C) when tip not broken (AvL 195‒267 (228 ± 30), N = 5; AvW 107‒132 (120 ± 12), N = 5). No pivot bar or obvious condyles, combined foramen drop-shaped; proximal cryptocyst steeply sloping, smooth.
Ovicell (Figure 16A) subimmersed; ooecium formed by distal kenozooid, with conspicuous transverse ectooecial tabula bordered by raised rim. Ovicell opening arches across distal margin of apertural plate, which is larger and transversely oval in ovicellate zooids (OoL 366, OoW 612, N = 1).
Uniporous mural septula present; basal pore-chambers absent. Ancestrula not seen.
Remarks
The sole colony of Seabourne rusti gen. nov. et sp. nov. encrusted the surface of a hexactinellid sponge, Aphrocallistes beatrix, at the type locality, captured in an upslope dredge tow. It is so far known only from the eastern Chatham Rise, New Zealand, at 497‒590 m (end and start depths of tow).
Superfamily LEPRALIELLOIDEA Vigneaux, Reference Vigneaux1949
Family ROMANCHEINIDAE Jullien, Reference Jullien1888
Genus Waeschenbachia gen. nov.
Type Species
Waeschenbachia splendida sp. nov.
Etymology
Honorific for Dr Andrea Waeschenbach, Natural History Museum, London, in recognition of her contribution to knowledge of bryozoan phylogeny.
Diagnosis
Colony encrusting, unilaminar, almost spot-like. Autozooidal frontal shield umbonuloid, convex suboral umbo conspicuous areolar-septular pores principally in two series. Orifices dimorphic, that of the autozooid longer than wide, poster broadly and deeply rounded. Peristomial rim thick, elevated, bearing a corona of up to 17 long articulated oral spines. No lyrula or peristomial denticle. No avicularia. Ovicellate orifice proportionally wider than in autozooids, and broadest proximally. Ovicell hyperstomial, escharelliform; ectooecium membranous, endooecium imperforate, surface irregular; orifice flanked by 6‒7 oral spines on each side. Ancestrula not seen.
Remarks
A new genus is required for a species of Romancheinidae with an exceptionally large number of orificial spines. Although superficially resembling Escharella Gray, Reference Gray1848, there is no median denticle and orifices are dimorphic. The related romancheinid genera Hippomenella Canu & Bassler, Reference Canu and Bassler1917 and Hippopleurifera Canu & Bassler, Reference Canu and Bassler1925 have similar orifices but are aviculiferous and never have so many oral spines.
Waeschenbachia splendida sp. nov.
(Figure 17A‒E)
Material Examined
Holotype: NIWA 132780, Station Z9752, east of Great Island (Manawatāwhi), Three Kings Islands, New Zealand, 190 m.

Fig. 17. Waeschenbachia splendida gen. et sp. nov., NIWA 132780, holotype, Station Z9752, Three Kings Islands: (A) unbleached autozooid with orificial spines intact. (B) detached bleached fragment showing mostly ovicellate zooids. (C) autozooidal orifice with 17 oral-spine bases. (D, E) ovicellate orifices. Scale bars: A, 500 μm; B, 1 mm; C, 200 μm; D, E, 300 μm.
Etymology
Latin splendidus, splendid, alluding to the spectacular corona of orificial spines.
Description
Colony encrusting, unilaminar, loosely pluriserial, attaining 11 mm maximum length.
Autozooids large, subhexagonal, frontal shield umbonuloid, steeply ascending to prominent pointed suboral umbo (Figure 17A) (ZL 1026‒1436 (1274 ± 133), N = 12; ZW 744‒1339 (927 ± 171), N = 12). Shield surface centrally imperforate, weakly granular-tubercular, flanked by conspicuous areolar-septular pores (Figure 17B), these principally in two series, but additional pores can occur in lateral wall. Autozooidal orifice (Figure 17C) longer than wide, a little wider distally than proximally, the proximal ends of the anter curved inwards laterally simulating condyles. Poster broadly and deeply rounded (OL 267‒275 (271 ± 6), N = 2; OW 232‒236 (234 ± 3), N = 2). Peristomial rim thick, elevated, bearing a spectacular corona of 12‒17 basally articulated oral spines up to 970 μm long, some with reparative joints. No lyrula or peristomial denticle.
No avicularia.
Ovicell (Figure 17B, D, E) hyperstomial, opening high above orifice. Ooecium formed by distal autozooid, escharelliform, skeletal surface of endooecium coarsely textured, somewhat irregular and granular-tubercular, continuous with calcified wall of distal zooid; ectooecium wholly cuticular, continuous with membranous frontal wall (359‒491 (446 ± 60), N = 4; OoW 433‒ 481 (459 ± 23), N = 4). Ovicellate zooids with dimorphic orifice that is proportionally wider than in autozooids (Figure 17E), and broadest proximally, orifice flanked by 6‒7 oral spines on each side (FoL 255‒337 (297 ± 58), N = 2; FoW 240‒297 (269 ± 41), N = 2).
Ancestrula not seen.
Remarks
The sole colony was growing on an irregular piece of substratum and is also irregular in shape, with zooids orientated in different directions. Interestingly, some oral spines, or parts of spines, were a bit crooked and bent at different angles, with joints, and evidence of repair. Since many zooids had surface debris obscuring their morphology, part of the colony was sacrificed for bleaching and SEM to reveal details of orifices, spine number and ooecia. The species is known only from the type locality at 190 m depth.
Discussion
Thirteen new genera, three new families and 16 new species of Zealandian cheilostome Bryozoa are described herein from 26.42° (northern Norfolk Ridge) to 54.02°S latitude (south-east Campbell Plateau) from coastal waters to lower bathyal depths (46‒1676 m). One of the new species belongs to the hitherto Eocene‒Miocene genus Vincularia now recognized in the present-day fauna. Two new combinations and a new non-Zealandian species are also established. Nearly half of the new Zealandian species form spot-like colonies on hard substrata but a range of colony forms is represented, including patch/sheet encrusters, erect rod-like and branching forms and a rooted species of soft sediments that forms the equivalent of planar spots but these are supported by long (>3 mm) rootlets on or above the sediment surface. Rocks are the commonest substratum, followed by scleractinian coral and molluscan shell. Arenaceous foraminiferal tubes are the sole substratum for one soft-sediment species.
Of the 14 genera described herein, 13 of them new to science and one new to the present-day fauna, five have plesiomorphic and or transitional skeletal morphologies in their autozooids and/or their ancestrulae. For example, Elementella gen. nov. has the simplest skeletal morphology of any known living cheilostome. The autozooidal cystid has no trace of cryptocyst and there are no spines, tubercles, heterozooids or reproductive structures. The two described species essentially have the appearance of simple runner-like ctenostomes that have calcified their lateral walls, resembling the very earliest-known cheilostome, late Jurassic Pyriporopsis pohowskyi, which also lacks a cryptocystal margin to the opesia. The ancestrula in Elementella is also bipolar, an ancestral character state found in Pyriporopsis, Herpetopora and some other nominal electrids, as well as a few other basalmost cheilostome families. Vincularia, previously known only from the early Eocene to the late Miocene, is represented by a living species. Radixenia gen. nov. unusually has multiple zooids (nine) produced from the ancestrula – apparently unique in the Cheilostomata but known in some Ctenostomata. Borioplebs gen. nov., with erect cylindrical stems from an encrusting base, has zooids with peristomial orifices that resemble those found in the erect cellariid genus Syringotrema.
Interestingly, these four genera can be found at just two NIWA stations. Station G3, north of Norfolk Island on the northern Norfolk Ridge, yielded Elementella simplex sp. nov., Radixenia radians sp. nov. and Borioplebs norfolkensis sp. nov. and station U582 on the Three Kings Ridge yielded Vincularia regia sp. nov. The distinctive morphologies of these likely relict cheilostomes recall some other taxon discoveries during the past 40 years of exploration of the northern Norfolk Ridge and waters around New Caledonia. A sphinctozoan sponge (Vacelet et al., Reference Vacelet, Cuif, Gautret, Massot, de Forges B. and Zibrowius1992), pleurotomariid gastropods (Bouchet & Métivier, Reference Bouchet and Métivier1982), a craniid brachiopod (Laurin, Reference Laurin1992) and primitive pedunculate crinoids (Améziane-Cominardi et al., Reference Améziane-Cominardi, Bourseau and Roux1987) were interpreted by Richer de Forges et al. (Reference Richer de Forges, Koslow and Poore2000) as ‘relicts of groups earlier believed to have disappeared in the Mesozoic’. Among the Bryozoa, Gordon (Reference Gordon1989) and Gordon & d'Hondt (Reference Gordon, Hondt and Crosnier1991) respectively discovered living species of Plagiopora MacGillivray, Reference MacGillivray1895 and Chelidozoum Stach, Reference Stach1935 alive on the Norfolk Ridge, genera that were previously known only from the Australian Miocene. In the popular literature, these and other examples from New Caledonia (northernmost Zealandia), such as the occurrence of the cephalopod Nautilus macromphalus and the hemichordate Cephalodiscus graptolitoides Dilly, Reference Dilly1993 (which revealed how graptolite coenecial spines are formed) led to the northern Norfolk Ridge being referred to as ‘un nid de fossiles vivants’ (e.g. Richer de Forges et al., Reference Richer de Forges, Jaffre and Chazeau1998). Terrestrial equivalents like the most basal living angiosperm genus (Amborella) in New Caledonia and the tuatara and leiopelmatid frogs (inter alia) in New Zealand underscore the apparent archaic elements of the Zealandian biota (Gibbs, Reference Gibbs2006).
The Three Kings Ridge lies well to the east of the Norfolk Ridge. Whereas both ridges are volcanic, only the Norfolk Ridge is underlain by continental crust (Mortimer & Campbell, Reference Mortimer and Campbell2014). Though separated from the Norfolk Ridge by the Norfolk Basin, the Three Kings Ridge, which continues south towards the North Island, is a volcanic-arc ridge associated with the eastern Zealandian continental margin. Vincularia as a relict bryozoan on the Three Kings Ridge is consistent with the Norfolk Ridge discoveries.
Grandcolas et al. (Reference Grandcolas, Nattier and Trewick2014) have argued that relict taxa imply regional extinctions, which is true, and that they therefore cannot simultaneously provide evidence of local biota permanence. Molecular evidence suggests that more than 90% of the land biota is derived from Australian, not Gondwanan or Zealandian ancestors, and probably all within the last 20 million years following a postulated total submergence of Zealandia during the Oligocene marine transgression ~25‒23 mya (Mortimer & Campbell, Reference Mortimer and Campbell2014). The marine realm, however, apart from coastal zones, need not have been similarly affected, and, indeed, Mortimer & Campbell (Reference Mortimer and Campbell2014) note that New Zealand's rich Cenozoic biota, well-studied for 150 years, would benefit from being reinterpreted from a Zealandian perspective. Analysing phylogeographic literature, Wallis & Jorge (Reference Wallis and Jorge2018) reviewed divergence times between New Zealand lineages and their closest overseas sister groups from a range of environments. They concluded that there is no evidence for a deficit of pre-Oligocene lineages, nor an excess of ones arriving just afterwards. In short, phylogeographic data provide no evidence for complete inundation of New Zealand during the Oligocene. One implication is that archaic Gondwanan or Zealandian elements are not precluded, especially in the marine environment.
One other area of notable bryozoan diversity in the New Zealand region is the Three Kings Shelf, especially the well-studied southern part off Spirits Bay abutting the New Zealand mainland. An intensive survey conducted in 1999 found a remarkable diversity of suspension feeders in the area, especially bryozoans and sponges, richness being highest between 30–80 m depth (Cryer et al., Reference Cryer, O'Shea, Gordon, Kelly, Drury, Morrison, Hill, Saunders, Shankar, Wilkinson and Foster2000). A total of 298 bryozoan species occurred in the area, a diversity, relative to area sampled, unmatched elsewhere in New Zealand, with a high average taxonomic distinctness (Rowden et al., Reference Rowden, Warwick and Gordon2004). At least 60 species of Bryozoa are endemic to the Three Kings Shelf region (e.g. Powell, Reference Powell1967; Taylor & Gordon, Reference Taylor and Gordon2003), now also including Carolanna schackae gen. et sp. nov. and Waeschenbachia splendida gen. et sp. nov.
Acknowledgements
Specimens from cruise KAH1706 were collected by NIWA for the Ministry of Primary Industries (MPI) funded project ‘Monitoring recovery of benthic fauna in Spirits Bay’. Specimens from cruises KAH0204, TAN0205, TAN0413, TAN0604 were collected by NIWA for the project ‘Seamounts: their importance to fisheries and marine ecosystems’, funded by the former New Zealand Foundation for Research, Science and Technology (C01X0028 (SFA023), C01X0224 (SFAS033), C01X0508 (SFBF063)) with additional funding from the former Ministry of Fisheries (project nos. ZBD2001/10, ZBD2004-01, ENV2005-16), NOAA Satellite Operations Facility (NRAM053) and the Census of Marine Life field programme on seamounts, CenSeam (UCS05301). Specimens from cruise TAN1301 were collected during NIWA trawl surveys funded by the New Zealand Ministry for Primary Industries. Specimens from cruises TAN1319 and TAN1501 were collected as part of a commercial project for Anadarko (Anadarko New Zealand Company and Anadarko New Zealand Taranaki Company). Specimens from cruise TAN1503 were collected by NIWA as part of the ‘Impact of resource use on vulnerable deep-sea communities’ project (CO1X0906), funded by the Ministry of Business, Innovation & Employment with support from Ministry for Primary Industries (project BEN2014-02) and NIWAs Enabling the Management of Marine Mining (MBIE contract CO1X1228). Dr Carina-Sim-Smith (Clearsight Consultants, Auckland) drafted Figure 1.
APPENDIX
Station data




















