1. Introduction
It has been well recognized that auditory phonological abilities, one of the essential skills for successful reading in one's native language (L1) (Wagner & Torgesen, Reference Wagner and Torgesen1987; Goswami, Reference Goswami1990; McBride-Chang, Bialystok, Chong & Li, Reference McBride-Chang, Bialystok, Chong and Li2004; Tingley, Dore, Lopez, Parsons, Campbell, Bird & Cleave, Reference Tingley, Dore, Lopez, Parsons, Campbell, Bird and Cleave2004), are also critical for reading development in a second language (L2) (Yamada, Reference Yamada2004). In addition, phonological skills may transfer from L1 to L2 (Holm & Dodd, Reference Holm and Dodd1996; Cardenas-Hagan, Carlson & Pollard-Durodola, Reference Cardenas-Hagan, Carlson and Pollard-Durodola2007; De Sousa, Greenop & Fry, Reference De Sousa, Greenop and Fry2010). For children who speak their L1 at home but are immersed and educated in English, phonological awareness in kindergarten is the single best predictor of their English reading performance in Grade 2 (Lesaux & Siegel, Reference Lesaux and Siegel2003). Such cross-language transfer of phonological skills occurs even when two languages are distinct from each other, such as Chinese and English. For instance, significant correlations between phonological measures in Chinese (L1) and English (L2) have been observed, with phonological measures in both L1 and L2 being significantly predictive of English reading skills (Gottardo, Yan, Siegel & Wade-Woolley, Reference Gottardo, Yan, Siegel and Wade-Woolley2001).
Neuroimaging studies have shown that the left superior temporal gyrus (LSTG) and the left inferior frontal gyrus (LIFG) play a critical role in phonological processing (Dehaene, Dupoux, Mehler, Cohen, Paulesu, Perani, van de Moortele, Lehericy & Le Bihan, Reference Dehaene, Dupoux, Mehler, Cohen, Paulesu, Perani, van de Moortele, Lehericy and Le Bihan1997; Chee, Caplan, Soon, Sriram, Tan, Thiel & Weekes, Reference Chee, Caplan, Soon, Sriram, Tan, Thiel and Weekes1999a; Pu, Liu, Spinks, Mahankali, Xiong, Feng, Tan, Fox & Gao, Reference Pu, Liu, Spinks, Mahankali, Xiong, Feng, Tan, Fox and Gao2001; Sakai, Miura, Narafu & Muraishi, Reference Sakai, Miura, Narafu and Muraishi2004). The LSTG is mainly associated with phonological representations of auditory languages (Scott, Blank, Rosen & Wise, Reference Scott, Blank, Rosen and Wise2000; Binder, Medler, Westbury, Liebenthal & Buchanan, Reference Binder, Medler, Westbury, Liebenthal and Buchanan2006; Kita, Yamamoto, Oba, Terasawa, Moriguchi, Uchiyama, Seki, Koeda & Inagaki, Reference Kita, Yamamoto, Oba, Terasawa, Moriguchi, Uchiyama, Seki, Koeda and Inagaki2013), and activated in both alphabetic and logographic languages during rhyming (Lin, Xiao, Shen, Zhang & Weng, Reference Lin, Xiao, Shen, Zhang and Weng2007; Cone, Burman, Bitan, Bolger & Booth, Reference Cone, Burman, Bitan, Bolger and Booth2008), spelling (Booth, Burman, Meyer, Lei, Choy, Gitelman, Parrish & Mesulam, Reference Booth, Burman, Meyer, Lei, Choy, Gitelman, Parrish and Mesulam2003; Lin et al., Reference Lin, Xiao, Shen, Zhang and Weng2007) and semantic processing (Booth et al., Reference Booth, Burman, Meyer, Lei, Choy, Gitelman, Parrish and Mesulam2003; Lin et al., Reference Lin, Xiao, Shen, Zhang and Weng2007) of aurally presented words. The LIFG is involved in phonological segmentation (Demonet, Chollet, Ramsay, Cardebat, Nespoulous, Wise, Rascol & Frackowiak, Reference Demonet, Chollet, Ramsay, Cardebat, Nespoulous, Wise, Rascol and Frackowiak1992; Hagoort, Indefrey, Brown, Herzog, Steinmetz & Seitz, Reference Hagoort, Indefrey, Brown, Herzog, Steinmetz and Seitz1999), motor planning for articulation (Rizzolatti & Craighero, Reference Rizzolatti and Craighero2004) and/or top-down modulation of posterior regions associated with phonological manipulation (Bitan, Booth, Choy, Burman, Gitelman & Mesulam, Reference Bitan, Booth, Choy, Burman, Gitelman and Mesulam2005). Besides these two regions, the left fusiform gyrus has also been shown to be activated during auditory rhyme decision tasks (Demonet, Price, Wise & Frackowiak, Reference Demonet, Price, Wise and Frackowiak1994; Booth, Burman, Meyer, Gitelman, Parrish & Mesulam, Reference Booth, Burman, Meyer, Gitelman, Parrish and Mesulam2002; Cone et al., Reference Cone, Burman, Bitan, Bolger and Booth2008). Notably, this region is believed to be responsible for processing the orthographic structure of well-learned visual word forms (Puce, Allison, Asgari, Gore & McCarthy, Reference Puce, Allison, Asgari, Gore and McCarthy1996; Cohen, Dehaene, Naccache, Lehericy, Dehaene-Lambertz, Henaff & Michel, Reference Cohen, Dehaene, Naccache, Lehericy, Dehaene-Lambertz, Henaff and Michel2000; Kronbichler, Hutzler, Wimmer, Mair, Staffen & Ladurner, Reference Kronbichler, Hutzler, Wimmer, Mair, Staffen and Ladurner2004; Kuo, Yeh, Lee, Chen, Lee, Chen, Ho, Hung, Tzeng & Hsieh, Reference Kuo, Yeh, Lee, Chen, Lee, Chen, Ho, Hung, Tzeng and Hsieh2004; Binder et al., Reference Binder, Medler, Westbury, Liebenthal and Buchanan2006). Because access to orthographic representations is not necessary for correct performance during an auditory rhyme decision task, activation of the left fusiform gyrus may suggest automatic access to orthographic representations during auditory rhyming in readers (Desroches, Cone, Bolger, Bitan, Burman & Booth, Reference Desroches, Cone, Bolger, Bitan, Burman and Booth2010).
Most neuroimaging studies on bilingualism have focused on reading processing, and revealed that both languages in proficient bilinguals share a common neural device (Perani & Abutalebi, Reference Perani and Abutalebi2005; Abutalebi & Green, Reference Abutalebi and Green2007). The same conclusion was obtained even for bilinguals who use very different languages, such as Chinese and English (Chee et al., Reference Chee, Caplan, Soon, Sriram, Tan, Thiel and Weekes1999a; Chee, Tan & Thiel, Reference Chee, Tan and Thiel1999b; Illes, Francis, Desmond, Gabrieli, Glover, Poldrack, Lee & Wagner, Reference Illes, Francis, Desmond, Gabrieli, Glover, Poldrack, Lee and Wagner1999; Tan, Spinks, Feng, Siok, Perfetti, Xiong, Fox & Gao, Reference Tan, Spinks, Feng, Siok, Perfetti, Xiong, Fox and Gao2003; Xue, Dong, Jin & Chen, Reference Xue, Dong, Jin and Chen2004). In addition, how bilinguals control or “switch” their languages has been extensively studied (Price, Green & von Studnitz, Reference Price, Green and von Studnitz1999; Hernandez, Reference Hernandez2000; Hernandez, Reference Hernandez2001; Crinion, Reference Crinion2006; Abutalebi, Annoni, Zimine, Pegna, Seghier, Lee-Jahnke, Lazeyras, Cappa & Khateb, Reference Abutalebi, Annoni, Zimine, Pegna, Seghier, Lee-Jahnke, Lazeyras, Cappa and Khateb2008; van Heuven, Schriefers, Dijkstra & Hagoort, Reference van Heuven, Schriefers, Dijkstra and Hagoort2008; Hernandez, Reference Hernandez2009; Wang, Kuhl, Chen & Dong, Reference Wang, Kuhl, Chen and Dong2009; Abutalebi, Della Rosa, Green, Hernandez, Scifo, Keim, Cappa & Costa, Reference Abutalebi, Della Rosa, Green, Hernandez, Scifo, Keim, Cappa and Costa2012) and a brain network for language control was proposed (Abutalebi & Green, Reference Abutalebi and Green2007). Furthermore, several studies have reported that learning a second language reshapes the structure and function of the brain (Mechelli, Crinion, Noppeney, O’Doherty, Ashburner, Frackowiak & Price, Reference Mechelli, Crinion, Noppeney, O’Doherty, Ashburner, Frackowiak and Price2004; Parker, Green, Grogan, Pliatsikas, Filippopolitis, Ali, Lee, Ramsden, Gazarian, Prejawa, Seghier & Price, Reference Parker, Green, Grogan, Pliatsikas, Filippopolitis, Ali, Lee, Ramsden, Gazarian, Prejawa, Seghier and Price2012; Zou, Abutalebi, Zinszer, Yan, Shu, Peng & Ding, Reference Zou, Abutalebi, Zinszer, Yan, Shu, Peng and Ding2012; Zou, Ding, Abutalebi, Shu & Peng, Reference Zou, Ding, Abutalebi, Shu and Peng2012).
Recently, researchers have begun to evaluate cognitive and neural deficits in ESL learners with reading difficulties. It has been reported that ESL children with reading difficulties in English also exhibit auditory phonological deficits (Geva, Yaghoub-Zadeh & Schuster, Reference Geva, Yaghoub-Zadeh and Schuster2000). A recent neuroimaging study investigated, for the first time, the neural deficits in ESL children with English reading impairment using reading tasks and observed that the deficits are similar to those in native English-speaking children with reading impairment (You, Gaab, Wei, Cheng-Lai, Wang, Jian, Song, Meng & Ding, Reference You, Gaab, Wei, Cheng-Lai, Wang, Jian, Song, Meng and Ding2011). Given the fact that auditory phonological skills are essential for reading development (Olofsson & Niedersoe, Reference Olofsson and Niedersoe1999; Schatschneider, Carlson, Francis, Foorman & Fletcher, Reference Schatschneider, Carlson, Francis, Foorman and Fletcher2002; Plaza & Cohen, Reference Plaza and Cohen2007), the exploration of the neural correlates underlying auditory processing in ESL children could help us to better understand the neural mechanism of L2 reading impairment.
So far, very little is known about the neural deficits involved in auditory phonological processing in ESL learners with reading difficulties. The present study aimed to address this issue using functional magnetic resonance imaging (fMRI). Brain activation during an auditory rhyme decision task was compared between Chinese children learning ESL with and without impaired English reading. In the group comparison, we particularly focused on the brain activation in three key brain regions for language and reading: the LSTG, the LIFG and the left fusiform gyrus.
Previous studies also found that children with English reading difficulty have weaker or absent connectivity between language regions compared with typically developing children (Horwitz, Rumsey & Donohue, Reference Horwitz, Rumsey and Donohue1998; Pugh, Mencl, Shaywitz, Shaywitz, Fulbright, Constable, Skudlarski, Marchione, Jenner, Fletcher, Liberman, Shankweiler, Katz, Lacadie & Gore, Reference Pugh, Mencl, Shaywitz, Shaywitz, Fulbright, Constable, Skudlarski, Marchione, Jenner, Fletcher, Liberman, Shankweiler, Katz, Lacadie and Gore2000; Shaywitz, Shaywitz, Pugh, Mencl, Fulbright, Skudlarski, Constable, Marchione, Fletcher, Lyon & Gore, Reference Shaywitz, Shaywitz, Pugh, Mencl, Fulbright, Skudlarski, Constable, Marchione, Fletcher, Lyon and Gore2002a). Particularly, it has been revealed through dynamic causal modelling that the mediating effect from the fusiform gyrus to superior parietal regions and inferior regions is significantly less than those in controls (Cao, Bitan & Booth, Reference Cao, Bitan and Booth2008). Then in addition to the brain activation, we further explored the group differences in functional brain connectivity among the three key brain regions.
2. Methods
2.1 Participants
Twenty-five children were selected from a pool of 857 children in grades four, five, and six (mean age: 10.85 years of age; range: 9.3–12.1 years old): 12 children with impaired English and Chinese reading and 13 typically developing readers. All participants were Mandarin-speaking, living in Mainland China, and learning English as a second language through their formal schooling, beginning at about age six. Their ESL training included three hours of formal instruction and approximately four hours of homework assignments per week. In-school English instruction in China is mainly composed of oral language and daily communication from grade one through grade two. Formal instruction of the English alphabet and its pronunciation, word reading, and spelling starts around grade three (when children are approximately eight years old). Their English teachers are native Chinese speakers who graduated with an English major from a university. Audio recordings (cassette tapes conveying the content of the textbook), read by native English speakers, are also used in class and for homework. These children learned all other academic subjects in Mandarin Chinese, the official language of Mainland China and the language of instruction in school.
None of the participants had a history of neurological diseases, head injury or psychiatric disorders. The DSM-IV Attention-Deficit/Hyperactivity Disorder (ADHD) Scale (American Psychiatric Association, 1994) was also used to exclude children with ADHD. All the participants were right-handed according to self-report (Oldfield, Reference Oldfield1971), and had normal or corrected-to-normal vision. Informed consent was obtained from each subject and his/her parent before participation. This study was approved by the ethical review board at the State Key Laboratory of Cognitive Neuroscience and Learning at Beijing Normal University.
The WRAT3 word Spelling subtest (Wide Range Achievement Test-Revision 3 (Wilkinson, Reference Wilkinson1993), an in-lab-developed spelling test, and a word reading test were used to screen impaired English readers (You et al., Reference You, Gaab, Wei, Cheng-Lai, Wang, Jian, Song, Meng and Ding2011). The Raven's Standard Progressive Matrices (Zhang & Wang, Reference Zhang and Wang1985) were also used to match nonverbal IQ of groups of children with and without English reading impairment.
Three criteria needed to be met for the inclusion of English-reading-impaired children (IR), as follows: first, the percentile in the Raven's test needed to be above the 50th percentile to ensure average IQ; second, the standard score for the in-lab-developed Spelling test needed to be below a standard score of 90; third, the raw scores for the Word Reading test needed to be below the grade average. The age- and grade-matched typically developing readers were selected among the reading-impaired children's peers. Children defined as typical English readers, in addition to having an average IQ as measured by Raven's test, needed to have a standard score above the 70th percentile on the lab-developed Spelling test and Word Reading performance above the grade average.
An additional battery of assessments was administered to measure reading, decoding and phonological abilities: the Word Identification and Word Attack subtasks of the Woodcock-Johnson Reading Mastery (Woodcock, Reference Woodcock1998), and the English Phonological Awareness test, which was specifically designed to assess the English phonological awareness in Chinese-speaking children (You et al., Reference You, Gaab, Wei, Cheng-Lai, Wang, Jian, Song, Meng and Ding2011). Four subtests were administered: Rhyme Detection, Oral Cloze, Syllable Identification and Initial Phoneme Deletion.
Chinese reading ability was tested through a reading fluency test and a Chinese written vocabulary test (You et al., Reference You, Gaab, Wei, Cheng-Lai, Wang, Jian, Song, Meng and Ding2011). The reading fluency test measured reading comprehension using a series of 95 sentences. Each sentence was paired with 5 multiple-choice pictures. Participants were asked to read each sentence and select the picture that best illustrated the meaning of the sentence. Rapid retrieval and retention of lexical information and construction of sentential representation were necessary to complete the task. Children were instructed to complete as many paragraphs as possible within a 10-minute time period. The performance score was determined by the total number of sentences that participants correctly comprehended.
The standardized written vocabulary test (Wang & Tao, Reference Wang and Tao1993) involved 210 characters divided into 10 groups based on their difficulty level in reading. Participants were asked to write down a compound word based on a constituent morpheme provided on the sheet. Participants had to know morpheme combination rules to form a compound word. Performance was measured by the total number of correct characters (morphemes) the participants could make use of in word compositions.
The impaired English readers showed reduced performance compared to the typical readers on both the Chinese reading fluency test and the Chinese written vocabulary test (see Table 1). These results indicated that children in the impaired English reading group also showed poorer reading in Chinese compared to the typically developed readers. However, they are not clinically impaired readers in Chinese because their standard mean reading scores in Chinese were above the 30th percentile. Thus, they were diagnosed as children with impairments in English reading rather than impairments in both English and Chinese.
Table 1. Participants’ behavioral characteristics and mean scores for reading measures, with minimum and maximum (in parentheses).

Note: a percentiles; b standard scores; c raw scores;
Items for each test listed in raw scores: WRAT3 word Spelling = 40, Word Identification = 58, Word Attack = 30, Word Reading = 45, Rhyme Detection = 10, Oral Cloze = 10, Syllable Identification = 8, Initial Phoneme Deletion = 8
Table 1 shows the average percentile for the Raven's test and the average standard score for the in-lab-developed spelling test in the two groups, with the minimum and maximum score for each test in parentheses. Means and ranges for raw scores of WRAT3 word Spelling, Woodcock Word Identification and Word Attack, and English Phonological Awareness tests are also shown.
2.2 Neuroimaging experiment
Stimulus
An auditory rhyme judgment task and a tone discrimination control task were used in the functional magnetic resonance imaging (fMRI) scanner. For the rhyme task, 48 highly familiar and high-frequency English words were selected from primary school English textbooks for Chinese-speaking children. Word frequency was assessed by averaging the familiarity score on a five-point scale assessed in 158 children in grades one, three and five. These words were combined into 12 rhyming pairs (e.g., Ten and Pen) and 12 non-rhyming pairs (e.g., Big and Sun). Each pair was presented twice and the word order within each pair was balanced. All words were read by an adult female who was a native speaker of English, and were recorded at a 44.1-kHz sampling rate with 16-bit resolution.
For the tone discrimination control task (baseline), two pure tones with frequencies of 700hz and 300hz, respectively, were created as non-linguistic control stimuli.
Task design
The fMRI run lasted seven minutes and 52 seconds, and contained eight blocks of the rhyme judgment task, which alternated with eight blocks of the tone discrimination task. During the rhyme judgment task, participants were instructed to judge whether each word pair rhymed. For the tone discrimination task, children were asked to judge whether the presented tones were the same or different according to the pitch. Half of the participants pressed a button with their left hand for pairs that were the same and with their right hand for pairs that were different. The other half of participants responded in the opposite manner. Measures of task accuracy and reaction time (RT) were obtained.
We presented both the rhyme judgment and tone discrimination blocks at varying durations in order to reduce the potential confusion from confounding factors, such as participants’ expectations, or periodic noise coming from human rhythms, such as breath or heartbeat, or the scanner itself. The average time for each block was 24 seconds and each block included between five and seven trials. For each trial, two words/tones were presented successively for 800 ms each with an interval of 200 ms. The participants were asked to respond within 3000 ms after the onset of the second stimulus. A fixation cross was presented visually in the middle of the screen and the children were asked to focus on it for the entire trial duration.
Prior to each block, the instructions (e.g., “Tone discrimination, same right, different left”) appeared in the middle of the screen for 4 seconds to cue the participants for the upcoming task and to remind them of the button locations.
2.3 Image acquisition
The MRI imaging and imaging-related procedures were performed at the Brain Imaging Center of State Key Laboratory of Cognitive Neuroscience and Learning, Beijing Normal University. A 3.0 T Siemens Trio scanner was used. A T2-weighted gradient-echo planar imaging (EPI) sequence sensitive to blood-oxygen-level-dependent contrast was used for fMRI scans with the following acquisition parameters: repetition time: 3000 ms; echo-time: 30 ms; flip-angle: 90°; field-of-view: 20 × 20 cm; matrix size: 64 × 64, 30 slices (4 mm). T1-weighted images were acquired using the MPRAGE (Magnetization Prepared Rapid Gradient Echo) sequence, with the following acquisition parameters: flip angel: 7°; echo-time: 3.39 ms; repetition time: 2530 ms; field-of-view: 256 mm; voxel size: 1.3 × 1.0× 1.3 mm; 128 slices (1.33 mm).
2.4 fMRI data analysis
Pre-processing
Data analysis was performed with Statistical Parametric Mapping software (SPM5; http://www.fil.ion.ucl.ac.uk/spm). After discarding the first four volumes of each subject in order to obtain T1 equilibration, the functional images were realigned to the first volume in the scanning session using affine transformations. The images were then co-registered to their corresponding anatomical volumes, normalized to Montreal Neurological Institute (MNI) stereotaxic space using parameters obtained from anatomical segmentation, and resampled to a voxel size of 2 × 2 × 2mm. Spatial smoothing was performed with a Gaussian filter (8mm full width at half maximum).
Statistical Parametric Mapping
The general linear model was used to estimate condition effects for individual participants. Three conditions, “instruction,” “rhyme,” and “tone,” were modeled using a canonical hemodynamic response function (HRF). For each subject, one contrast of interest was computed: rhyme vs. tone. Parameter estimates from contrasts in individual subject models was entered into random-effects analysis. One-sample t-tests were used for each group to test if a contrast was significant. The statistical threshold was set at p < 0.05 (FDR-corrected) with an extent threshold of 20 voxels. Two-sample t-tests were performed to assess the group difference in brain activation (rhyme vs. tone) between the two group participants. The statistical threshold was also set at p < 0.05 (FDR-corrected) with an extent threshold of 20 voxels.
Independent regions of interest (ROIs) analysis
Besides the whole brain analysis, we also conducted independent regions of interest (ROIs) analyses with Marsbar (http://marsbar.sourceforge.net) to assess the group differences between children with and without reading impairment for the contrast rhyme vs. tone. We chose three brain regions as ROIs based on previous studies, including the left superior temporal gyrus (LSTG), left inferior frontal gyrus (LIFG), and left fusiform gyrus. The first two regions have previously been reported to be activated during phonological processing (Zatorre, Meyer, Gjedde & Evans, Reference Zatorre, Meyer, Gjedde and Evans1996; Burton, Reference Burton2001) and the last one during orthographic processing (Cohen, Lehericy, Chochon, Lemer, Rivaud & Dehaene, Reference Cohen, Lehericy, Chochon, Lemer, Rivaud and Dehaene2002; Kronbichler et al., Reference Kronbichler, Hutzler, Wimmer, Mair, Staffen and Ladurner2004; Glezer, Jiang & Riesenhuber, Reference Glezer, Jiang and Riesenhuber2009) and specific auditory processing tasks (Demonet et al., Reference Demonet, Price, Wise and Frackowiak1994; Booth et al., Reference Booth, Burman, Meyer, Gitelman, Parrish and Mesulam2002; Cone et al., Reference Cone, Burman, Bitan, Bolger and Booth2008). Three spherical volumes in the left hemisphere were created with a radius of 6mm. The LSTG centered at the coordinates [MNI: -59, -6, -1], which was derived from an average of the coordinates in Friederici et al. (Friederici, Kotz, Scott & Obleser, Reference Friederici, Kotz, Scott and Obleser2010) ([-58,-4, 4]) and Obleser and Kotz (Obleser & Kotz, Reference Obleser and Kotz2010) ([-60,-8, -6]). The LIFG centered at the coordinates [MNI: -51, 28, 19], which was defined from Bokde et al. (Bokde, Tagamets, Friedman & Horwitz, Reference Bokde, Tagamets, Friedman and Horwitz2001). The left fusiform gyrus centered at the coordinates [MNI: -44, -58, -15], defined from Jobard et al. (Jobard, Crivello & Tzourio-Mazoyer, Reference Jobard, Crivello and Tzourio-Mazoyer2003). The mean activation (α estimates) within each region for each participant was extracted. An independent two-sample t-test was then used to compare activation differences between the two groups for each ROI at the threshold of p < 0.0167 (Bonferroni corrected for β = 0.05).
Functional connectivity analysis
We further performed a functional connectivity analysis in order to investigate whether the brain connectivity among the ROIs of LSTG, LIFG and left fusiform gyrus differed between children with and without reading impairment. The ROIs were the same as those defined in the ROIs analysis (see above). We first extracted the time series for each ROI, averaged across all voxels within the region, and then applied simple Pearson correlation analyses to calculate the functional connectivity between each pair of ROIs during the rhyme judgment for each group. A two-sample t-test was conducted between Fisher's Z score transformations of the correlation coefficients from each group with a threshold of p < 0.05, two-tailed.
2.5 Correlations between brain activation/functional connectivity in ROIs and reading measures
To further investigate the relations between brain activations and reading measures, a correlation analysis was conducted between scores of the English language behavioral tests and the mean activation within the three regions of interest for all 25 participants. The English language tests are listed in Table 3. We further explored whether the functional connectivity among the three ROIs also correlated with the behavioral language measures. Statistical tests to compare the correlation coefficients were performed with SPSS 17.0, and a threshold of p < 0.05 (FDR-corrected) was employed.
3. Results
3.1 Behavioral data
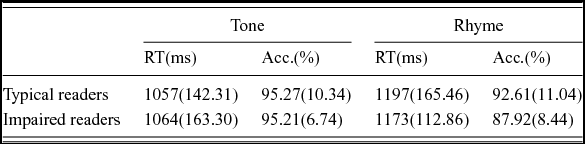
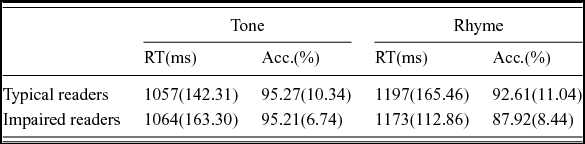
Mean accuracy and reaction time data for the rhyme and control tasks for each group are shown in Table 2. Mixed-model ANOVAs were conducted for both accuracy and reaction time separately with Task (rhyme judgment/tone discrimination) as the within-subjects factor and Group (typical/impaired English readers) as the between-subjects factor. For accuracy, a main effect of Task was observed, indicating better performance on the tone discrimination vs. the rhyme judgment task [F(1,23) = 8.76, p <.01]. Neither the main effect of Group nor the interaction of the Group and Task was significant. For reaction time, similar to what we observed with accuracy, a main effect of Task was observed, such that faster reaction times were observed in the tone task than in the rhyme task [F(1,23) = 40.30, p < .001]. Again, neither the main effect of Group nor the interaction of the Group and Task was significant. The data showed that both groups had similar in-scanner performance, suggesting that the task difficulty was equivalent for both groups of participants.
Table 2. In-scanner behavioral results.
3.2 fMRI
Whole-brain activation patterns in typical and impaired English readers
Whole-brain analyses were conducted for the contrast rhyme judgment greater than the control task (tone discrimination) (FDR-corrected p < 0.05, ET = 20) for each group separately. For the typically developing group, regions that showed greater activation in rhyming compared to tone discrimination were the bilateral temporal lobes, bilateral inferior frontal gyrus, bilateral precentral gyrus, left occipital lobes, left supplementary motor area, right insula, and several subcortical areas (Table 3, Figure 1). For the impaired group, neural activation was greater for rhyme judgment vs. tone discrimination in bilateral temporal, left inferior frontal, right superior occipital, right fusiform, left lingual, right precentral, bilateral insula, left supplementary motor areas and in some subcortical regions (Table 3, Figure 1).
Table 3. Coordinates of activation peaks for each group, auditory rhyme vs. tone discrimination.

L, left; R, right; N/A, not applicable

Figure 1. Whole-brain activation for the contrast auditory rhyme > tone discrimination is rendered on a 3D brain. For the condition effect in each group, the threshold is FDR corrected p < 0.05 combined with a cluster size > 20. For the group difference (Typical > Impaired), the threshold is uncorrected p < 0.005 combined with a cluster size > 20.
Group differences in activation: whole-brain analysis
The question whether there were group differences in the neural activation pattern was investigated using two-sample t-tests. Using a strict threshold of FDR-corrected p < 0.05, we did not find significant group difference. However given the lower SNR in children (Thomason, Burrows, Gabrieli & Glover, Reference Thomason, Burrows, Gabrieli and Glover2005), we lowered the threshold at uncorrected p < 0.005 at the voxel level (k > 20) in order to still verify the presence of more subtle potential differences between groups at the whole-brain level. At this more liberal threshold the typically developing group did exhibit significantly greater activation than impaired English readers in the left inferior occipital/fusiform, left precentral, bilateral superior parietal, inferior temporal regions and the bilateral cerebellum (Table 4, Figure 1). No regions exhibited significantly greater activation in the impaired group than the typical group with any threshold.
Table 4. Group difference, auditory rhyme vs. tone discrimination.

L, left; R, right.
Group differences in activation: ROI analysis
Figure 2 shows the result of the independent ROI analysis. The mean activation (β estimates) within each ROI was calculated by averaging the activation of all voxels within each ROI. Significant group differences were observed in two ROIs, including the LIFG (βED = 0.24, βNM = 0.59, t(23) = 2.89, p < 0.05), and the left fusiform gyrus (βED = 0.04, βNM = 0.33, t(23) = 2.76, p < 0.05), where the typical readers showed stronger activation than the impaired readers. There was no significant group difference in the ROI of LSTG.

Figure 2. Brain activation in the three brain regions of interest (ROIs). The left-hand brain images show the positions of the ROIs. Bar graphs represent the mean activation (contrast β values) of each ROI. The error bars represent SEM. *: p < 0.01. The coordinates of the LSTG (the left superior temporal gyrus) were determined according to Friederici et al. (Reference Friederici, Kotz, Scott and Obleser2010) and Obleser and Kotz (Obleser & Kotz, Reference Obleser and Kotz2010). The LIFG (the left inferior frontal gyrus) was defined from Bokde et al. (Bokde et al., Reference Bokde, Tagamets, Friedman and Horwitz2001). The left fusiform gyrus was defined from Jobard et al. (Jobard et al., Reference Jobard, Crivello and Tzourio-Mazoyer2003).
Functional connectivity
Figure 3 illustrates the correlation coefficients (r) for each pair of ROIs in each group for the functional connectivity analyses. The number above the line in each pair of correlation coefficients is that of impaired children while the number below the line is that of typical children. One-sample t-tests revealed that both typical and impaired children have significant functional connectivity among all pairs of ROIs, suggesting that these regions are not functionally isolated but rather interact with each other during the auditory rhyming task. For the group comparison, only the difference of the connectivity between LSTG and the left fusiform gyrus reaches statistical significance (p < 0.05, two-tailed). The typical readers showed higher connectivity than the impaired readers. The group difference of the connectivity between LSTG and LIFG is marginally significant (p = 0.094), in which the typical readers showed higher value than the impaired readers. There is no group difference for the connectivity between LIFG and the left fusiform gyrus.

Figure 3. Functional connectivity among the three ROIs in the impaired English readers (the top one in each pair) and typical English readers (the bottom one in each pair).
3.3 Correlations between brain activation/functional connectivity in ROIs and reading measures
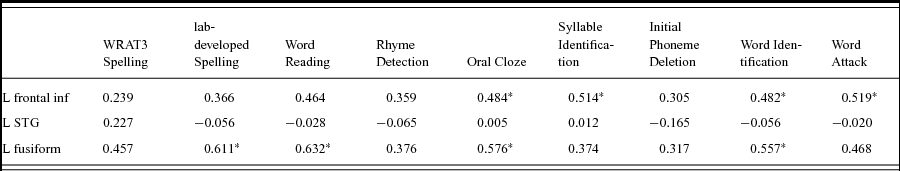
The correlations between the mean activation in each of the three ROIs and the behavioral tests were calculated for all participants. Mean activation within the LIFG had a significant positive correlation with multiple reading tests, including Word Identification, Word Attack (non-word decoding), Oral Cloze and Syllable Identification (Table 5). Most of these tests are related to phonological processing. Mean activation within the left fusiform ROI were significantly positively correlated with spelling performance, word reading, oral cloze, and word identification. Interestingly, the mean activation within the LSTG ROI showed no correlation with any of the tests (Table 5).
Table 5. Correlations between ROI activations and reading measurements.
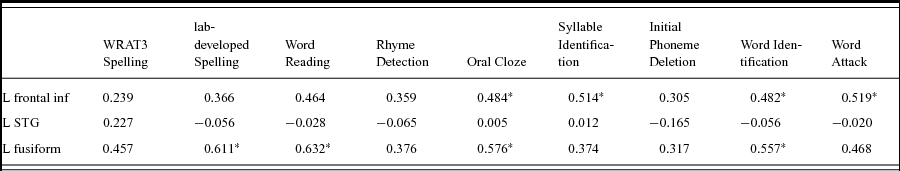
*<0.05; FDR-corrected.
We further explored whether the functional connectivity among the three ROIs also correlates with behavioral language measures. The results revealed that the connectivity of LSTG-LIFG and LSTG-fusiform, but not LIFG-fusiform, significantly correlated with the rhyme judgment test scores across all participants (LSTG-LIFG: r = 0.515; p = 0.008; LSTG-fusiform: r = 0.494; p = 0.012; LIFG-fusiform: r = 0.194; p = 0.352).
4. Discussion
In this study, we compared the behavioral performance and neural activation during spoken word rhyme judgments compared to tone judgment between typical and impaired ESL readers in China. Behavioral results showed no group differences (as well as no interaction between group and task) for either accuracy or reaction time. However, we observed a task difference between rhyme judgment and tone judgment across groups. Neuroimaging results showed that when contrasting rhyme judgment with a control tone discrimination task, activations in the left inferior temporal and superior temporal areas were observed in both groups. Whole-brain analyses showed that typically developing children compared to the reading impaired children exhibited greater activation in several brain regions, including the left inferior occipital/fusiform, left precentral, bilateral superior parietal, inferior temporal areas, and the right cerebellum. However, these effects could only be observed when using an uncorrected threshold of p < 0.005, extent = 20 voxels. Applying a stricter threshold (FDR-corrected p < 0.05, extent = 20 voxels) did not reveal significant group differences.
Because the sample size in the study is rather small for testing group differences in the whole-brain analysis, we further performed an independent region of interest (ROI) analysis. The results showed that, compared to the typically reading ESL group, the impaired ESL readers showed reduced activation in the LIFG and left fusiform gyrus, but not in the LSTG. Additionally, activation within the LIFG and fusiform gyrus, but not the LSTG, significantly correlated with multiple reading measures across both groups. We also found that functional connectivity between the LSTG and the left fusiform gyrus was significantly higher in typical readers than in impaired readers. Furthermore, connectivity between the LSTG and both the LIFG and the left fusiform revealed a relationship with performance in the in-scanner rhyme judgment task across groups. These findings suggest an association between brain activation/functional connectivity patterns during auditory phonological processing and behavioral reading skills in ESL children.
Both group showed greater activation within LSTG during the rhyming judgment compared to the tone judgment task and no significant group differences within LSTG were observed. The LSTG has been suggested to be the primary region responsible for phonological representations of spoken words (Petersen, Fox, Posner, Mintun & Raichle, Reference Petersen, Fox, Posner, Mintun and Raichle1988; Zatorre, Evans, Meyer & Gjedde, Reference Zatorre, Evans, Meyer and Gjedde1992; Buchsbaum, Hickok & Colin., Reference Buchsbaum, Hickok and Colin2001; Kita et al., Reference Kita, Yamamoto, Oba, Terasawa, Moriguchi, Uchiyama, Seki, Koeda and Inagaki2013). However, some studies have also revealed that this region is engaged in tone processing (Binder, Frost, Hammeke, Rao & Cox, Reference Binder, Frost, Hammeke, Rao and Cox1996; Zatorre et al., Reference Zatorre, Meyer, Gjedde and Evans1996; Celsis, Boulanouar, Doyon, Ranjeva, Berry, Nespoulous & Chollet, Reference Celsis, Boulanouar, Doyon, Ranjeva, Berry, Nespoulous and Chollet1999). Since pure tones differ from auditory words not only in terms of phonological processing, but also in terms of low-level acoustic features, the greater activation in the LSTG in the contrast of rhyming words vs. pure tones might be a mixture effect of both phonological and low-level acoustic features. However, our key finding is a lack of group differences in this region, suggesting that the phonological representation (also low-level acoustic processing) may be intact in the ESL children with reading difficulty. This finding is consistent with previous studies showing that individuals with dyslexia have intact phonological representation within LSTG but suffer from deficits in the access to higher-level phonological processing or phonological manipulation within LIFG (Steinbrink, Groth, Lachmann & Riecker, Reference Steinbrink, Groth, Lachmann and Riecker2012; Boets, Op de Beeck, Vandermosten, Scott, Gillebert, Mantini, Bulthe, Sunaert, Wouters & Ghesquiere, Reference Boets, Op de Beeck, Vandermosten, Scott, Gillebert, Mantini, Bulthe, Sunaert, Wouters and Ghesquiere2013). However, this interpretation should be considered carefully since these findings may be heavily influenced by the nature of the experiment task employed in each study. Previous studies have observed reduced activation in the LSTG in children with dyslexia compared to their typical developing peers for various reading and reading-related tasks including rhyming and matching judgment to letter pairs (Temple, Poldrack, Salidis, Deutsch, Tallal, Merzenich & Gabrieli, Reference Temple, Poldrack, Salidis, Deutsch, Tallal, Merzenich and Gabrieli2001), phonological manipulation tasks (Kita et al., Reference Kita, Yamamoto, Oba, Terasawa, Moriguchi, Uchiyama, Seki, Koeda and Inagaki2013) and tasks that require phonological analysis (Shaywitz, Shaywitz, Pugh, Fulbright, Constable, Mencl, Shankweiler, Liberman, Skudlarski, Fletcher, Katz, Marchione, Lacadie, Gatenby & Gore, Reference Shaywitz, Shaywitz, Pugh, Fulbright, Constable, Mencl, Shankweiler, Liberman, Skudlarski, Fletcher, Katz, Marchione, Lacadie, Gatenby and Gore1998). However, all these previous studies addressed phonological processing of visually-presented stimuli (words or letters) which differ from the present study focusing solely on auditory phonological processing. Some recent studies which employed auditory phonological processing are consistent with our findings (Steinbrink et al., Reference Steinbrink, Groth, Lachmann and Riecker2012; Boets et al., Reference Boets, Op de Beeck, Vandermosten, Scott, Gillebert, Mantini, Bulthe, Sunaert, Wouters and Ghesquiere2013), suggesting a possible modality effect for phonological processing deficits in dyslexia.
Kita et al. (Reference Kita, Yamamoto, Oba, Terasawa, Moriguchi, Uchiyama, Seki, Koeda and Inagaki2013) suggested to differentiate phonological representation and manipulation and suggested that the LSTG is the primary brain area for processing phonological representations based on auditory input and that the LIFG is critical for the active manipulation of language as required during rhyming judgments. We observed hypoactivation in the LIFG (BA45) in impaired English readers compared to controls, which could suggest a deficit in phonological manipulation rather than phonological representation (which has been primarily attributed to activation in LSTG) in these children (Steinbrink et al., Reference Steinbrink, Groth, Lachmann and Riecker2012; Kita et al., Reference Kita, Yamamoto, Oba, Terasawa, Moriguchi, Uchiyama, Seki, Koeda and Inagaki2013).
The finding of hypoactivation in the LIFG is in concert with previous studies in native English-speaking children with developmental dyslexia during auditory rhyme judgment (Corina & McBurney, Reference Corina and McBurney2001), visual rhyme judgment of conflicting spellings (Cao, Bitan, Chou, Burman & Booth, Reference Cao, Bitan, Chou, Burman and Booth2006), non-word reading (Georgiewa, Rzanny, Hopf, Knab, Glauche, Kaiser & Blanz, Reference Georgiewa, Rzanny, Hopf, Knab, Glauche, Kaiser and Blanz1999; Shaywitz, Shaywitz, Pugh, Mencl, Fulbright, Skudlarski, Constable, Marchione, Fletcher, Lyon & Gore, Reference Shaywitz, Shaywitz, Pugh, Mencl, Fulbright, Skudlarski, Constable, Marchione, Fletcher, Lyon and Gore2002b), letter-sound mapping (Aylward, Richards, Berninger, Nagy, Field, Grimme, Richards, Thomson & Cramer, Reference Aylward, Richards, Berninger, Nagy, Field, Grimme, Richards, Thomson and Cramer2003) and phonological manipulations (Georgiewa et al., Reference Georgiewa, Rzanny, Hopf, Knab, Glauche, Kaiser and Blanz1999). It has been argued that dorsal and ventral portions of the LIFG are differentially activated for phonological and semantic processing (Poldrack, Wagner, Prull, Desmond, Glover & Gabrieli, Reference Poldrack, Wagner, Prull, Desmond, Glover and Gabrieli1999; Bokde et al., Reference Bokde, Tagamets, Friedman and Horwitz2001; Devlin, Matthews & Rushworth, Reference Devlin, Matthews and Rushworth2003). According to Bokde et al. (Reference Bokde, Tagamets, Friedman and Horwitz2001), the dorsal LIFG (-50, 28, 16) was selectively activated for phonological processing, whereas the ventral LIFG (-48, 36, -14) was preferentially activated for semantic processing. The region within the LIFG shown for the contrast rhyme judgment vs. tone detection in our study (-51, 28, 17) was nearly identical to the phonological region within the LIFG reported in Bokde et al. (Reference Bokde, Tagamets, Friedman and Horwitz2001). This suggests that the involvement of this left inferior frontal region is associated with phonological processes during an auditory rhyme judgment task.
The hypoactivation within the LIFG in Chinese children with impaired English reading could indicate a disruption in phonological manipulation similar to that of English-speaking children with dyslexia during spoken language processing (Corina & McBurney, Reference Corina and McBurney2001), or German-speaking children with dyslexia (Steinbrink et al., Reference Steinbrink, Groth, Lachmann and Riecker2012). In fact, a number of remediation studies have reported increased IFG activation during phonological processing following remediation in both children (Temple, Reference Temple2003; Shaywitz, Shaywitz, Blachman, Pugh, Fulbright, Skudlarski, Mencl, Constable, Holahan, Marchione, Fletcher, Lyon & Gore, Reference Shaywitz, Shaywitz, Blachman, Pugh, Fulbright, Skudlarski, Mencl, Constable, Holahan, Marchione, Fletcher, Lyon and Gore2004) and adults (Eden, Jones, Cappell, Gareau, Wood, Zeffiro, Dietz, Agnew & Flowers, Reference Eden, Jones, Cappell, Gareau, Wood, Zeffiro, Dietz, Agnew and Flowers2004) with developmental dyslexia. For instance, increases in IFG were observed after a year-long remediation for children; and this training effect still existed even one year after the intervention (Shaywitz et al., Reference Shaywitz, Shaywitz, Blachman, Pugh, Fulbright, Skudlarski, Mencl, Constable, Holahan, Marchione, Fletcher, Lyon and Gore2004). These studies support the present finding that the hypoactivation in the LIFG is associated with less developed phonological manipulation skills in readers with dyslexia.
Our correlation results further support, to some degree, our hypothesis that the impaired readers show a deficit in phonological manipulation but not representation. We observed that activation of LIFG, but not LSTG, correlated with various reading tasks including Word Identification, Word Attack (non-word decoding), Oral Cloze and Syllable Identification (Table 5). This suggests that the LIFG may be closer related to the task performance required during these behavioral assessments. Notably, the absence of a correlation between LSTG and behavioral reading performance does not suggest that the LSTG is not involved in phonological representation. Instead, the LSTG may process the basic components of phonological processing which are not directly associated with individual difference. We also observed that functional connectivity between LIFG and LSTG significantly correlates with the performance on the rhyme judgment test, suggesting that the LIFG and LSTG may interact during phonological processing. Interestingly, Boets et al. have shown that functional and structural connectivity between LIFG and LSTG is significantly lower in adult readers with dyslexia compared to typical adults (Boets et al., Reference Boets, Op de Beeck, Vandermosten, Scott, Gillebert, Mantini, Bulthe, Sunaert, Wouters and Ghesquiere2013). Here we only observed a marginal group difference in functional connectivity between the two group of children but the reading profiles in the two studies differ fundamentally. Future studies are needed to further characterize the interplay between LSTG and LIFG in phonological processing.
We also observed reduced activations in the left inferior occipital/fusiform (BA37) regions that encompass the visual word fusiform area (VWFA) (Cohen et al., Reference Cohen, Lehericy, Chochon, Lemer, Rivaud and Dehaene2002; Kronbichler et al., Reference Kronbichler, Hutzler, Wimmer, Mair, Staffen and Ladurner2004; Glezer et al., Reference Glezer, Jiang and Riesenhuber2009) in impaired ESL learners relative to typical ESL learners. The left fusiform gyrus seems crucial for visual word recognition (Cohen, Jobert, Lebihan & Dehaene, Reference Cohen, Jobert, Lebihan and Dehaene2004; Dehaene, Jobert, Naccache, Ciuciu, Poline, Le Bihan & Cohen, Reference Dehaene, Jobert, Naccache, Ciuciu, Poline, Le Bihan and Cohen2004) and has been reported to respond automatically and rapidly to visually presented words (Dehaene, Naccache, Cohen, Bihan, Mangin, Poline & Riviere, Reference Dehaene, Naccache, Cohen, Bihan, Mangin, Poline and Riviere2001). More recently, a larger Visual Word Form (VWF) system that plays a vital role in the processing of orthographic representations of visual letter strings (Mechelli, Crinion, Long, Friston, Ralph, Patterson, Mcclelland & Price, Reference Mechelli, Crinion, Long, Friston, Ralph, Patterson, Mcclelland and Price2005; Brem, Bucher, Halder, Summers, Dietrich, Martin & Brandeis, Reference Brem, Bucher, Halder, Summers, Dietrich, Martin and Brandeis2006; Vinckier, Dehaene, Jobert, Dubus, Sigman & Cohen, Reference Vinckier, Dehaene, Jobert, Dubus, Sigman and Cohen2007; van der Mark, Klaver, Bucher, Maurer, Schulz, Brem, Martin & Brandeis, Reference van der Mark, Klaver, Bucher, Maurer, Schulz, Brem, Martin and Brandeis2011) was proposed. A recent fMRI study reported that print sensitivity in the VWF system emerged after letter-sound correspondence was learned (Brem, Bach, Kucian, Guttorm, Martin, Lyytinen, Brandeis & Richardson, Reference Brem, Bach, Kucian, Guttorm, Martin, Lyytinen, Brandeis and Richardson2010). Meanwhile, several neural imaging studies in typical readers have observed activation of the left fusiform during auditory word repetition (Castro-Caldas, Petersson, Reis, Stone-Elander & Ingvar, Reference Castro-Caldas, Petersson, Reis, Stone-Elander and Ingvar1998), auditory consonant discrimination (Burton, Locasto, Krebs-Noble & Gullapalli, Reference Burton, Locasto, Krebs-Noble and Gullapalli2005), auditory lexical decision (Orfanidou, Marslen-Wilson & Davis, Reference Orfanidou, Marslen-Wilson and Davis2006) and auditory rhyme (Booth, Burman, Meyer, Gitelman, Parrish & Mesulam, Reference Booth, Burman, Meyer, Gitelman, Parrish and Mesulam2004; Burton et al., Reference Burton, Locasto, Krebs-Noble and Gullapalli2005; Yoncheva, Zevin, Maurer & McCandliss, Reference Yoncheva, Zevin, Maurer and McCandliss2010) tasks. Given that none of these tasks had orthographic input, activation in the left fusiform during spoken language processing suggests an automatic access to orthographic representations during auditory phonological processing. Previous studies have suggested that learning to read reorganizes various parts of the reading network including the fusiform gyrus (Dehaene, Pegado, Braga, Ventura, Nunes Filho, Jobert, Dehaene-Lambertz, Kolinsky, Morais & Cohen, Reference Dehaene, Pegado, Braga, Ventura, Nunes Filho, Jobert, Dehaene-Lambertz, Kolinsky, Morais and Cohen2010). It has been suggested that the acquisition of reading skills may lead to an automatic access to orthographic representations during language processing in a top-down fashion (Dehaene et al., Reference Dehaene, Pegado, Braga, Ventura, Nunes Filho, Jobert, Dehaene-Lambertz, Kolinsky, Morais and Cohen2010). The reduced activation in the left fusiform gyrus in impaired ESL readers may suggest that they have reduced or absent automatic access to orthographic representations during the auditory task. This finding is also consistent with a functional neuroimaging study that investigated the relation between orthography and phonology in English during auditory word rhyming (Desroches et al., Reference Desroches, Cone, Bolger, Bitan, Burman and Booth2010). Desroches et al. (Reference Desroches, Cone, Bolger, Bitan, Burman and Booth2010) reported that unlike control children, those with reading disabilities did not reveal reliable activation within fusiform regions during an auditory rhyming task. A direct comparison between impaired and typical readers revealed significant activation differences in this region. However, another study did not observe differences within the left fusiform gyrus between children with and without dyslexia during auditory word rhyming (Kovelman, Norton, Christodoulou, Gaab, Lieberman, Triantafyllou, Wolf, Whitfield-Gabrieli & Gabrieli, Reference Kovelman, Norton, Christodoulou, Gaab, Lieberman, Triantafyllou, Wolf, Whitfield-Gabrieli and Gabrieli2012). The differences between these studies with respect to this finding may relate to differing baseline conditions (i.e., a linguistic vs. a nonlinguistic baseline) or the nature of reading impairments. We also observed that Chinese ESL children with impaired English reading showed reduced functional connectivity between the LSTG and left fusiform gyrus compared to typically reading ESL children. This finding suggests that reduced or absent automatic access to orthographic representations during auditory phonological processing in reading-impaired ESL learners may be due to less developed connectivity between these two brain regions. It is possible that ESL learners with reading impairment have difficulties developing functional connectivity between brain regions for phonological, orthographic and semantic processing.
Our findings extend previous fMRI studies in native English-speaking children with impaired English reading that showed weaker activation in the left occipital cortex (Corina & McBurney, Reference Corina and McBurney2001) and decreased activation in left fusiform regions (Desroches et al., Reference Desroches, Cone, Bolger, Bitan, Burman and Booth2010). Moreover, as proposed by Desroches et al. (Reference Desroches, Cone, Bolger, Bitan, Burman and Booth2010) the lack of automatic access to orthographic representations exhibited by children with impaired reading during spoken language might be the result of phonological deficits. Deficits in phonological manipulation might have led to an impaired development of the grapheme-to-phoneme mappings that are essential for reading. Consequently, the connections between orthography and phonology are not strengthened (or possibly not formed), leaving impaired readers with limited access to orthographic representations during auditory phonological processing. In the current study, the reduced interregional functional connectivity between LSTG and the left fusiform gyrus for impaired readers supports this hypothesis. Previous studies have also observed that impaired readers show reduced (van der Mark et al., Reference van der Mark, Klaver, Bucher, Maurer, Schulz, Brem, Martin and Brandeis2011) or absent interregional neural connectivity (Shaywitz, Shaywitz, Fulbright, Skudlarski, Mencl, Constable, Pugh, Holahan, Marchione, Fletcher, Lyon & Gore, Reference Shaywitz, Shaywitz, Fulbright, Skudlarski, Mencl, Constable, Pugh, Holahan, Marchione, Fletcher, Lyon and Gore2003; Stanberry, Richards, Berninger, Nandy, Aylward, Maravilla, Stock & Cordes, Reference Stanberry, Richards, Berninger, Nandy, Aylward, Maravilla, Stock and Cordes2006; Cao et al., Reference Cao, Bitan and Booth2008) between regions involved in phonological processing (e.g., LIFG, left inferior parietal lobule) and regions responsible for orthographic processing (e.g., left fusiform) during visual word processing. Our result is consistent with these findings.
Since our findings are in concert with studies in native English impaired readers (Desroches et al., Reference Desroches, Cone, Bolger, Bitan, Burman and Booth2010), it can be suggested that similar neural deficits are involved in spoken language processing for both L1 and L2 learners. This study is an extension of our previous fMRI study, which investigated visual word processing in Chinese children with impaired English reading (You et al., Reference You, Gaab, Wei, Cheng-Lai, Wang, Jian, Song, Meng and Ding2011). These children showed reduced activation in parietotemporal regions for visual letter rhyming (phonological processing) and reduced activation in the left inferior occipital gyrus for visual letter matching (orthographic processing), which was consistent with previous studies in native English speakers (Temple et al., Reference Temple, Poldrack, Salidis, Deutsch, Tallal, Merzenich and Gabrieli2001; Shaywitz et al., Reference Shaywitz, Shaywitz, Pugh, Mencl, Fulbright, Skudlarski, Constable, Marchione, Fletcher, Lyon and Gore2002b). Taken together, our results suggest that the neural deficits involved in impaired English reading are similar across L1 and L2 learners in both the visual and auditory modalities.
5. Conclusion
Using functional magnetic resonance imaging, we investigated the neural mechanism underlying spoken word processing in Chinese children with and without English reading impairment. We revealed that children with English reading impairment showed relatively intact phonological representations (e.g., in the LSTG) but impaired phonological manipulation (e.g., in the LIFG). We also observed reduced or absent automatic access to orthographic representations (the left fusiform, BA 37) which may be due to reduced functional connectivity between the LSTG and the left fusiform. These findings are similar to findings in native English speakers and may suggest that brain areas underlying phonological and orthographic representation/processing and their connectivity are impaired in children with English reading impairment during spoken language processing. This suggests a common neural mechanism underlying impaired English reading across L1 and L2 learners.