Significant outcomes
-
• Chronic whole-egg treatment exerted an antidepressant-like effect in the forced swimming test (FST) on both rat strains.
-
• The tryptophan/large neutral amino-acid ratio in the plasma, which provides an index of the incorporation of tryptophan into the brain, was increased by chronic whole-egg treatment.
-
• This study demonstrated that chronic whole-egg treatment increased the tryptophan concentration in the prefrontal cortex.
Limitations
-
• Immobility is the only index of depression-like behaviour in the FST; hence, the beneficial effect found in the FST does not necessarily link to an antidepressant effect in humans.
-
• Wistar Kyoto (WKY) rats are the only animal model of depression. Therefore, the results of this study should be treated with caution.
-
• The experimental design used in this study was not able to specify the mechanism for the antidepressant-like effect found with the daily ingestion of whole egg.
Introduction
Background
Major depression is a common mental disorder with the following symptoms: depressed mood, loss of interest or pleasure, feelings of guilt or low self-worth, disturbed sleep or appetite, poor concentration, self-injury and/or suicidal thoughts. These symptoms can become chronic and lead to an inability to fulfil everyday responsibilities. The World Health Organization estimated that more than 350 million patients were suffering from depression in 2012 (1). Data from the American Psychiatric Association show that an estimated 10–25% of women and 5–12% of men experience major depression in their lifetime (Reference Pincus and Pettit2). Thus, overcoming major depression is a task of pressing urgency throughout the world. In addition, antidepressants that are used to reduce the symptoms of major depression have some problems. First, antidepressants have a lot of side effects, such as dry mouth, constipation, sexual problems, nausea, dizziness and daytime drowsiness (Reference Koenig and Thase3,Reference Rief, Nestoriuc and Von Lilienfeld-Toal4). Second, current antidepressants take at least several weeks to exert therapeutic benefits (Reference Gelenberg and Chesen5) and only 50–75% of depressed patients are responsive to antidepressants (Reference Sartorius, Baghai and Baldwin6). Third, antidepressant therapy has a higher relapse rate when the medication is stopped (Reference Fava, Ruini, Rafanelli, Finos, Conti and Grandi7). Because of these problems, antidepressant therapy for major depression may not be the best choice of treatment. Therefore, the exploration of novel therapies throughout the world is also required.
It was previously known that antidepressants exerted their effects via the monoamine systems (Reference Coppen8,Reference Yoshitake, Fujino, Kehr, Ishida, Nohta and Yamaguchi9). In these systems, it has been thought that the serotonin (5-HT) system plays a key role in the onset of major depression. The reasons are as follows: depressed patients showed a lower concentration of 5-HT in the brain; most antidepressants functionally increased the serotonergic neurotransmission during long-term treatment; and several selective serotonin reuptake inhibitors are effective in treating major depression (Reference Owens and Nemeroff10). However, recent studies have demonstrated that antidepressants affect not only the monoamine system but also amino-acid metabolism (Reference Kokras, Antoniou, Polissidis and Papadopoulou-Daifoti11–Reference Nagasawa, Murakami, Tomonaga and Furuse13). Therefore, it is possible that antidepressants may exert their effects via alteration of the metabolism of amino acids. In addition, the study, which used an animal model of depression, indicated that a depressive-like state may induce abnormality in amino-acid metabolism (Reference Nagasawa, Ogino and Kurata14). In a study using humans, in fact, depressed patients showed abnormal amino-acid metabolism in the plasma and platelets and a low tryptophan/large neutral amino-acid (Trp/LNAA) ratio – an index of the incorporation of tryptophan into the brain – compared with healthy subjects (Reference Mauri, Boscati and Volonteri15). Furthermore, it is reported that tryptophan, which is the precursor of 5-HT, has an antidepressant-like effect in mice (Reference Wong and Ong16) and in humans (Reference Charney, Heninger, Reinhard, Sternberg and Hafstead17). Thus, there is abundant evidence showing the relationship between depression and amino-acid metabolism, especially tryptophan metabolism.
Several studies have reported that vegetarian diets are related to poorer mental health, with a particularly high ratio of vegetarians experiencing major depression in comparison with subjects with non-vegetarian diets (Reference Baines, Powers and Brown18–Reference Perry, Mcguire, Neumark-Sztainer and Story20). These data raise the hypothesis that the intake of animal products may lead to better mental health. In fact, our previous results showed that animal protein extracts, such as chicken, pork and beef, altered the brain monoamine metabolism-regulating emotion (Reference Nagasawa, Murakami, Sato, Takahata, Morimatsu and Furuse21). In addition, the administration of chicken breast extract rich in carnosine (β-alanyl-L-histidine) increases carnosine concentration in the brain (Reference Tomonaga, Hayakawa and Yamane22), and carnosine induces an antidepressant-like effect (Reference Tomonaga, Yamane and Onitsuka23). These data suggest that various eligible animal products have a role in regulating affective status through monoamine metabolism and other regulatory systems in the brain.
Egg produces various effects, including antimicrobial, immunomodulatory, anticancer, antihypertensive and antioxidant activity, and these effects are related to a number of functional peptides and proteins in egg (Reference Kovacs-Nolan, Phillips and Mine24). Therefore, identified and/or unidentified peptides and proteins in egg may have antidepressant-like effects. In fact, fertilised egg powder (Young Tissue Extract; YTE®, Med-Eq AS, Tonsberg, Norway) induced antidepressant-like effects in depressed patients (Reference Solberg25). Furthermore, it is well known that the amino-acid balance of egg is excellent, and egg protein is used as the standard to evaluate the nutritional value of food protein (Reference Ihekoronye26). Therefore, we focused on the function of egg in an animal model of depression.
WKY rats were isolated from normal Wistar (WIS) rats and were used as the control animals of spontaneously hypertensive rats. However, WKY rats are also used in a study elucidating the mechanism for major depression. They display longer immobility in the FST compared with outbred Sprague–Dawley rats and other inbred strains (Reference Nagasawa, Ogino and Kurata14,Reference Lahmame and Armario27–Reference Tejani-butt, Kluczynski and Paré30); however, chronic antidepressant treatment has been found to alleviate this depression-like behaviour in the FST (Reference Lahmame, Del Arco, Pazos, Yritia and Armario31). In addition, WKY rats showed hypoactivity in the open-field test (OFT) compared with WIS rats (Reference Nagasawa, Ogino and Kurata14,Reference Redei, Solberg, Kluczynski and Pare29,Reference O'mahony, Clarke, Gibney, Dinan and Cryan32). This abnormal behaviour in WKY rats corresponds with the findings of a study using human subjects, in which depressed patients exhibited hypoactivity compared with healthy subjects (Reference Hauge, Berle, Oedegaard, Holsten and Fasmer33). Therefore, WKY rats are proposed as an animal model of depression.
Aims of the study
The purpose of the present study was to evaluate the effects of chronic whole-egg treatment on depression-like behaviours in normal WIS rats and WKY rats, an animal model of depression. In addition, we aimed to elucidate the effects of oral whole egg on tryptophan metabolism and the incorporation of tryptophan into the brain to provide an insight into the pathogenesis of major depression. This study was conducted to demonstrate the possibility that daily whole-egg intake is useful for mental health in humans.
Materials and methods
Animals
Three-week-old male WIS and WKY rats were bought from Charles River Japan (Yokohama, Japan). The rats were housed three or four per cage with free access to food (MF diet; Oriental Yeast Co., Tokyo, Japan) and water. They were maintained on a 12-h light/dark cycle (lights on at 08:00, lights off at 20:00) at a room temperature of 23 ± 1°C and with humidity at 60%. This study conformed to the guidelines for animal experiments conducted in the Faculty of Agriculture and in the Graduate Course of Kyushu University, and to Law No. 105 and Notification No. 6 of the government. The experimental plan for the present study was approved by Kyushu University (A22-135-5).
Experimental procedure
The WIS and WKY rats were acclimated for 7 days on arrival. On a body weight basis, rats in each strain were assigned to two groups of seven rats each, that is: (i) control WIS rat group, (ii) egg-treated WIS rat group, (iii) control WKY rat group and (iv) egg-treated WKY rat group. Either whole chicken-egg solution (5 ml/kg) or distilled water (5 ml/kg) was orally administrated for 35 days, but administration was not carried out on the behavioural test days (21st, 27th and 28th days) to avoid the acute effects of single administration on behavioural tests. The duration for recovery from the last behavioural test was set at 7 days. On the 36th day, all rats were decapitated under anaesthesia with isoflurane (Escain®, Mylan, Osaka, Japan), and trunk blood was collected. The plasma was prepared by centrifuging at 3000 × g for 15 min at 4°C (KUBOTA 3740). The brains were quickly removed and dissected in the prefrontal cortex, hippocampus, striatum, thalamus, hypothalamus, brain stem and cerebellum, and weighed. The dissection of the brain was performed according to Paxinos and Watson (Reference Paxinos and Watson34). The samples were frozen in liquid nitrogen, and stored at −80°C until analysis was performed.
Open-field test
The OFT was conducted on the 21st day after the treatments were started. Motor activity in a novel environment was evaluated by the OFT. Each rat was transferred to the open-field area from its home cage. The open field was a square arena (length 90 cm, width 90 cm and height 45 cm) made of black-coloured wood. Measurement of the motor activity was started as soon as the rats were placed at the centre of the arena in 70 lux light. The motor activity of each rat was recorded by video camera for 5 min. After each test, rats were returned to their home cages and the open-field area was cleaned with a solution of ethanol and water to unify the test conditions. For the analysis, the bottom aspect of the open-field arena was drawn with lines measuring 18 cm both longitudinally and transversely; it was divided into 25 square compartments. Line crossing (the number of times crossed) during the 5 min of video recording was used as the parameter representing motor activity, and it was counted in a blind manner. Originally, OFT was the behavioural test used to evaluate anxiety-like behaviour. In this experiment, however, OFT was adopted to evaluate whether the chronic egg treatment alleviates hypoactivity in WKY rats, this being a depression-like behaviour specific to WKY rats.
Forced swimming test
FST was conducted on the 27th and 28th days after the treatments were started to evaluate whether the rats were in a depressive-like state. This test was conducted according to a previous report (Reference Porsolt, Anton, Blavet and Jalfre35), with some modifications. In the present study, the FST comprised two exposure sessions: a pre-test session and a main test session. In the pre-test session, the rats were individually placed in an acrylic cylinder (30 cm in diameter, 45 cm high) containing water to a depth of 30 cm maintained at 24–26°C for 15 min. Twenty-four hours later, the rats were placed back into the cylinder in a similar manner, and their behaviour was recorded by a video camera for 5 min (main test session). After each session, the rats were returned to their home cages and the water in the acrylic cylinder was replaced with fresh water to unify the test conditions. The total duration of immobility was counted manually in a blind manner. Immobility was defined as the index of a depressive state. A rat was evaluated as immobile when it was floating motionless or making only small movements to keep its head above water.
Amino-acid analysis
To investigate the effects of chronic egg treatment on tryptophan metabolism and tryptophan incorporation into the brain, free amino-acid concentrations in the plasma and brain were analysed by high-performance liquid chromatography (HPLC). The tissue samples were homogenised in ice-cold 0.2 mol/l perchloric acid solution containing 0.01 mmol/l EDTA·2Na and left for deproteinisation on ice for 30 min. Then the tissue homogenates were centrifuged at 20 000 × g for 15 min at 0°C. Supernatants were adjusted to pH 7 with 1 mol/l sodium hydroxide. Plasma samples were filtrated through an ultrafiltration tube (Millipore, Billerica, Massachusetts, USA). Each 20-μl sample of the brain and each 10-μl sample of the plasma was dried under reduced pressure. The dried residues were dissolved in 10 μl of 1 mol/l sodium acetate–methanol–triethylamine (2 : 2 : 1) and re-dried under reduced pressure, and then dissolved in 20 μl of methanol-distilled water–triethylamine–phenylisothiocyanate (7 : 1 : 1 : 1), which was a derivatisation solution. After phenylisocyanate had finished reacting with the amino groups at room temperature for 20 min, the samples were dried again and dissolved in 200 μl of Pico-Tag diluent (Waters, Milford, Massachusetts, USA). These diluted samples were filtrated through a 0.20-mm filter (Millipore). The same methods were carried out on standard solutions that were prepared by diluting a commercially available L-amino-acid solution (type ANII, type B, L-asparagine, L-glutamine and L-tryptophan; Wako, Osaka, Japan) with distilled water. The derivatised samples were applied to a Waters HPLC system (Pico-Tag free amino-acid analysis column (3.9 mm × 300 mm), Alliance 2690 separation module, 2487 dual-wavelength UV detector, and Millennium 32 chromatography manager; Waters). They were equilibrated with buffer A [70 mM sodium acetate (pH 6.45 with 10% acetic acid)–acetonitrile (975 : 25)] and eluted with a linear gradient of buffer B [water–acetonitrile–methanol (40 : 45 : 15) (0%, 3%, 6%, 9%, 40% and 100%)] at a flow rate of 1 ml/min at 46°C. The concentrations of free amino acids were determined by the absorbance at a 254-nm wavelength. The plasma amino-acid concentrations were expressed as pmol/μl, and the amino-acid concentrations in the brain were expressed as pmol/mg wet tissue.
5-HT analysis
To investigate the effects of chronic egg treatment on the metabolism of brain tryptophan, 5-HT concentrations in the brain were analysed by HPLC. The supernatants that were collected during the amino-acid analysis were adjusted to pH 3 with 1 mol/l sodium acetate and were filtrated through a 0.20-μm filter. 5-HT tissue concentrations in filtrates were analysed using an HPLC system (Eicom, Kyoto, Japan), with a 150 × 3.0 mm ODS column (EICOMPAK SC-5ODS, Eicom) and an electrochemical detector (ECD-300, Eicom) at an applied potential of +750 mV versus an Ag/AgCl reference analytical electrode. The changes in electric current (nA) were recorded on a computer using an interface system (Power Chrom ver 2.3.2.J; AD Instruments, Tokyo, Japan). The mobile phase consisted of an aceto-citric acid buffer (pH 3.5, 0.1 mol/l), methanol, sodium 1-octane sulfonate (0.46 mol/l) and EDTA·2Na (13.4 mmol/l) (830 : 170 : 1.9 : 1). The retention time and the height of the peaks in the tissue homogenates were measured and compared with the samples of external calibrating standard solution containing 5-HT. Concentrations of these substances in the samples were calculated and expressed as pg/mg wet tissue.
Statistics
All data were expressed as means ± SEM. Data analyses for bodyweight changes were performed by a repeated-measures three-way ANOVA. Crossing in OFT, immobility in FST, and free amino-acid and 5-HT concentrations were analysed by a two-way ANOVA and later by a t-test on the same strains and on corresponding treatment groups (control group in WIS rats vs. control group in WKY rats and egg-treated group in WIS rats vs. egg-treated group in WKY rats). Significance was set at p < 0.05. All the analyses were performed with StatView (version 5, SAS Institute Cary, United States, SAS 1998). Outlying data were eliminated by Thompson's test criterion for outlying observations (p < 0.05).
Results
Body weight
Changes in the body weight (g) after the administration of whole-egg solution or water in WIS and WKY rats are shown in Fig. 1. The body weight increased over time in both strains, but the body weight gain was significantly lower in WKY rats than in WIS rats. No significant effect of egg treatment was observed. A significant interaction of strain × time was observed, implying that the difference in body weight gain between WIS and WKY rats widened as time went on.
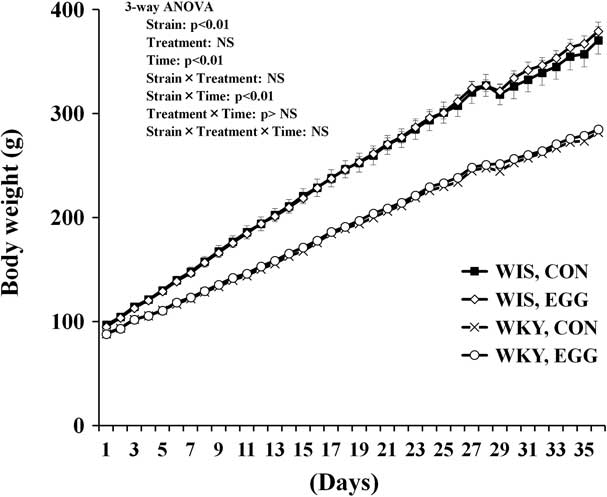
Fig. 1 Effects of strain, treatment and dosing period (time) on body weight (g). The number of samples used for analysis was between five and seven. All results are expressed as mean ± SEM. WIS, Wistar rats; WKY, Wistar Kyoto rats; CON, control group; EGG, chronic whole-egg treatment group; NS, not significant.
Behavioural tests
The effects of strain difference and egg treatment on line crossing (the number of times crossed) in the OFT and on total duration of immobility (s) in the FST are shown in Fig. 2. In the OFT, WKY rats displayed significantly less crossing than WIS rats, but no significant effects of egg treatment and interaction were observed. In the FST, immobility was significantly longer in WKY rats than in WIS rats, and egg treatment caused a significant decrease in immobility. No significant interaction between egg treatment and strain was observed in the FST.
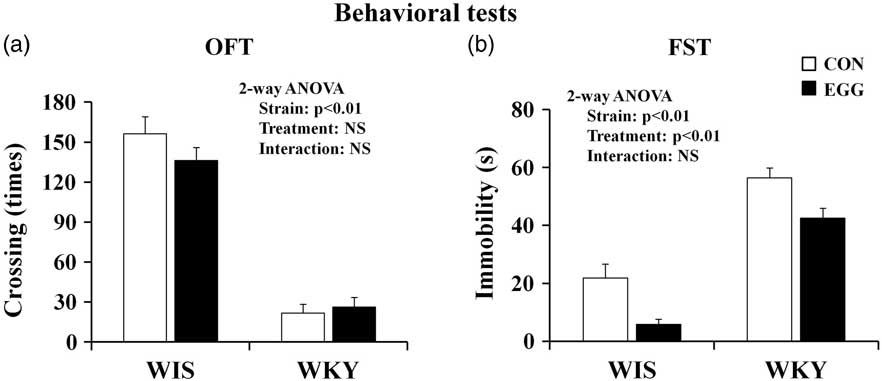
Fig. 2 Effects of strain and treatment on behavioural tests. (a) Effects of strain difference and egg treatment on line crossing (the number of times crossed) in OFT. (b) Effects of strain difference and egg treatment on the duration of immobility (s) in FST. The number of samples used for analysis was between six and seven. All results are expressed as mean ± SEM. OFT, open-field test; FST, forced swimming test; WIS, Wistar rats; WKY, Wistar Kyoto rats; CON, control group; EGG, chronic whole-egg treatment group; NS, not significant.
Incorporation of tryptophan into the brain and tryptophan concentrations in the brain
Although the plasma tryptophan concentration (pmol/μl) did not change with strain (WIS rat group: 33.3 ± 1.7; WKY rat group: 28.6 ± 1.6) or with egg treatment (control group: 28.7 ± 1.8; egg-treatment group: 33.1 ± 1.6), the plasma Trp/LNAA ratio increased with egg treatment (Fig. 3a). No significant effects of strain difference were observed and there was no interaction between strain and egg treatment.
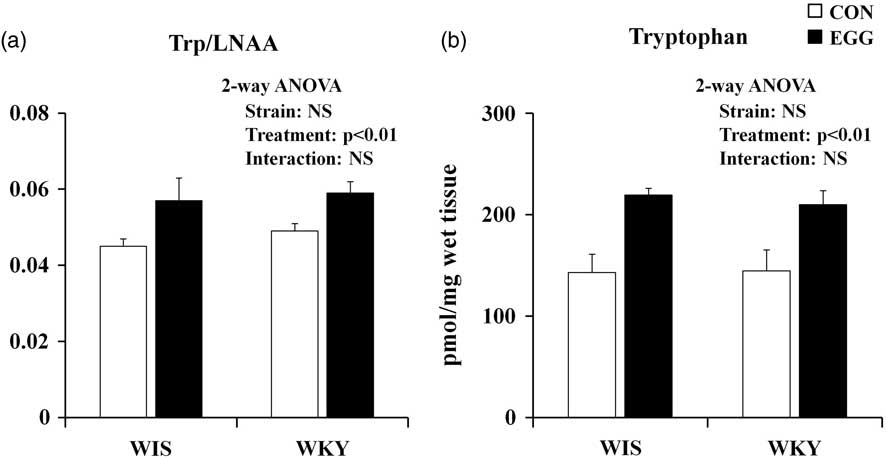
Fig. 3 Effects of strain and treatment on the Trp/LNAA ratio in the plasma and on tryptophan concentration in the prefrontal cortex. (a) Effects of strain difference and egg treatment on tryptophan incorporation into the brain. The number of samples used for analysis was between five and seven. All results are expressed as mean ± SEM. (b) Effects of strain difference and egg treatment on tryptophan concentration in the prefrontal cortex. The number of samples used for analysis was between five and six. All results are expressed as mean ± SEM. Concentration was expressed in pmol/mg wet tissue. Trp, tryptophan; LNAA, large neutral amino acids; WIS, Wistar rats; WKY, Wistar Kyoto rats; CON, control group; EGG, chronic whole-egg treatment group.
Tryptophan concentration in the prefrontal cortex is also shown in Fig. 3b. Egg treatment significantly increased the tryptophan concentration, but no significant effects of strain difference and interaction were observed. In the other brain regions, tryptophan concentration (pmol/mg wet tissue) was not changed by egg treatment (hippocampus: control group 146 ± 5.5, egg-treatment group 154 ± 1.3; striatum: control group 101 ± 4.9, egg-treatment group 99.7 ± 9.0; thalamus: control group 66.0 ± 5.4, egg-treatment group 51.0 ± 4.6; hypothalamus: control group 44.3 ± 12, egg-treatment group 45.9 ± 14; brain stem: control group 106 ± 6.4, egg-treatment group 127 ± 4.7; and cerebellum: control group 143 ± 3.9, egg-treatment group 143 ± 4.1).
5-HT concentrations in the brain
Egg treatment did not affect the 5-HT concentrations in the prefrontal cortex, hippocampus, striatum, thalamus, hypothalamus, brain stem or cerebellum, whereas the effect of strain difference was confirmed in the striatum, thalamus and cerebellum (data not shown). WKY rats showed lower 5-HT concentrations in these three regions compared with WIS rats.
Discussion
In the OFT, WKY rats displayed less line crossing (in terms of the number of times crossed) compared with WIS rats, meaning that WKY rats are hypoactive, in agreement with the findings of previous studies (Reference Nagasawa, Ogino and Kurata14,Reference Redei, Solberg, Kluczynski and Pare29,Reference O'mahony, Clarke, Gibney, Dinan and Cryan32). In addition, in the FST, WKY rats displayed higher immobility – the index of a depressive-like state – compared with WIS rats. These results are in agreement with those of previous studies (Reference Nagasawa, Ogino and Kurata14,Reference Lahmame and Armario27–Reference Tejani-butt, Kluczynski and Paré30). Therefore, here we reconfirmed the possibility that the WKY rat strain is an animal model of depression.
In the present study, the effects of chronic egg treatment on body weight, depression-like behaviours, Trp/LNAA ratio, tryptophan concentrations and 5-HT concentrations were evaluated. Daily egg treatment did not influence the body weight gain in either WIS or WKY rats. In addition, these data showed that daily egg treatment did not improve the low body weight in WKY rats. These facts suggest that in both strains a relatively small amount of energy was supplied from the daily whole egg compared with the total energy intake. However, we cannot clarify this point any more precisely because we did not measure the food intake or the energy intake. On the other hand, WKY rats grew more slowly in comparison with WIS rats, suggesting that growth is strictly controlled by genetic background.
Chronic egg treatment had no effect on line crossing (the number of times crossed) in the OFT in either WIS or WKY rats. This result suggests that chronic egg treatment did not improve hypoactivity, one of the parameters of depression-like behaviours, in WKY rats. On the other hand, chronic egg treatment significantly decreased immobility in the FST without any relation to the rat strain. This result indicates that chronic egg treatment alleviated the depression-like behaviours in the FST without any relation to genetic background. The data obtained here produced a conflicting result whereby chronic egg treatment alleviated depression-like behaviour only in the FST, and not in the OFT. A previous study revealed that chronic antidepressant treatment improved depression-like behaviour in the FST, but had no effect on motor activity in the OFT (Reference Réus, Stringari and Kirsch36). Therefore, it may be speculated that motor activity in the OFT is regulated by a different mechanism to that which regulates immobility in the FST. The discrepancy in the present study may be because of the different mechanism-regulating behaviours, and egg treatment might specifically act only on the depression-like behaviour exhibited in the FST.
From our analysis of the free amino-acid and 5-HT concentrations in the plasma and brain, we drew a conclusion concerning the possible mechanism of the antidepressant-like effect induced by daily egg treatment. Daily egg treatment increased the Trp/LNAA ratio. LNAA includes tryptophan, tyrosine, phenylalanine, leucine, isoleucine, valine, methionine and histidine, and these amino acids compete with one another for the same amino-acid transport system when they are incorporated into the brain (Reference Kanai, Segawa, Miyamoto, Uchino, Takeda and Endou37). The data obtained in the present study indicate that daily egg treatment may induce an elevated rate of incorporation of tryptophan into the brain. Interestingly, tryptophan concentration increased only in the prefrontal cortex, and not in the other brain regions. It has been thought that the reduction in 5-HT – a metabolite of tryptophan – in the brain induces major depression (Reference Lanni, Govoni, Lucchelli and Boselli38). Therefore, we expected that in this study the 5-HT concentration would increase in the prefrontal cortex, because chronic egg treatment increased the tryptophan concentration in the prefrontal cortex. However, the 5-HT concentration in the prefrontal cortex was found to be identical between the control and whole-egg groups. Therefore, the present study demonstrated that antidepressant-like effects of egg treatment may be not mediated by an increase in 5-HT concentration in the prefrontal cortex. In this regard, however, only the 5-HT concentration was evaluated and not its release into the synaptic clefts. Therefore, in a future study, the alteration of 5-HT release into the synaptic clefts that is caused by chronic whole-egg treatment needs to be evaluated. On the other hand, it is known that tryptophan is mainly metabolised by kynurenine in mammals (Reference Beadle, Mitchell and Nyc39). Therefore, the increase in tryptophan concentration in the prefrontal cortex caused by daily egg treatment may affect the kynurenine pathway. It is known that kynurenine catabolises into kynurenic acid, quinolinic acid and 3-hydroxy kynurenine. Previous studies have reported that depressed patients and the social isolation stress model in mice showed lower kynurenic-acid concentrations in the plasma compared with the control group (Reference Myint, Kim, Verkerk, Scharpé, Steinbusch and Leonard40,Reference Möller, DU Preez, Emsley and Harvey41), and an unpredictable chronic mild stress model, one of the animal models of depression, showed lower kynurenic-acid concentrations in the striatum compared with the control group (Reference Laugeray, Launay, Callebert, Surget, Belzung and Barone42). Furthermore, repetitive treatment of kynurenic acid reduced immobility in the FST (Reference Maj, Rogóz, Skuza and Kołodziejczyk43). In addition, kynurenic-acid treatment produces neuroprotective effects against the excitotoxic action of quinolinic acid (Reference Kim and Choi44). Furthermore, kynurenic acid attenuates the stress-related behaviours induced by the corticotrophin-releasing hormone in chicks (Reference Yoshida, Tomonaga, Ogino, Nagasawa, Kurata and Furuse45). Taking into consideration the previous reports and the results obtained here, it may be speculated that egg treatment exerts an antidepressant-like effect via activation of the tryptophan–kynurenine pathway in the prefrontal cortex. However, it is reported that kynurenine administration induces depression-like behaviours in mice (Reference O'connor, Lawson and André46). In addition, the elevation of kynurenine concentration also increases the concentration of quinolinic acid, which induces excitotoxicity (Reference Möller, DU Preez, Emsley and Harvey41). Therefore, it may be thought that the activation of the kynurenine–kynurenic-acid pathway is more important than that of the kynurenine–quinolinic-acid pathway. The relationship between depression and the kynurenine pathway should be examined carefully in future investigations.
Finally, we refer to the problem of whether the attenuation of depression-like behaviour is specific to chronic whole-egg treatment, or whether it is the additional energy intake through daily diet that is the significant factor in bringing about the attenuation. In the present study, we could not make any judgement about this, although the body weight between different dietary treatments was almost identical (Fig. 1), as we had planned the experiment in such a way as to clarify only the effect of additional supplementation of the daily diet through chronic whole eggs. This point should, however, be clarified in future work.
In conclusion, the present study has demonstrated that chronic whole-egg treatment induces antidepressant-like behaviour in both WIS and WKY rats only in the FST. The findings suggest that whole egg might function as a means of preventing and alleviating the condition of major depression.
Author Contributions
Mao Nagasawa designed this study, analysed all the experimental data and drafted this article. Tsuyoshi Otsuka and Junki Yoshida measured the behavioural tests. Yumi Ogino assisted in the sampling process. Shozo Tomonaga and Shinobu Yasuo provided critical advice about the experimental design and the writing of this article. Mitsuhiro Furuse was involved in the design of this study and discussed the experimental design with the first author. In addition, he provided advice concerning this study and revised this article.
Financial Support
Part of this project has been supported by the Kieikai Research Foundation and by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (No. 23248046).
Conflicts of Interest
There are no conflicts of interest.
Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional guides on the care and use of laboratory animals.





