Kaolin is a clay composed mainly of kaolinite (Al2Si2O5[OH]4) used in numerous engineering applications, particularly in the aluminium foundry industry, where Al2O3-rich compositions with remarkable chemical resistance are traditionally selected due to their low SiO2 content (White, Reference White1970; Wynn, Reference Wynn1992). The stability of kaolinite significantly affects the evolution of the aluminosilicate structure, as the presence of Al2O3 reduces the material's liquid immiscibility. In addition, the combination of SiO2 and Al2O3 with CaO, MgO, Cr2O3 and/or BaO is commonly employed to impart specific properties related to phase formation, sintering temperature, porosity, resistance to chemical attack or thermal shock, wettability, etc. (Allahevrdi et al., Reference Allahevrdi, Afshar and Allaire1998; Mishra et al., Reference Mishra, Anand, Panda and Das2002). During sintering, kaolinite is transformed into meta-kaolin with a highly disordered structure due to dehydroxylation and subsequent reorganization (Bundy, Reference Bundy, Murray, Bundy and Harvey1993; Chandrasekhar & Ramaswamy, Reference Chandrasekhar and Ramaswamy2002). The addition of iron decreases the transition temperature of meta-kaolin, whereas crystallization of primary and secondary mullite and cristobalite is promoted (Soro et al., Reference Soro, Aldon, Jumas and Blanchart2003). Primary mullite (mullite 1:1 or Al2O3•SiO2) forms at ~1100°C due to Si–O–Al linkages in the meta-kaolinite. At temperatures >1200°C, the primary mullite crystals begin to grow by reacting with amorphous aluminosilicate phases, forming secondary mullite (mullite 3:2 or 3Al2O3•2SiO2) (Ribeiro et al., Reference Ribeiro, Tulyagavov, Ferreira and Labrincha2005). Above 1100°C, the diffraction maxima of mullite appear, becoming more intense with increasing sintering temperature (Bouzidi et al., Reference Bouzidi, Bouzidi, Bouguermouh, Nunes, Benabdeslem, Mahtout and Merabet2014).
BaCO3 finds many important commercial applications in the glass, ceramic and building industries (Ersoy et al., Reference Ersoy, Kavas, Evcin and Önce2008). In the glass industry, barium is added to glass as barium carbonate or barium oxide to improve the refractive index of optical glass, to promote sintering and to lower the viscosity of the molten glass. It is used as a fluxing agent in ceramic industry enamels and glazes and in ceramic materials. Barium monoaluminate (BaO•Al2O3) has the ability to hydrate and may be used as cement or grouting material. Barium aluminosilicate (BaAl2Si2O8 or BAS) is a refractory material with a melting point of 1760°C (Semler & Foster, Reference Semler and Foster1970), existing primarily in three different polymorphic forms: celsian or monocelsian (monoclinic), hexacelsian (hexagonal) and α-hexacelsian (orthorhombic). Hexacelsian is thermodynamically stable at temperatures between 1590°C and the melting point, whereas celsian is stable below 1590°C (Lee & Aswath, Reference Lee and Aswath2000). Celsian is commonly synthesized by solid-state reactions from mixtures of BaCO3, Al2O3 and SiO2 reagent-grade powders or using either kaolin or topaz as alternative sources of Al2O3 and SiO2. Celsian has also been synthesized by the glass–ceramic route (Eichler et al., Reference Eichler, Solow, Otschik and Schaffrath1999) using mixtures of BaCO3, Al2O3 and SiO2, and in some cases with the addition of MgO. Basic oxides added to aluminosilicate compositions may act as fluxes, resulting in a displacement of the liquidus curve towards lower temperatures. Additional displacement of phase transformations may also occur at a lower SiO2 content due to the formation of immiscible liquid phases that decrease the solubility of SiO2 in the melt. Liquid immiscibility may occur in the oxides of divalent metal cations, except in the case of Ba2+ due to its large ionic radius. In fact, as the cation radius increases, the miscibility gap begins to decrease (Amritphale et al., Reference Amritphale, Anshul, Chandra and Ramakrishnan2007; López-Cuevas et al., Reference López-Cuevas, Long-González and Gutiérrez-Chavarría2012a,Reference López-Cuevas, Long-González, Gutiérrez-Chavarría, Calderon, Salinas-Rodriguez and Balmori-Ramirezb). For these oxides, immiscibility occurs over the primary crystallization field of silica, thus leading to the formation of silicates (López-Cuevas et al., Reference López-Cuevas, Long-González and Gutiérrez-Chavarría2012a,Reference López-Cuevas, Long-González, Gutiérrez-Chavarría, Calderon, Salinas-Rodriguez and Balmori-Ramirezb). This can be correlated with the ability of the cations to fit into the interstices of the silicate structure (Tkalcec et al., Reference Tkalcec, Prodanovic, Falz and Hennicke1985).
While porosity leads to a decrease in the mechanical strength of ceramic materials, it actually improves the thermal shock resistance. For this reason, pullout, crack branching and deflection mechanisms in alumina-mullite materials require a weak interface between the additives and matrix, in which needle-like mullite whiskers interlock with alumina grains (Lamidieu & Gault, Reference Lamidieu and Gault1988). In multiphase polycrystalline materials, their mechanical properties are sensitive to these microstructure modifications and phase changes.
The aim of the study was to investigate the phase formation of the composites sintered at 1100 and 1200°C for 3 h composed mainly of a clay rich in kaolinite and halloysite mixed with BaCO3 at various weight ratios. The study is followed by an investigation of the microstructure and the quantity of amorphous phases in the composites that control their mechanical properties.
MATERIALS AND METHODS
Commercial powders of BaCO3 (>99.9 purity, Alfa Aesar, Ward Hill, MA) and Algerian pure kaolin (naturally rich in kaolinite and halloysite) from Djebel Debbagh were used as the starting materials. Milling was carried out in a planetary ball mill (Pulverisette 7, Fritsch, Germany). In total, 220 mg of kaolin and BaCO3 mixed with 80 mL of distilled water was placed in an agate vessel containing 22 × 10-mm zirconia balls. The vessel was rotated at 200 rpm at room temperature for 2 h.
The BaCO3 powder was added to the kaolin powder at concentrations of 40, 50 and 60 wt.%. Pure kaolin powder without BaCO3 was also used. The slurries obtained were dried at 90°C for 24 h. The mixtures were labelled K1 (BaCO3-free), KB2 (with 40 wt.% BaCO3), KB3 (with 50 wt.% BaCO3) and KB4 (with 60 wt.% BaCO3) and then ground manually for 15 min. Finally, the powders were pressed under uniaxial pressure, without added binder, at 55 MPa for 10 min to form pellets of 30 mm diameter and 4 mm thickness. The pellets were fired at 1100 and 1200°C with a heating rate of 10°C/min and a soaking time of 3 h under air atmosphere.
Characterization of the powders and the sintered specimens was performed on a Bruker D2 Phaser X-ray diffractometer using Cu-Kα radiation, a step size of 0.02°2θ and a counting time of 10 s per step. The elemental compositions of the materials were determined from X-ray fluorescence (PANalytical Perl'X 3). Quantitative mineralogical analysis was carried out by calculating the mineral content from the chemical analyses and the stoichiometric formula for each mineral (Bennet & Reed, Reference Bennet and Reed1971; Brindley, Reference Brindley1978). Differential thermal analysis (DTA) was performed with a Netzsch STA 409 CD instrument using α-Al2O3 as a reference material, operating in air atmosphere at a heating rate of 10°C/min from room temperature to 1200°C. The amorphous SiO2 phase was quantified by dissolving the materials in 10% hydrofluoric acid (HF) for 1 min and monitoring the dissolution rate of the amorphous phase compared to that of the crystalline phases.
The selective dissolution in HF is a simple and frequently used technique for quantifying the silica amorphous phase. The principle is based on estimation of the dissolution kinetics in an aqueous solution of HF. The dissolution rate of the amorphous phase is quicker than that of the crystalline phases. Vitreous silica, with a relatively open structure, dissolves faster than quartz. If a product made of a mixture of amorphous and crystalline phases (Al-Si rich) is attacked by HF for various durations, the dissolution variation curve presents two distinct slopes. The first slope represents the superposed dissolutions of the two phases. The second, weaker slope, starting after a complete dissolution of the amorphous phase, corresponds exclusively to the crystalline phase dissolution. An extrapolation of the second linear part towards the starting time (t = 0) allows estimation of the vitreous phase content (Mikeska et al., Reference Mikeska, Bennison and Grise2000).
Bulk and apparent density measurements of the samples were carried out by the Archimedes method in distilled water (ASTM C 830-00). The microstructure of the sintered samples was studied on polished and gold-coated surfaces using a scanning electron microscope (Philips FEI XL30). The microhardness of the samples was measured with a Vickers tester (H-400-H1-Leco) on polished samples using an indentation of 9.8 N for 15 s. An average value was obtained from ten indentations.
RESULTS AND DISCUSSION
Microstructure and phase formation
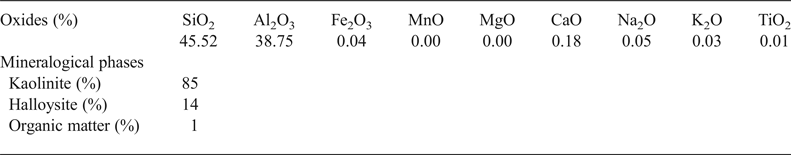
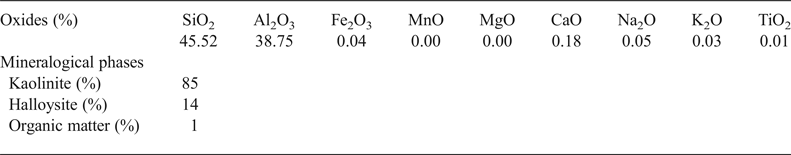
The Djebel Debbagh kaolin consists mainly of SiO2 and Al2O3 (Table 1). The raw material is pure as elements such as Fe, Ti and Mn were not present in the structure of kaolinite and halloysite. Quantitative mineralogical analysis showed that the sample contains a large amount of kaolinite (85 wt.%) and halloysite (14 wt.%).
Table 1. Chemical and mineralogical compositions of the kaolin raw material.
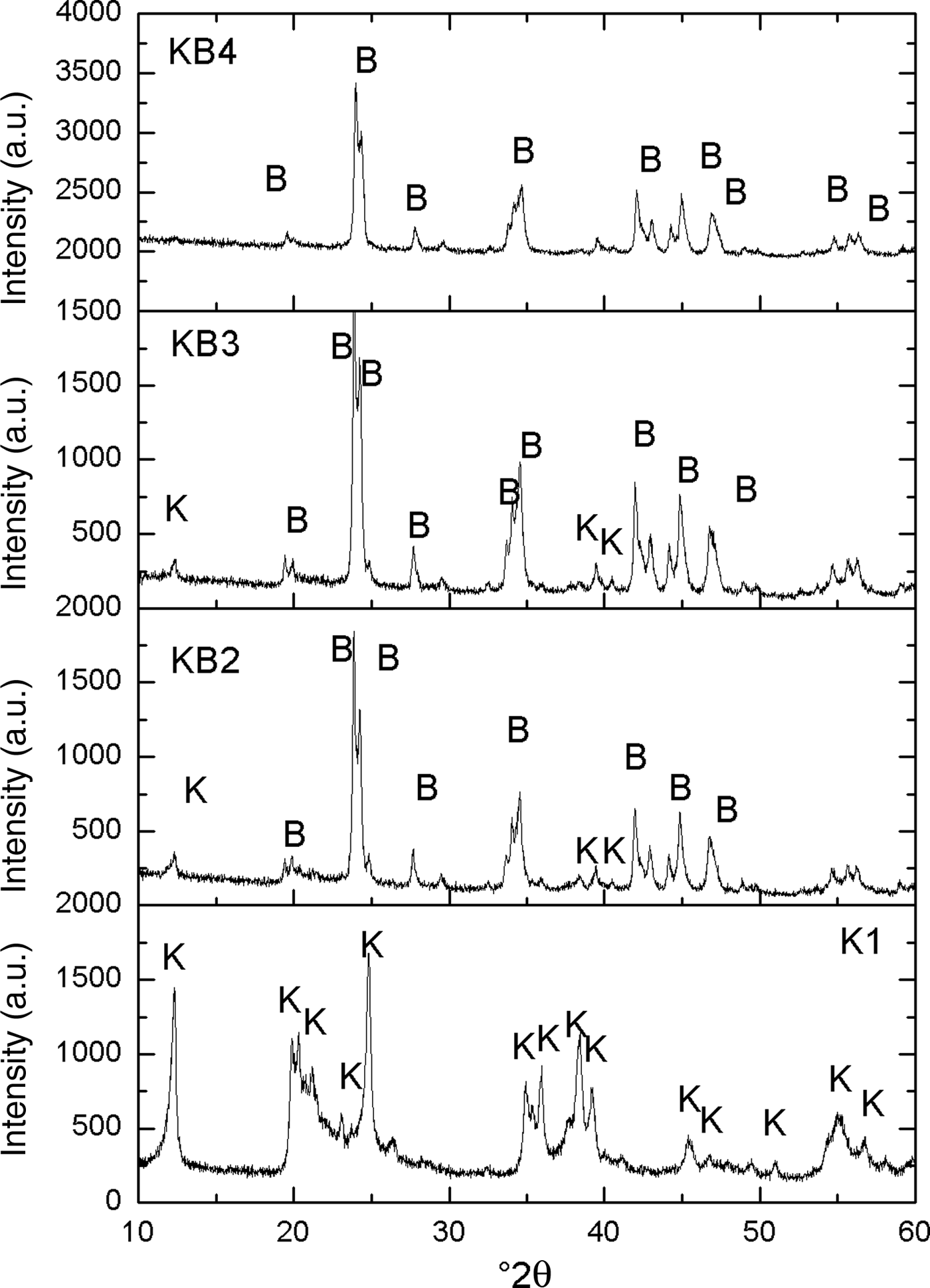
The X-ray diffraction (XRD) traces of the original kaolin (K1) mixed with various amounts of BaCO3 and milled for 2 h (KB2, KB3 and KB4) are shown in Fig. 1. In K1, the main diffraction maxima are attributed to kaolinite. The intensity of the (001) peak of kaolinite at ~12°2θ decreased considerably with increasing BaCO3 content, almost completely disappearing in the KB4 sample, which consists almost entirely of witherite (BaCO3). The significant decrease of basal peaks of kaolinite is related to delamination produced by ball milling, which increased the reactivity of kaolinite, and halloysite (Sánchez-Soto et al., Reference Sánchez-Soto, Carmen Jiménez de Haro, Pérez-Maqueda, Varona and Pérez-Rodríguez2000).
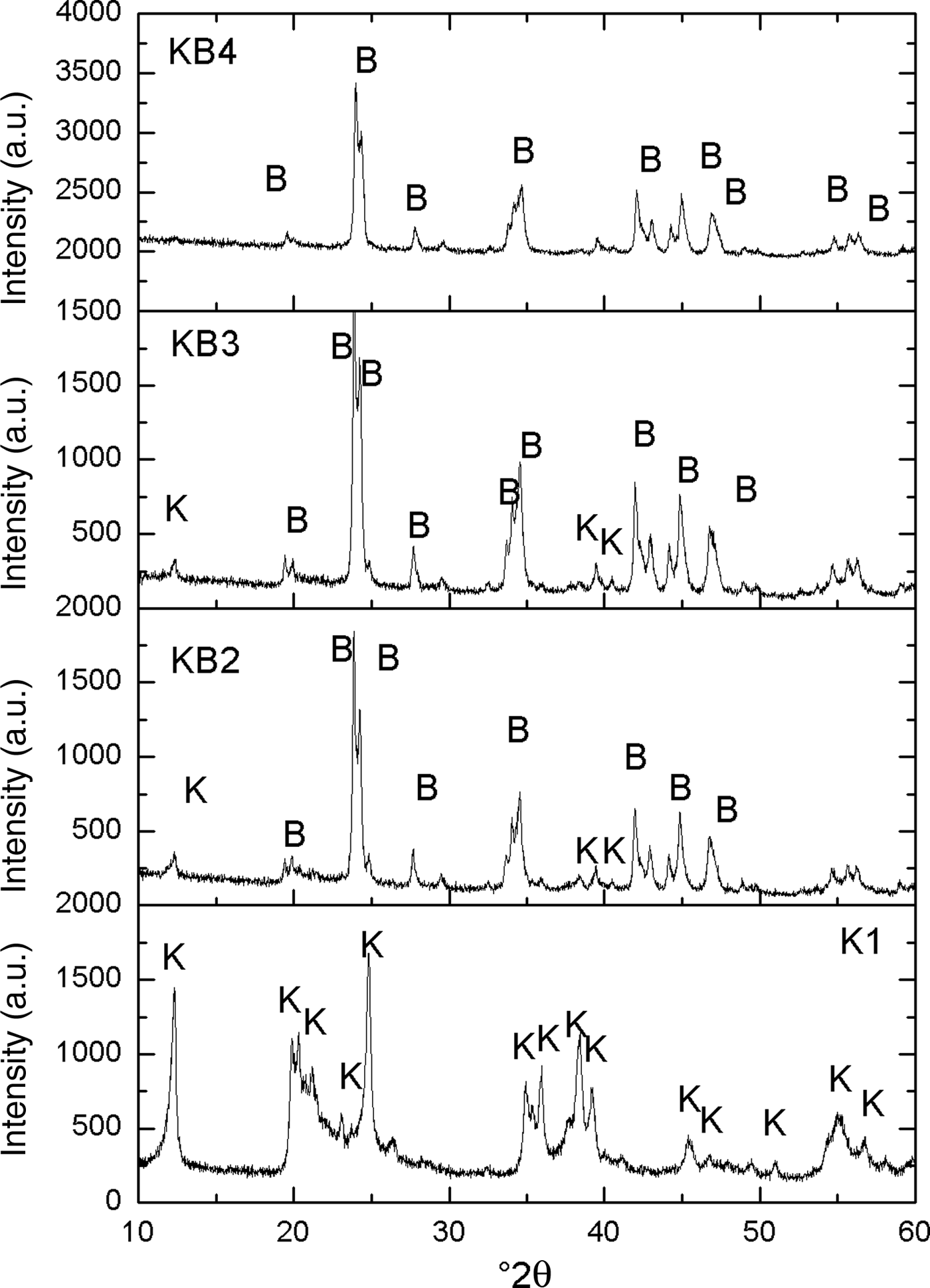
Fig. 1. XRD patterns of the uncalcined halloysite-kaolinite/BaCO3 mixed powders. B = BaCO3; k = kaolinite.
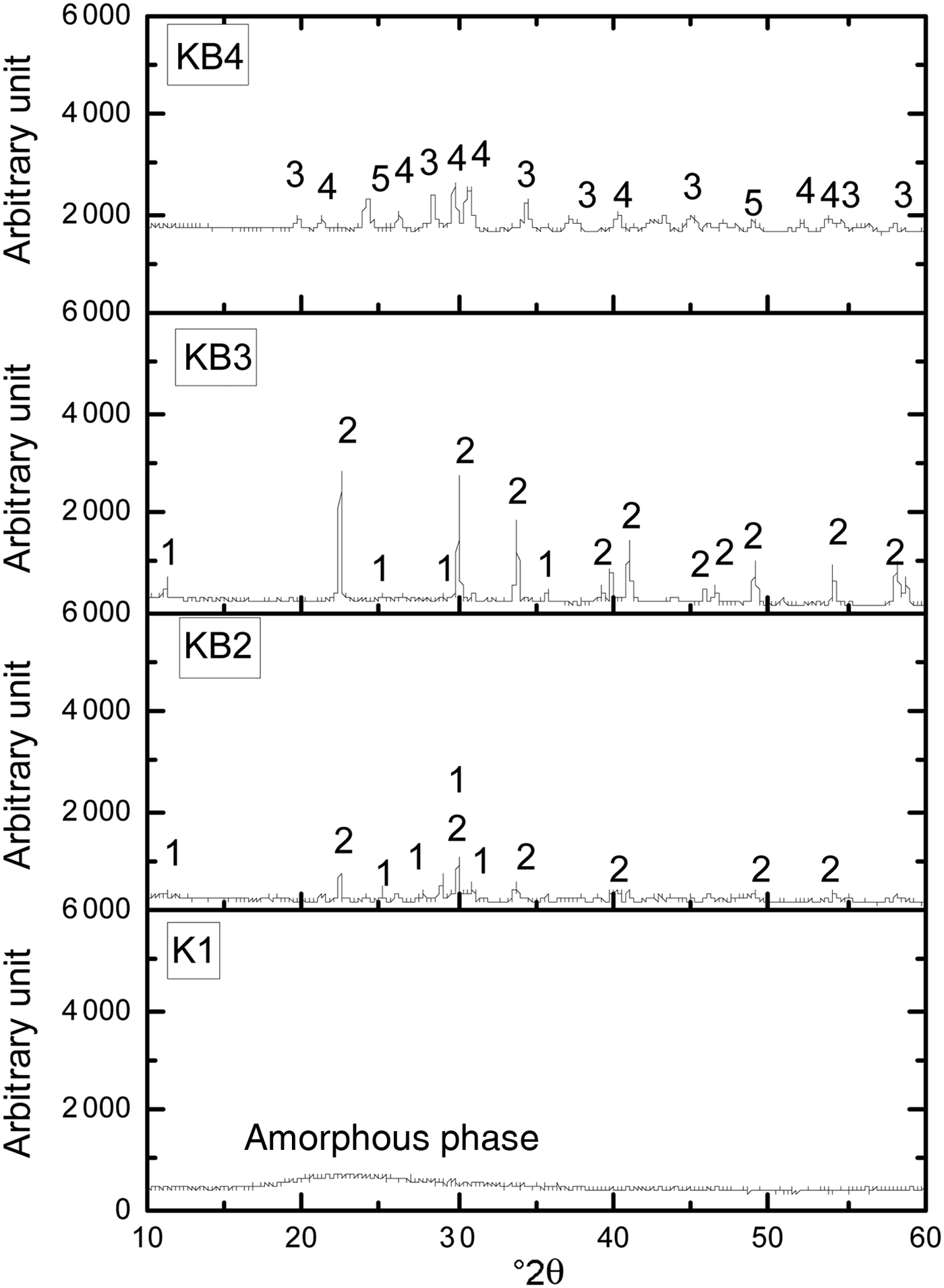
After sintering at 1100°C, sample K1 appeared amorphous, as only a broadened hump at 20–25°2θ is evident (Fig. 2). With the addition of BaCO3, new crystalline phases of hexagonal celsian (BaAl2SiO6 and BaAl2Si2O8) were formed. With increasing BaCO3 content, the peak intensities of BaAl2SiO6 and BaAl2Si2O8 increased (samples KB2 and KB3). These phases were not present in sample KB4, being replaced by BaAl2O4, Ba2SiO4 and BaSiO3.
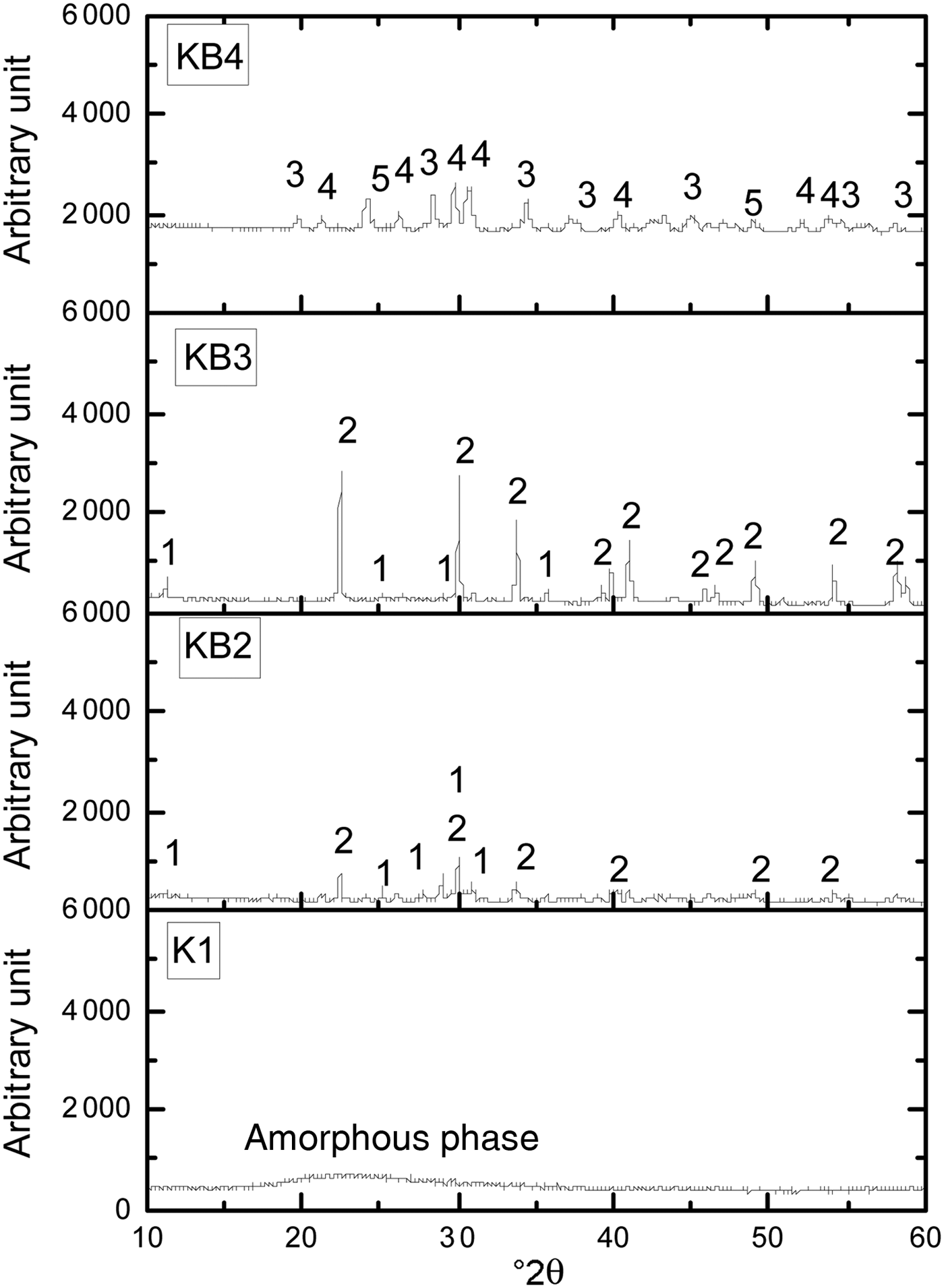
Fig. 2. XRD patterns of halloysite–kaolinite/BaCO3 composites sintered at 1100°C. 1 = BaAl2SiO6; 2 = BaAl2Si2O8; 3 = BaAl2O4; 4 = Ba2SiO4; 5 = BaSiO3.
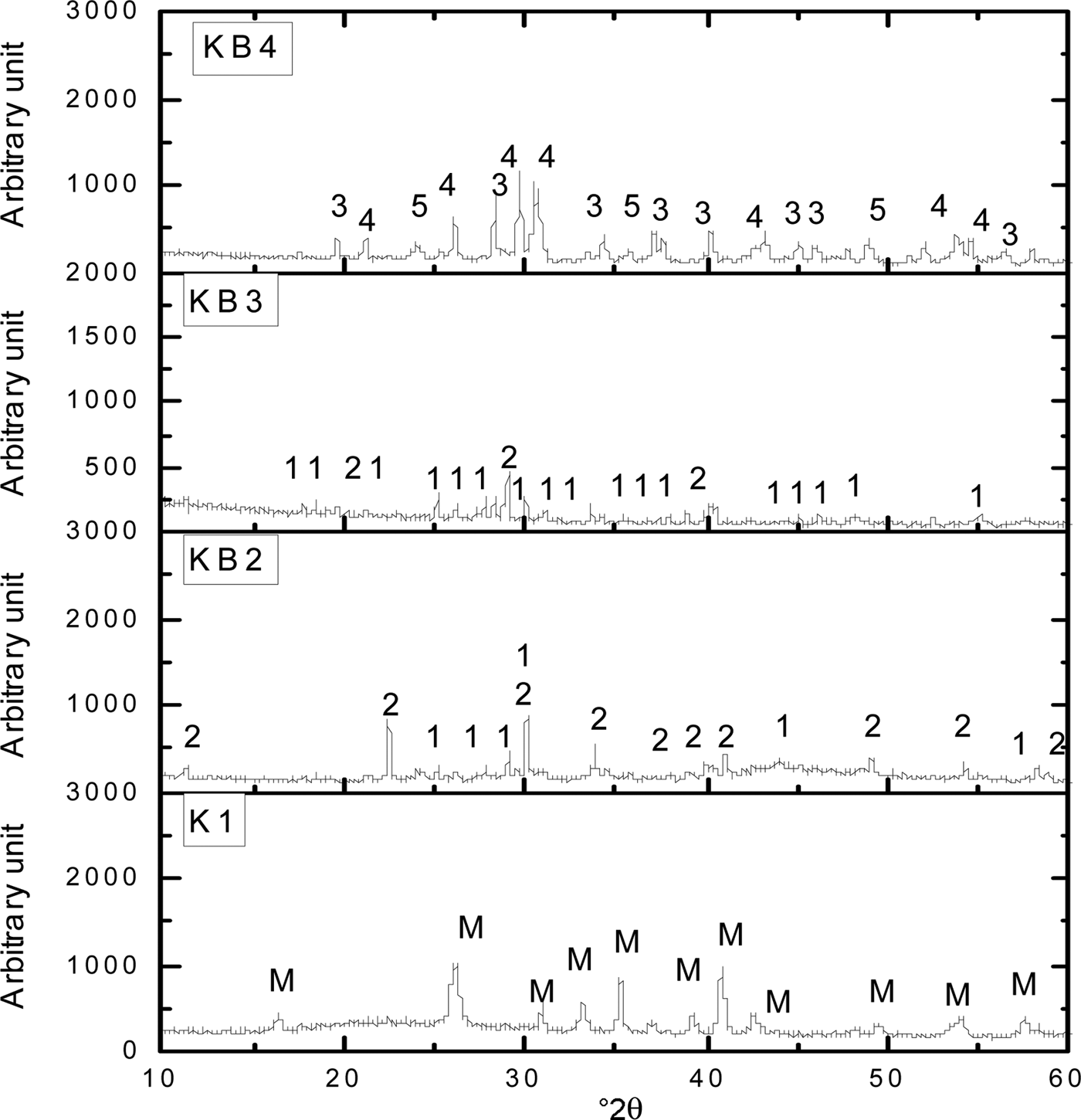
At 1200°C, mullite is the primary phase detected in the K1 sintered sample (Fig. 3). With increasing temperature, the peak intensities of BaAl2SiO6 and BaAl2Si2O8 increased in sample KB2 and diminished in sample KB3. Mechanical activation enhanced the reaction of BaAl2SiO6 and the formation of BaAl2Si2O8 at BaCO3 contents <50 wt.%. However, because the Ba2+ ions are reactive enough to form an immediate chemical bond with the kaolinite–mullite liquid state during sintering (Bouzidi et al., Reference Bouzidi, Aissou, Concha-Lozano, Gaudon, Janin, Mahtout and Merabet2014), samples KB2 and KB3 became more densified. In KB4, the peak intensities of BaAl2O4 and Ba2SiO4 increased with increasing temperature and increasing BaCO3 content to 60 wt.%, leading to the formation of refractory phases that required a higher sintering temperature.
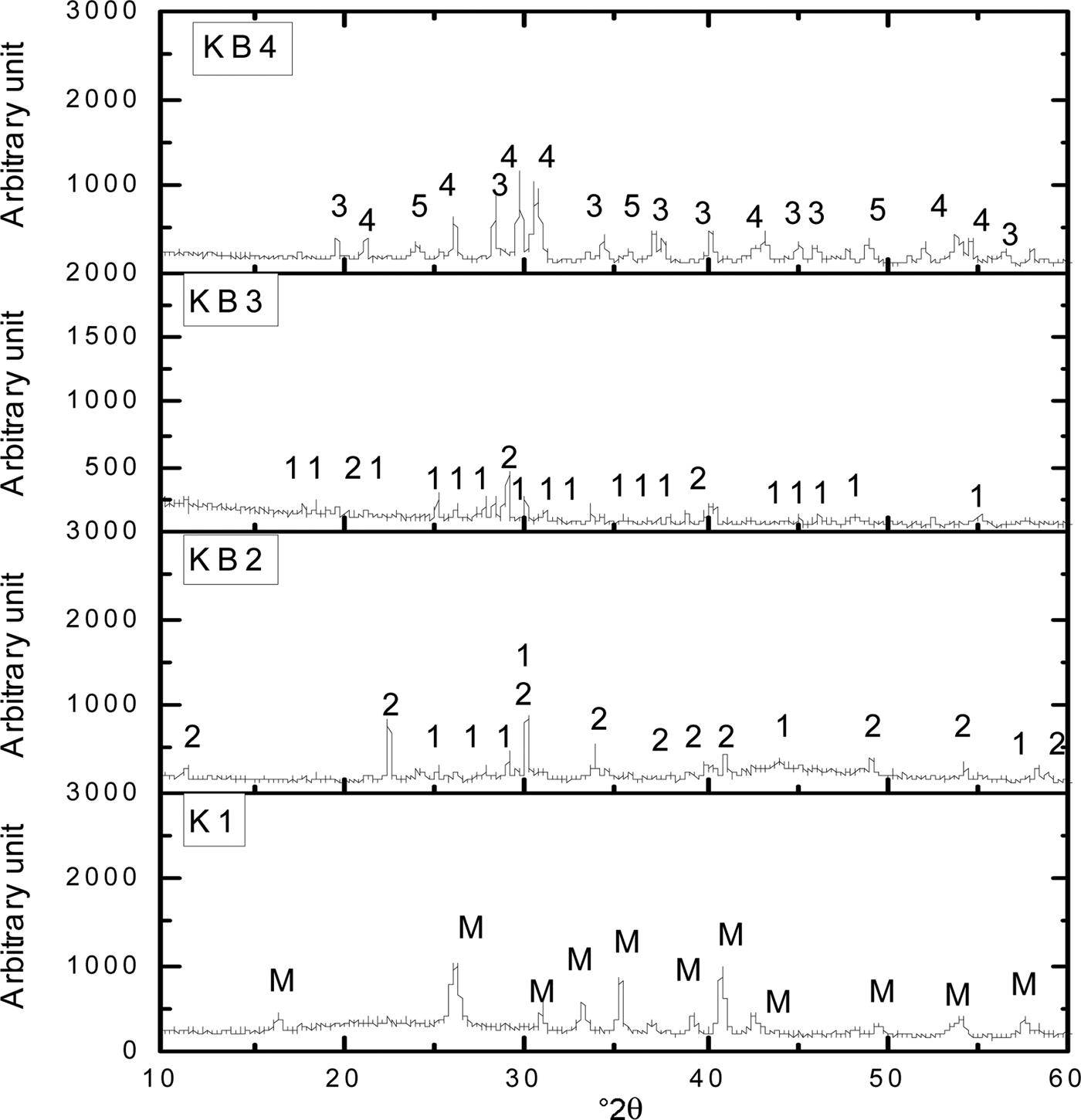
Fig. 3. XRD patterns of halloysite–kaolinite/BaCO3 composites sintered at 1200°C. 1 = BaAl2SiO6; 2 = BaAl2Si2O8; 3 = BaAl2O4; 4 = Ba2SiO4; 5 = BaSiO3; M = mullite.
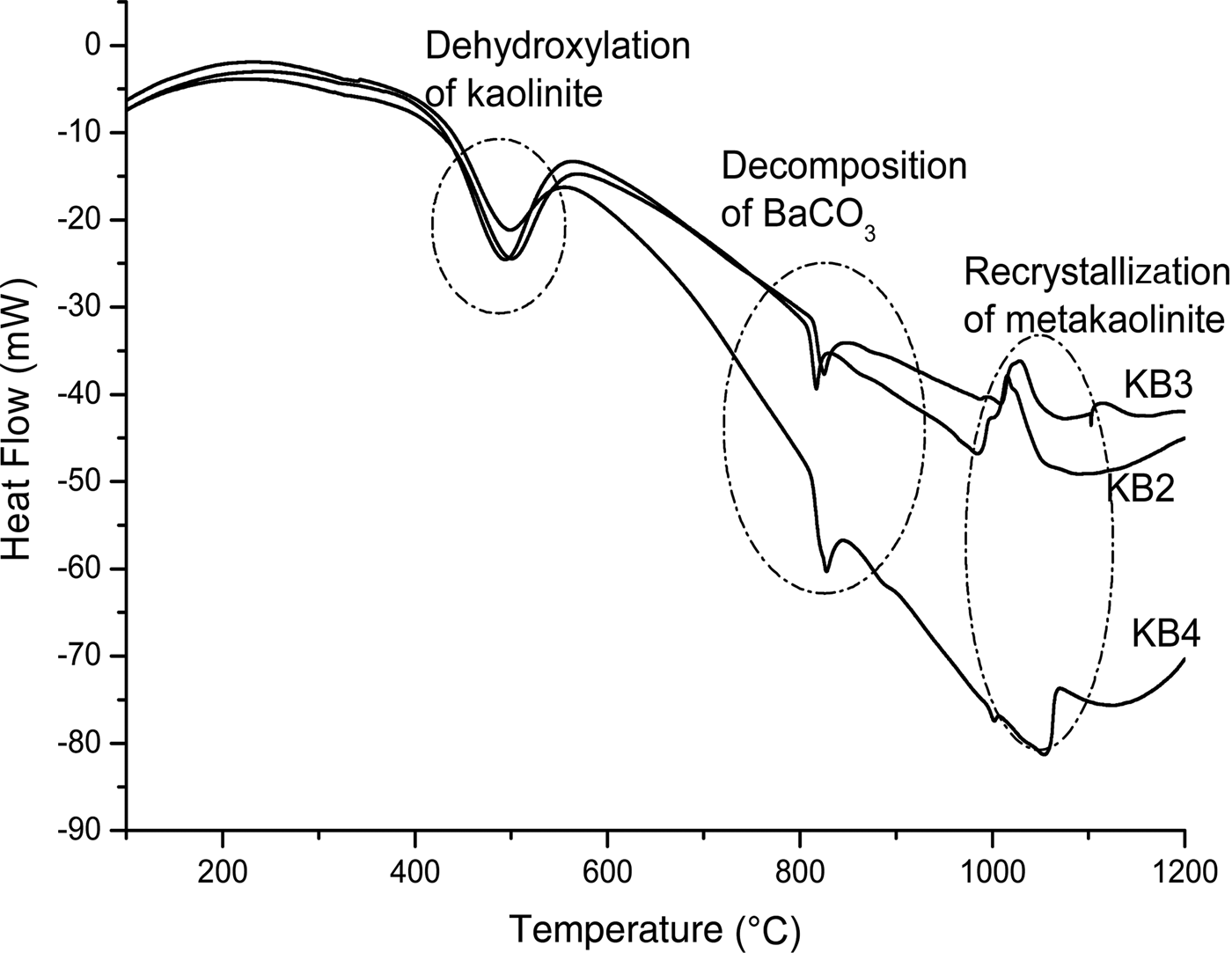
The DTA results showed a series of thermal events during heating of the samples to 1200°C (Fig. 4). The first endothermic peaks recorded at 494, 497 and 501°C for KB2, KB3 and KB4, respectively, are attributed to the dehydroxylation of kaolinite and halloysite (Bouzidi et al., Reference Bouzidi, Bouzidi, Bouguermouh, Nunes, Benabdeslem, Mahtout and Merabet2014). In fact, with increasing amounts of BaCO3, the temperature of dehydroxylation decreased. Small amounts of some oxides (Fe2O3, TiO2, BaO, Na2O, etc.) may act as fluxes, decreasing the melting point of the materials.
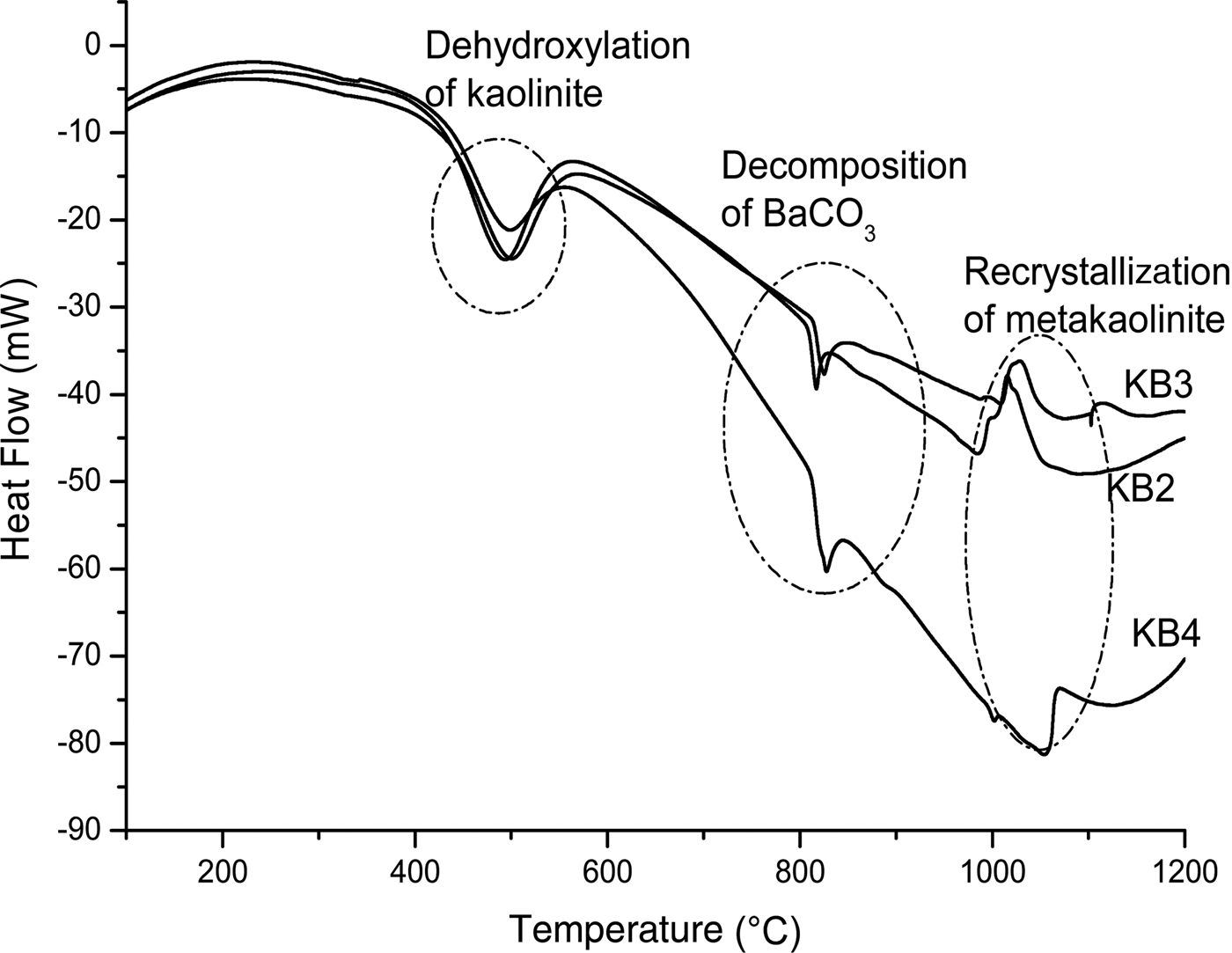
Fig. 4. Differential thermal analysis traces of the different mixtures KB2, KB3 and KB4.
Transformation of meta-kaolinite to form primary mullite is shown by the exothermic peaks in KB2 and KB3 at 1019 and 1026°C, respectively. As the recrystallization of meta-kaolinite occurs at higher temperatures (i.e. ~1100°C), the observed lower temperature is probably due to the refractory character of the sample and the amount of BaCO3. The decomposition temperatures of BaCO3 were recorded at 816, 825 and 827°C for KB2, KB3 and KB4, respectively, as was indicated by the second endothermic peak (Fig. 4). In contrast, the decomposition takes longer for sample KB4, occurring at 998 and 1054°C for BaAl2O4 and Ba2SiO4, respectively (Fig. 4). Because BaO was not detected in the XRD patterns of the heated samples, it is concluded that BaO reacts with Al2O3 to form BaAl2O4, which was observed even in the samples treated at 1100 and 1200°C.
The microstructures of the KB4 samples sintered at 1100 and 1200°C obtained from polished surfaces are shown in Fig. 5. The large pores observed were formed by the release of CO2 due to decomposition of carbonates, as well as the phase transformation that occurs during sintering. The surface of KB4 heated at 1100°C for 3 h exhibits large grains consisting of polyhedral crystals, indicating an inadequate sintering temperature (Fig. 5a). It is assumed that these grains are agglomerated rather than consolidated by the glassy phase, forming very fine grains of non-transformed BaCO3 distributed on the blocky surface. As the temperature rises to 1200°C, the sample exhibits multiphase inhomogeneity in the microstructure (Fig. 5b). Dense crystals also appear white in the back-scattered electron images with low aspect ratios in highly siliceous residual glass with acicular morphology. This indicates that BaCO3 acts as a nucleating agent. The particles of BaCO3 appear to be well dispersed in the matrix. For both samples, the porosity is highlighted.

Fig. 5. SEM images of the polished surface of halloysite-kaolinite/BaCO3 composites sintered at: (a) 1100°C for KB4 and (b) 1200°C for KB2 samples. G = glassy phase; P = pores.
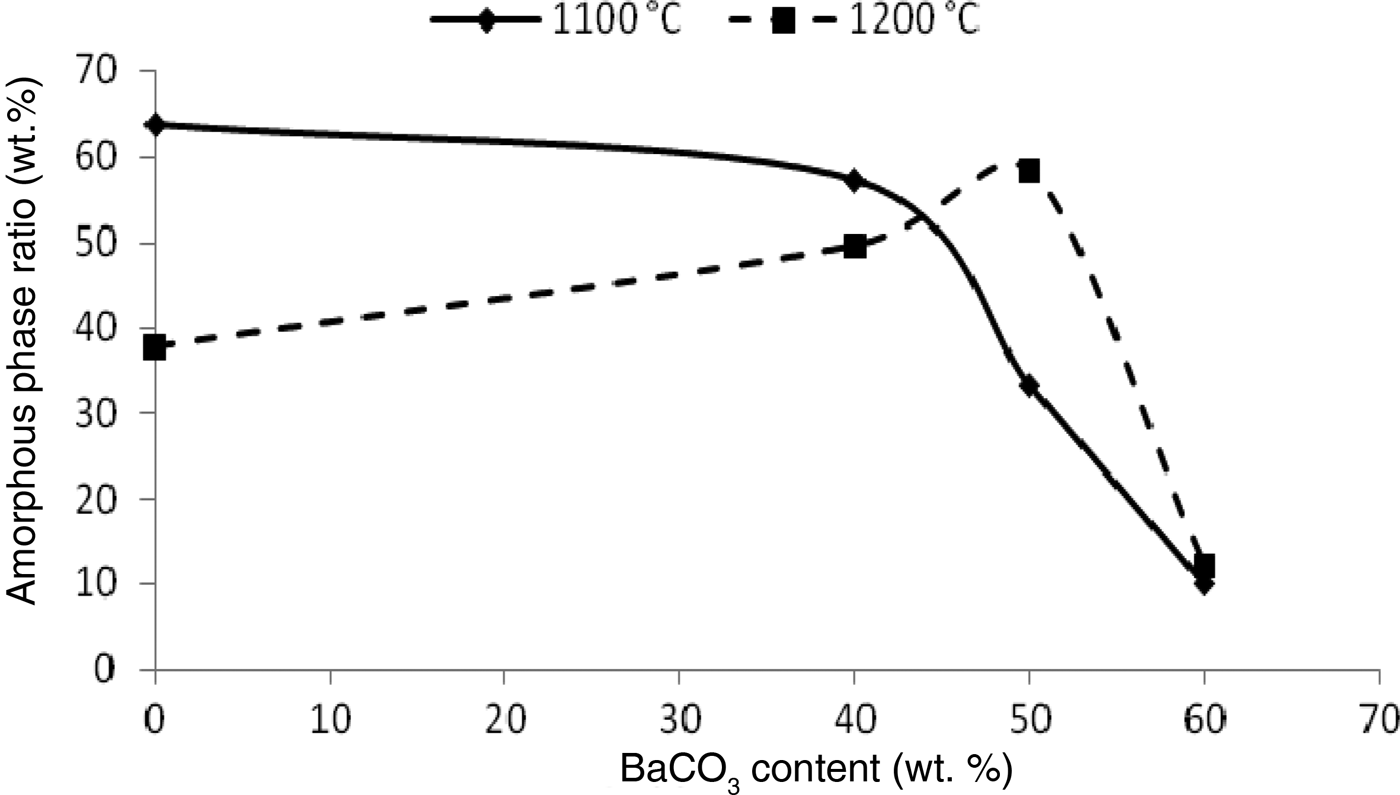
With increasing BaCO3 content, the abundance of the amorphous phase decreases in samples sintered at 1100°C and increases at 1200°C (especially for KB3 and KB4) (Fig. 6). BaCO3 acts as a nucleating agent, leading to the formation of celsian and more of the amorphous phase. The decomposition of BaCO3 is associated with the formation of the amorphous phase, which is caused by diffusion of BaO (simultaneous diffusion of Ba2+ and O2–) through the disordered structure of meta-kaolinite.
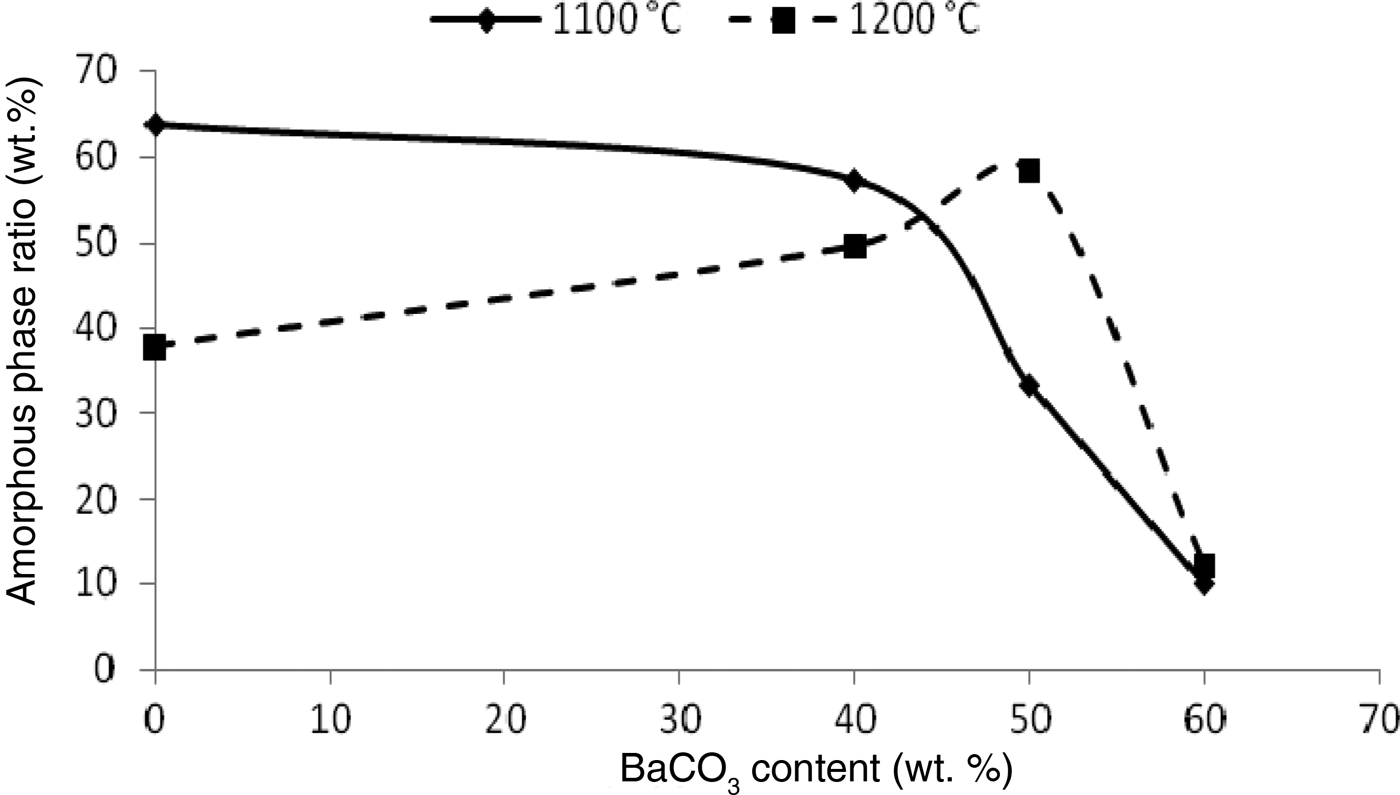
Fig. 6. Amorphous phase ratio as a function of temperature and BaCO3 content.
The rate of chemical attack is controlled by the reactions at the liquid–solid interfaces and by the grain microstructure of the powder. Corrosion occurs within the interconnected pores where the vitreous phase may be present. In addition, the BaAl2SiO6 and BaAl2Si2O8 phases may be considered as glass–ceramic phases and would also be subjected to corrosion, similar to other ceramics (Mikeska et al., Reference Mikeska, Bennison and Grise2000). When the BaCO3 content exceeds 50 wt.% (e.g. sample KB4), the sintering temperature is insufficient to form spar, which might promote growth of the amorphous phase.
Furthermore, 57 wt.% of the amorphous phase is obtained when the sintering temperature is 1100°C after addition of 40 wt.% BaCO3. As the BaCO3 content increased to 50 wt.%, the amount of amorphous phase also increased. For example, 58 wt.% amorphous phase formed at 1200°C. It is possible that Ba2+ ions react with the kaolinite layers, yielding a liquid phase that acts as a fluxing agent and decreases the firing temperature, forming a glassy phase (Kang et al., Reference Kang, Park, Kim and Hwang2000; Shen et al., Reference Shen, Chen, Qi, Wang, Chan, Chen and Jiang2009), as seen in the KB2 and KB3 samples.
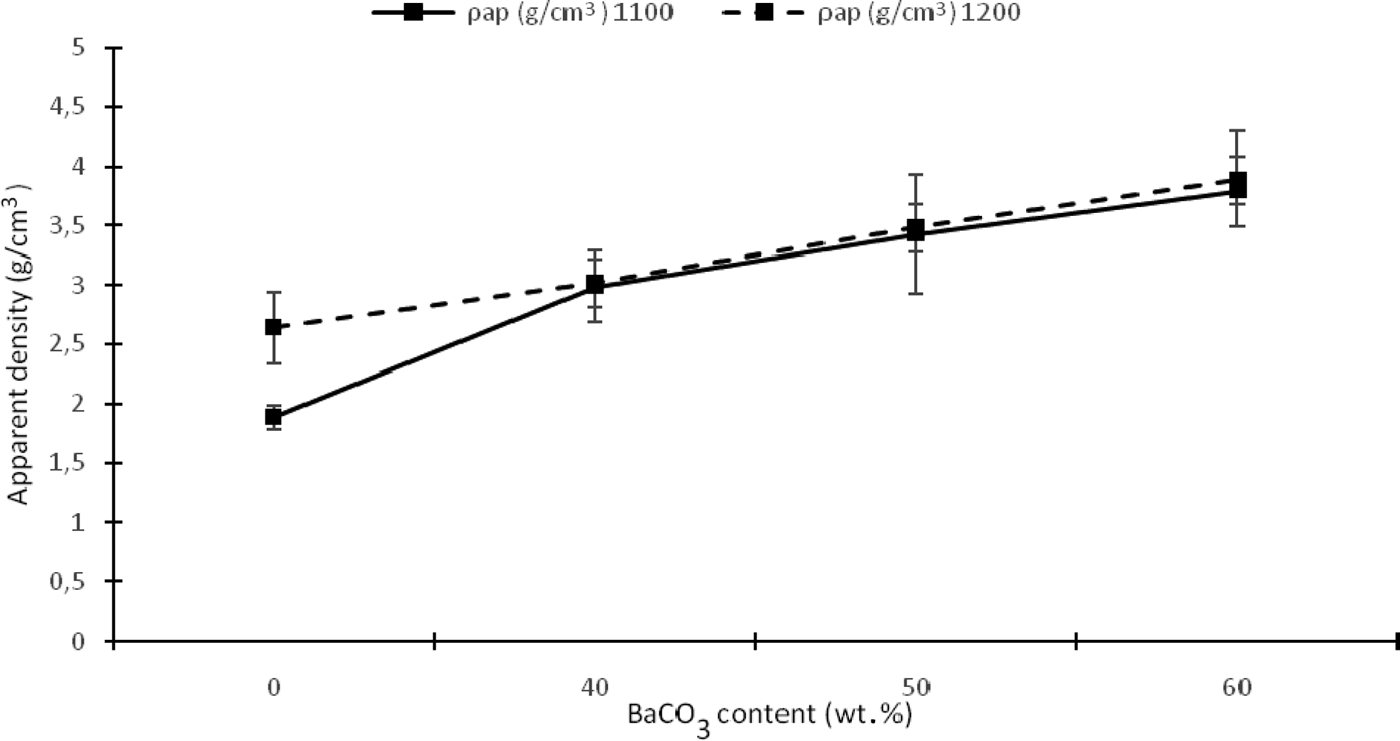
The apparent density of the samples increases with addition of BaCO3 at 1100 and 1200°C (Fig. 7). In contrast, sample K1 exhibits a low density (1.89 g/cm3) at 1100°C, which is close to that of the amorphous silica (2.48 g/cm3) that is formed by structural reorganization of meta-kaolinite and nucleation of mullite. However, this parameter increases considerably at 1200°C due to densification of the sample to 2.64 g/cm3 caused by crystallization of mullite in the K1 sample. The greater apparent densities of KB2 and KB3 are probably due to the BaAl2Si2O8 phase, which exhibits a theoretical apparent density of close to 3.40 g/cm3. The apparent density of the KB4 sample is greater than the other samples at both 1100 and 1200°C. Consequently, there is less of the liquid phase at 60 wt.% BaCO3, resulting in a low degree of wetting of the solid particles by the liquid phase and poor consolidation of the material during sintering. Therefore, it can be concluded that the temperature of 1200°C is insufficient to obtain fully sintered composites.
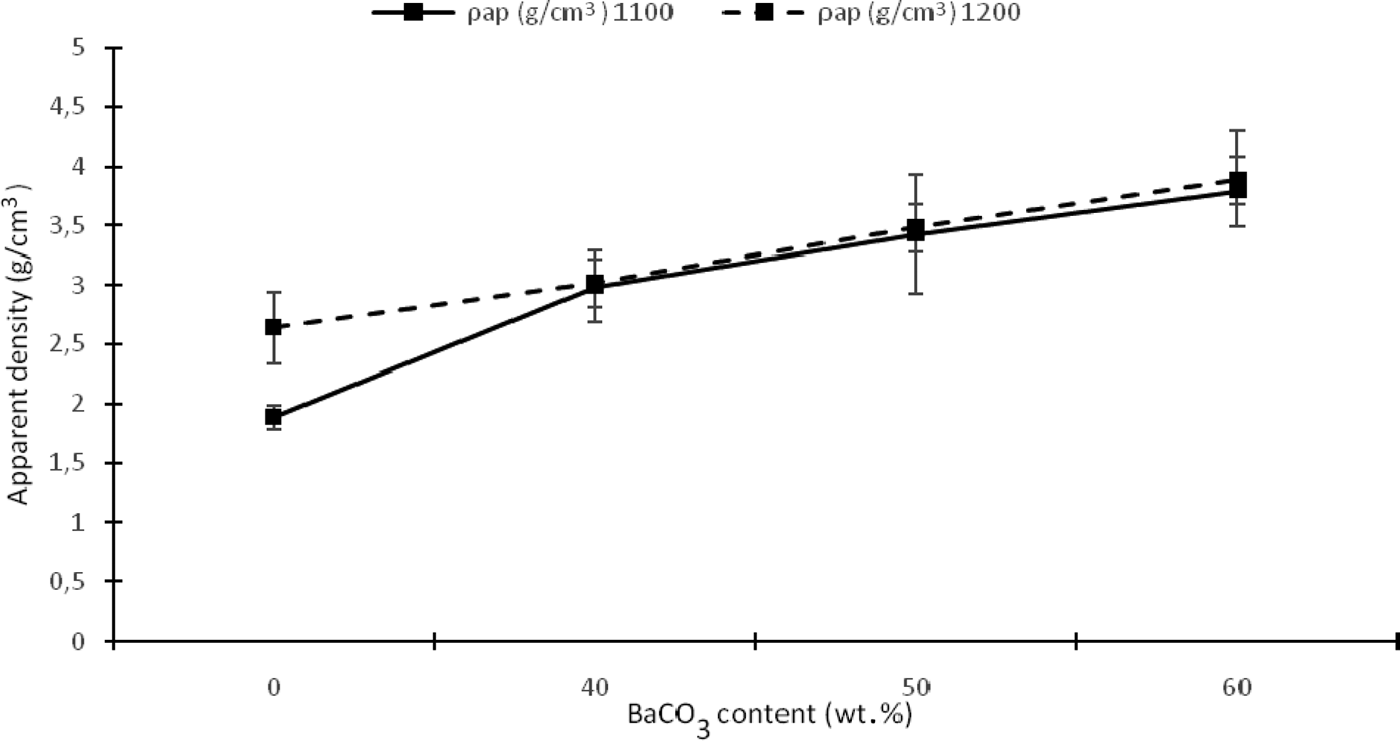
Fig. 7. Apparent density as a function of temperature and BaCO3 content.
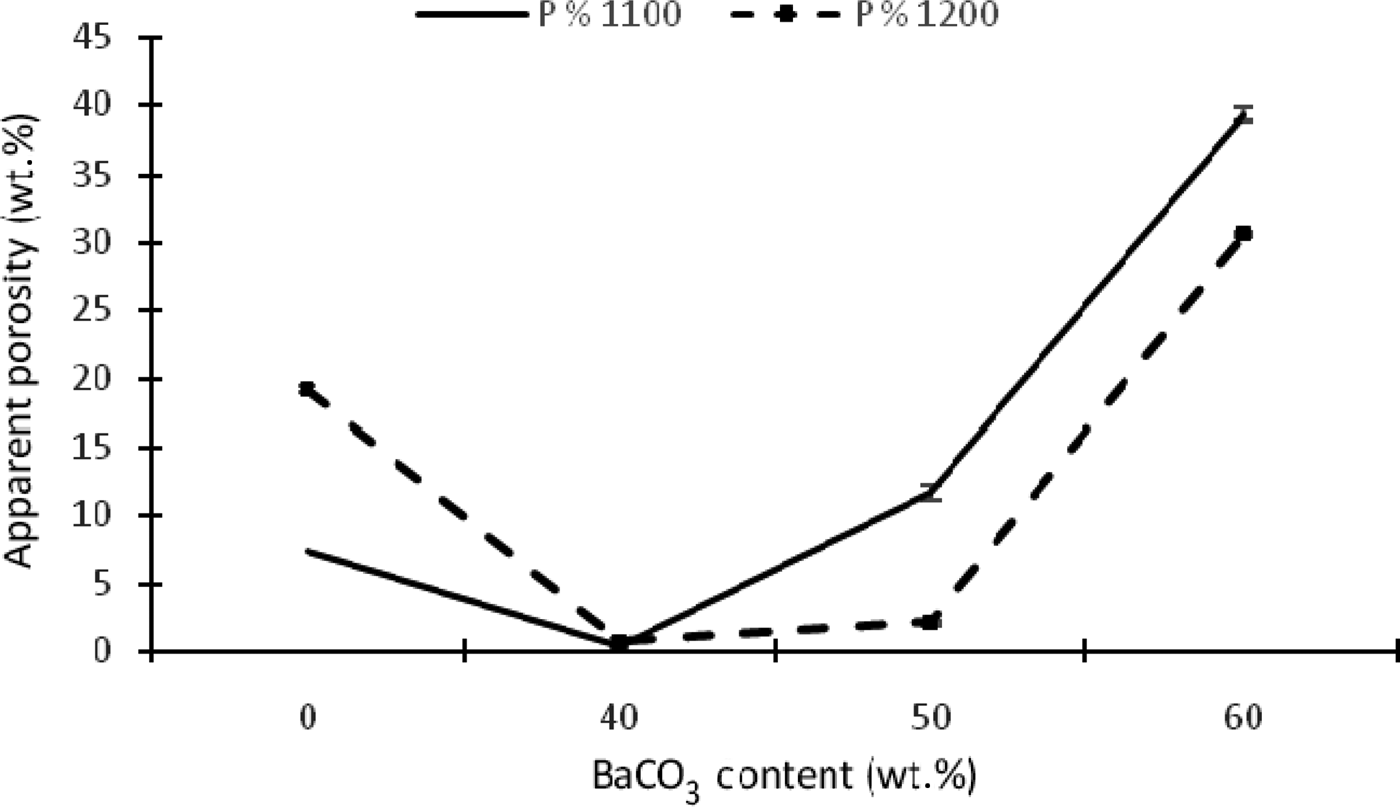
The porosity of the samples is also affected by the addition of BaCO3 at 1100 and 1200°C (Fig. 8), decreasing with the first addition of BaCO3 (KB2) at 1100 and 1200°C, while further additions have the opposite effect. In fact, very low porosity was observed in the KB3 sample (50 wt.% BaCO3). This phenomenon is probably attributed to the formation of abundant hexacelsian (BaAl2SiO6, BaAl2Si2O8). As the addition of BaCO3 exceeds 50 wt.%, porosity becomes increasingly important. New phase transformations (BaAl2O4, Ba2SiO4 and BaSiO3) take place, affecting the completion of the firing process that cannot be considered as soon as porosity does not decrease.
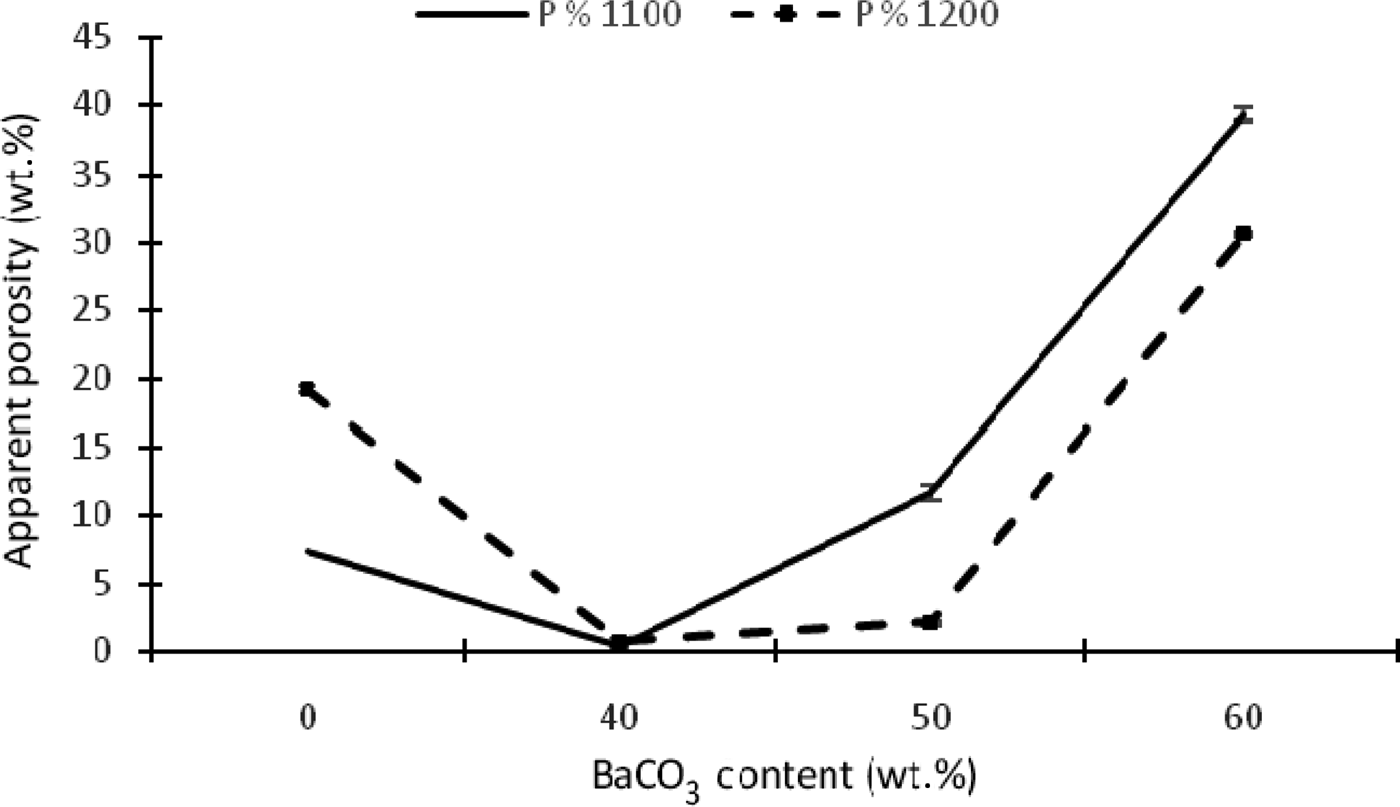
Fig. 8. Apparent porosity as a function of temperature and BaCO3 content.
Mechanical properties of the composites (microhardness)
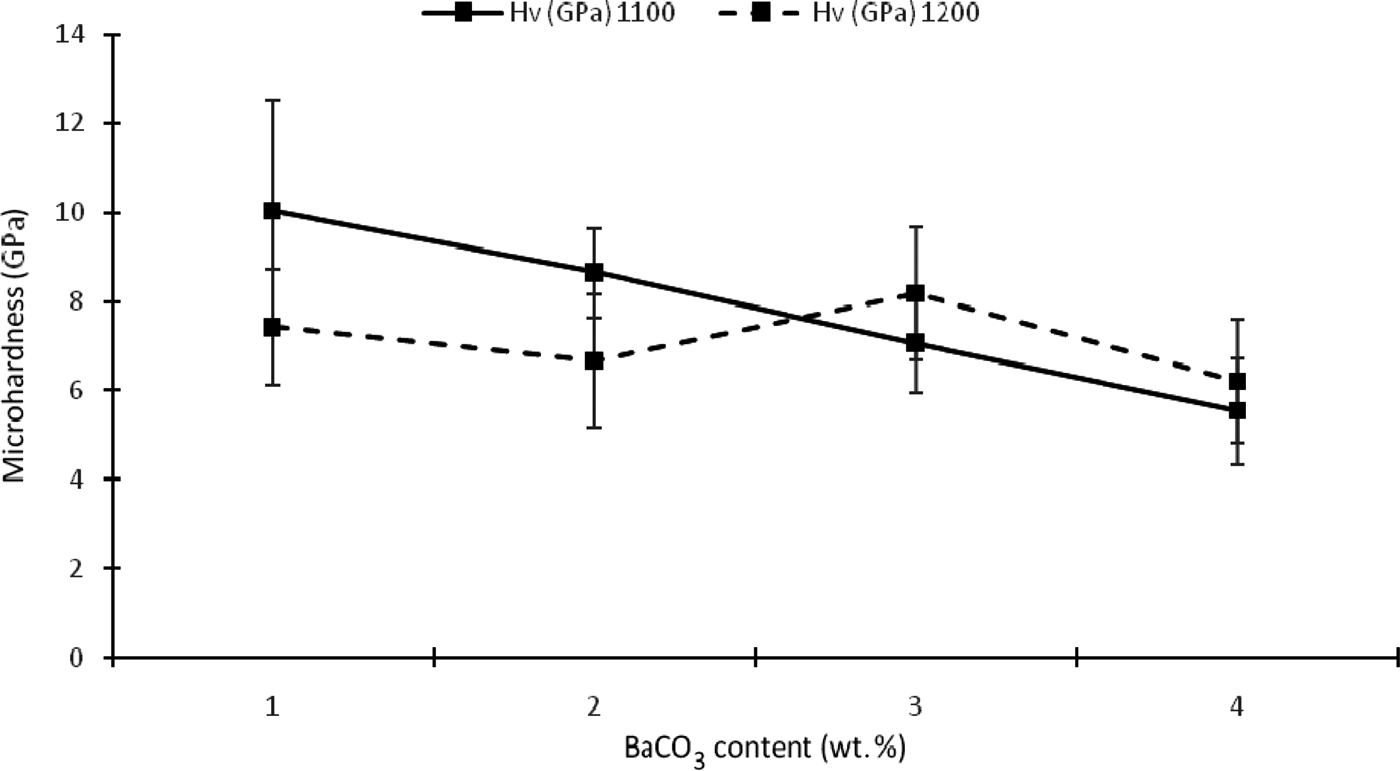
The addition of BaCO3 causes a decrease in microhardness for samples sintered at 1100°C due to increasing porosity (Fig. 9). However, the amorphous phase has a positive effect on this mechanical property. Microhardness decreases with decreasing amount of amorphous phases. The microhardness of sample K1 reached a maximum value (10.02 GPa) when it was completely amorphous, decreasing considerably when the sintering temperature was increased to 1200°C. The enhancement of the mechanical properties with increasing sintering temperature for samples KB3 and KB4 was probably due to an increase in the glassy phase and a reduction in porosity. Alternatively, it may be a function of separate crystal phases (e.g. barium aluminium silicate), especially in sample KB4.
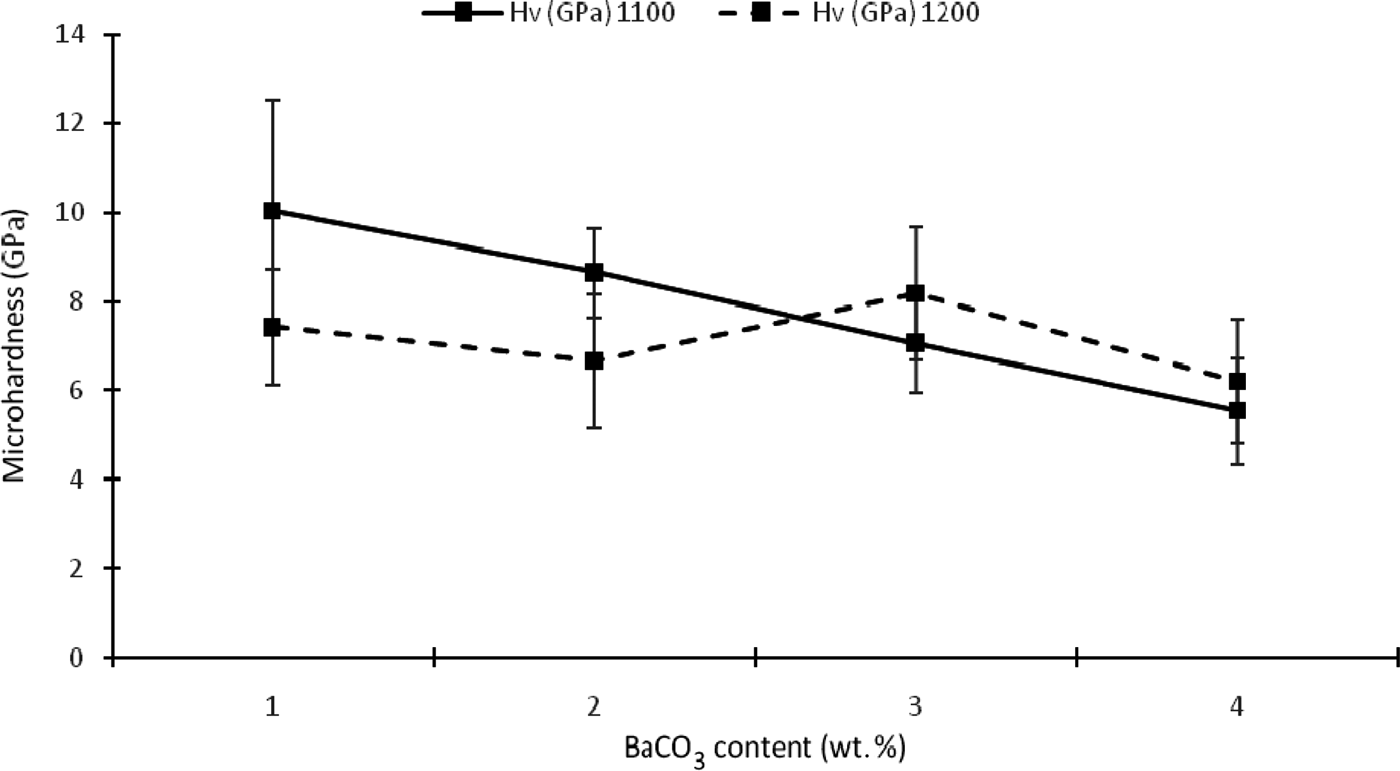
Fig. 9. Microhardness of the composites as a function of temperature and BaCO3 content.
For samples sintered at 1100°C, the hardness increased with decreasing BaCO3 content and was very dependent on the formation of a glassy phase. This is attributed to the effect of Ba2+ ions on the nucleation and accelerated formation of the liquid phase. For samples sintered at 1200°C, hardness increased with decreasing BaCO3 content from sample KB4 to KB3, but otherwise remained fairly constant.
In summary, this work has shown that ball milling promotes the mixture of these raw materials, whereas the microstructure of kaolinite is destroyed. Despite the large Al content of the Djebel Debbagh kaolin, the consolidation and densification of the samples occur at 1100 and 1200°C for BaCO3 contents of <50 wt.%. When the amount of BaCO3 exceeds 50 wt.%, the diffraction maxima of kaolinite disappear in favour of the BaCO3 peaks. The addition of 10 wt.% of kaolinite is the optimal additive amount to form hexacelsian (Shen et al., Reference Shen, Chen, Qi, Wang, Chan, Chen and Jiang2009). In the present study, the formation of hexacelsian still occurs even at 50 wt.% BaCO3. Hexacelsian leads to a decrease in porosity and an increase in the amorphous phase content, as was also reported by López-Cuevas et al. (Reference López-Cuevas, Long-González, Gutiérrez-Chavarría, Calderon, Salinas-Rodriguez and Balmori-Ramirez2012b), whereas the formation of barium aluminium and barium silicates increases the porosity and decreases the amorphous phase content. This behaviour affects the mechanical properties (microhardness) of the samples directly. Sintering temperature (1100 and 1200°C) affects the microhardness of the samples, but appears to be insufficient for complete consolidation of the materials when the amount of BaCO3 exceeds 50 wt.%.
CONCLUSIONS
Addition of <50 wt.% of BaCO3 in ceramic-grade kaolin leads to the formation of new crystalline phases such as hexagonal celsian (BaAl2SiO6 and BaAl2Si2O8). The porosity of the composites fired at 1100 and 1200°C was <12 wt.%. Addition of >50 wt.% of BaCO3 to the raw material inhibits the consolidation and densification of the samples. Consequently, new phase transformations (BaAl2O4, Ba2SiO4 and BaSiO3) were developed and affected the completion of the firing process that cannot then be considered; therefore, porosity does not decrease. The microhardness decreased progressively with the addition of BaCO3 for samples sintered at 1100°C due to the increase in porosity; however, the amorphous phase increased the microhardness with increasing phase content and firing temperature.
ACKNOWLEDGEMENTS
The assistance of Ekaterina Novitskaya and James Cahill from the Department of Mechanical and Aerospace Engineering, University of California, San Diego (USA) is gratefully acknowledged.