Introduction
The Gyliauchenidae Fukui, 1929 is a peculiar lineage of the Lepocreadioidea Odhner, 1905, comprising 13 genera and 41 species (Bray et al., Reference Bray, Waeschenbach, Cribb, Weedall, Dyal and Littlewood2009; Bray and Cribb, Reference Bray and Cribb2012; WoRMS, 2018). Gyliauchenids are found almost exclusively in the intestines of herbivorous marine, and mostly tropical, teleosts and approximately 75% of species are known only from the Indo-West Pacific region (Hall and Cribb, Reference Hall, Cribb, Jones, Bray and Gibson2005a; Cribb et al., Reference Cribb, Bray, Diaz, Huston, Kudlai, Martin, Yong and Cutmore2016). Species of the Gyliauchenidae reach their definitive hosts via cercariae which encyst on vegetation (Al-Jahdali and Hassanine, Reference Al-Jahdali and Hassanine2012), rather than infecting a second intermediate host. Such external encystment is the typical life-cycle strategy for digeneans which exploit herbivorous fishes, e.g. species of the Atractotrematidae Yamaguti, 1939, Gorgocephalidae Manter, 1966, Haplosplanchnidae Poche, 1926 and Microscaphidiidae Looss, 1900 (Cable, Reference Cable1954; Hassanine et al., Reference Hassanine, Al-Zahrani, Touliabah and Youssef2016; Huston et al., Reference Huston, Cutmore and Cribb2016, Reference Huston, Cutmore and Cribb2018).
Gyliauchenids are most readily recognized by their unusual morphology, which appears to represent specializations for life in herbivorous fishes. The oral sucker is thought to have been replaced by the pharynx as the anterior muscular structure, in many species the oesophagus has become coiled and greatly elongated, and there is a muscular oesophageal bulb that acts as a valve between the oesophagus and caeca (Jones et al., Reference Jones, Hughes-Stamm, East and Cribb2000; Hall and Cribb, Reference Hall, Cribb, Jones, Bray and Gibson2005a). The loss of the oral sucker is thought to be an indication of a transition to a fluidic diet, and the position of the ventral sucker near the posterior extremity in most species, is thought to facilitate better grazing on the hosts’ luminal contents (Jones et al., Reference Jones, Hughes-Stamm, East and Cribb2000). Gyliauchenids also appear to be closely associated with other intestinal symbionts, hosting complex ecto-commensal communities of microorganisms (Hughes-Stamm et al., Reference Hughes-Stamm, Cribb and Jones1999), as well as ingesting free-swimming species (Jones et al., Reference Jones, Hughes-Stamm, East and Cribb2000). The relationships between gyliauchenids and other endosymbionts are virtually unknown. Ecto-commensals are not thought to be harmful to gyliauchenids, though it is unknown if the relationship is beneficial to either the gyliauchenids or the microorganisms (Hughes-Stamm et al., Reference Hughes-Stamm, Cribb and Jones1999). Similarly, it is unknown whether the ingestion of intestinal microorganisms by gyliauchenids is purposeful or incidental.
The major host groups for gyliauchenids in their adult form are herbivorous fishes from the surgeonfishes (Acanthuridae), butterflyfishes (Chaetodontidae), angelfishes (Pomacanthidae), rabbitfishes (Siganidae), parrotfishes (Scaridae), porgies (Sparidae) and the Moorish idol (Zanclidae) (Hall and Cribb, Reference Hall, Cribb, Jones, Bray and Gibson2005a). Herbivorous species of damselfishes (Pomacentridae) have also been reported to host gyliauchenids occasionally (Dyer et al., Reference Dyer, Williams and Williams1988; Nahhas and Wetzel, Reference Nahhas and Wetzel1995). There are also scattered records from non-herbivores in the families Ariidae, Blenniidae, Clupeidae, Engraulidae and Synodontidae (Nahhas and Wetzel, Reference Nahhas and Wetzel1995; Hall and Cribb, Reference Hall, Cribb, Jones, Bray and Gibson2005a). Oddly, just one major group of large herbivorous marine fishes, the drummers or sea chubs (Kyphosidae), have never been reported to host gyliauchenids, although they have been extensively studied for parasites. Instead, they host species of the Enenteridae, the sister taxon of the Gyliauchenidae. This pattern of host-partitioning remains unexplained.
In December 2017, we collected specimens of Kyphosus cornelii (Whitley, 1944), Kyphosus gladius Knudsen & Clements, 2013, and Kyphosus sydneyanus (Günther, 1886) from off southwestern Australia. From K. cornelii we recovered a species of gyliauchenid, the first known from a kyphosid host. Here, using morphological and molecular data, we describe this species as new, propose a new genus to accommodate it and discuss the colonization of a kyphosid by a gyliauchenid.
Materials and methods
Specimen collection
This is the second in a series of reports on the trematode fauna of kyphosid fishes of the Indo-West Pacific (Perciformes: Kyphosidae). Between 1994 and 2018 we have collected 116 individual Kyphosus fishes of seven species from multiple localities across the Indo-West Pacific. The majority of these fishes were collected in Australian waters (Great Barrier Reef and Moreton Bay, Queensland; Yorke Peninsula, South Australia; Ningaloo Reef and off Perth, Western Australia), but the collection also includes fishes collected from French Polynesia, Japan, Palau and South Africa. For more specific information with regards to this collection, including collection numbers, localities and identification of specimens, readers are referred to the first paper of the series, Reference Martin, Huston, Cutmore and CribbMartin et al. (in press). Additionally, numerous other herbivorous marine fishes have been collected from the same localities during this period.
Morphological analyses
Herbivorous marine fishes were collected by spear and examined for trematodes following Cribb and Bray (Reference Cribb and Bray2010). Trematodes were removed live from the host, fixed without pressure in near boiling saline and preserved in 70% ethanol. Trematode specimens used for morphological examination were removed from their preservative, washed in fresh water, overstained in Mayer's haematoxylin, destained in a solution of 1.0% hydrochloric acid and neutralized in a 0.5% ammonium hydroxide solution. Specimens were then dehydrated in a graded ethanol series. Some dehydrated specimens were selected for scanning electron microscopy (SEM); these specimens were transferred to hexamethyldisilazane, air-dried overnight and mounted on 12.5 mm pin-stubs using an adhesive carbon tab. Before SEM, specimens were coated with 15 nm of iridium with a Quorumtech Q150TS sputter coater. SEM images were obtained on a Hitachi SU3500 scanning electron microscope in secondary electron mode. Specimens for whole mounts were cleared for ~24 h in a 1:1 methyl salicylate/100% ethanol solution, then transferred to 100% methyl salicylate for ~24 h. After the 48 h clearing period, Canada balsam was added incrementally to the methyl salicylate over several days. Specimens were then permanently mounted on slides in Canada balsam. Both laterally and dorso-ventrally mounted specimens were used in this study; measurements for length, width and depth are provided. Length is taken from both dorso-ventrally and laterally mounted specimens, whereas width is taken only from dorso-ventrally mounted specimens and depth is taken only from laterally mounted specimens. Measurements were made with cellSens standard imaging software paired with an Olympus SC50 digital camera and drawings were made with a camera lucida, both mounted on an Olympus BX-53 compound microscope. Drawings were digitized in Adobe Illustrator. All vouchers are lodged in the Western Australian Museum (WAM), Perth, Australia; accession numbers are presented in the taxonomic section of this paper.
Molecular sequencing
Three molecular markers were targeted in this study, the second internal transcribed spacer region (ITS2), 28S rRNA and the mitochondrial cytochrome c oxidase I (COI). DNA was extracted from small tissue samples excised from individual specimens, with the remainder of the specimen being processed for morphological study as described above to serve as both a morphological and molecular voucher [hologenophore sensu Pleijel et al. (Reference Pleijel, Jondelius, Norlinder, Nygren, Oxelman, Schander, Sundberg and Thollesson2008)]. Total genomic DNA was extracted from trematodes using phenol/chloroform extraction techniques (Sambrook and Russell, Reference Sambrook and Russell2001). The D1–D3 regions of 28S nuclear ribosomal DNA (D1–D3 regions) were amplified using the primers LSU5 (5′-TAG GTC GAC CCG CTG AAY TTA AGC-3′) (Littlewood, Reference Littlewood1994) and 1500R (5′-GCT ATC CTG AGG GAA ACT TCG-3′) (Snyder and Tkach, Reference Snyder and Tkach2001) and the ITS2 region using 3S (5′-GGTACC GGT GGATCA CGT GGC TAG TG-3′) (Morgan and Blair, Reference Morgan and Blair1995) and ITS2.2 (5′-CCT GGT TAG TTT CTT TTC CTC CGC-3′) (Cribb et al., Reference Cribb, Adlard and Bray1998). Partial COI mtDNA was amplified using the primers Dig_cox1Fa (5′-ATG ATW TTY TTY TTY YTD ATG CC-3′) and Dig_cox1R (5′-TCN GGR TGH CCR AAR AAY CAA AA-3′) (Wee et al., Reference Wee, Cribb, Bray and Cutmore2017).
Polymerase chain reaction (PCR) for the ITS2 and 28S regions was performed with a total volume of 20 µL consisting of 2 µL DNA template (~10 ng), 5 µL of 5× MyTaq Reaction Buffer (Bioline), 0.75 µL of each primer (10 µm), 0.25 µL of Taq DNA polymerase (Bioline MyTaq™ DNA Polymerase) and 11.25 µL H2O (Invitrogen™ ultraPURE™ distilled water). PCR for the COI region was performed in the same manner using 4 µL DNA template, 5 µL reaction buffer, 2 µL of each primer, 0.25 µL Taq and 6.25 µL H2O. Amplification was carried out on a MJ Research PTC-150 thermocycler with the following profiles: ITS2: an initial single cycle of 95 °C denaturation for 3 min, 45 °C annealing for 2 min, 72 °C extension for 90 s, followed by four cycles of 95 °C denaturation for 45 s, 50 °C annealing for 45 s, 72 °C extension for 90 s, followed by 30 cycles of 95 °C denaturation for 20 s, 52 °C annealing for 20 s, 72 °C extension for 90 s, followed by a final 72 °C extension for 5 min; 28S: an initial 95 °C denaturation for 4 min, followed by 30 cycles of 95 °C denaturation for 1 min, 56 °C annealing for 1 min, 72 °C extension for 2 min, followed by a single cycle of 95 °C denaturation for 1 min, 55 °C annealing for 45 s and a final 72 °C extension for 4 min; COI: an initial 94 °C denaturation for 3 min, followed by 40 cycles of 94 °C denaturation for 30 s, 50 °C annealing for 30 s and 72 °C extension for 30 s, with a final extension at 72 °C for 10 min. Sequence data were generated using the amplification primers, and the additional 28S internal primers 300F (5′-CAA GTA CCG TGA GGG AAA GTT-3′) (Littlewood et al., Reference Littlewood, Curini-Galletti and Herniou2000) and ECD2 (5′-CTT GGT CCG TGT TTC AAG ACG GG-3′) (Littlewood et al., Reference Littlewood, Rohde and Clough1997) via cycle sequencing with an capillary sequencer and ABI Big Dye™ v.3.1 chemistry, performed by the Australian Genome Research Facility, Brisbane. Collection data and GenBank accession numbers for taxa sequenced are presented in the taxonomic section of this paper.
Phylogenetic analyses
The partial 28S rRNA sequences generated in this study were aligned with sequences of species of Gyliauchenidae and selected outgroup taxa available on GenBank (Table 1). Species of the Enenteridae were chosen as outgroup taxa as the family has been repeatedly demonstrated as the sister group to the Gyliauchenidae (Bray et al., Reference Bray, Waeschenbach, Cribb, Weedall, Dyal and Littlewood2009, Reference Bray, Cribb and Cutmore2018; Bray and Cribb, Reference Bray and Cribb2012). We note that there are currently several gyliauchenid sequences on GenBank which relate to nomina nuda; these sequences were excluded from analyses. Alignment for the 28S rRNA sequences were performed with MUSCLE (Edgar, Reference Edgar2004) as implemented in MEGA7 (Kumar et al., Reference Kumar, Stecher and Tamura2016). The resultant alignment was trimmed to the maximal length of the shortest 50% of sequences. Phylogenetic trees based on the 28S dataset were constructed with maximum likelihood and Bayesian inference analyses on the CIPRES portal (Miller et al., Reference Miller, Pfeiffer and Schwartz2010) implementing best-fit nucleotide substitution models selected using jModelTest 2 (Darriba et al., Reference Darriba, Taboada, Doallo and Posada2012) with the Akaike information criterion and Bayesian information criterion. Maximum likelihood analyses were performed using RAxML (Stamatakis, Reference Stamatakis2014) with 1000 bootstrap pseudoreplicates. Bayesian inference was performed using MrBayes v3.2.6 (Ronquist et al., Reference Ronquist, Teslenko, van der Mark, Ayres, Darling, Höhna, Larget, Liu, Suchard and Huelsenbeck2012). Four chains were sampled every 1000 generations for 10 000 000 generations with the first 2500 samples being discarded as burn-in, at which point average standard deviation of split frequencies were <0.01 for all analyses.
Table 1. Gyliauchenidae and selected outgroup taxa from GenBank used in phylogenetic analyses, including host, provenance data, accession number and original reference

a As Flagellotrema amphitrite on GenBank (see Hall and Cribb, Reference Hall and Cribb2004).
Results
Of the 116 Kyphosus fishes collected between 1994 and 2018, trematodes of the family Gyliauchenidae were recovered from only six individuals, and only from K. cornelii collected from locations off Perth, Western Australia. This species of gyliauchenid is the first known from the family Kyphosidae, and based on combined morphological and molecular evidence, the species is considered new to science, and a new genus is proposed to accommodate it.
Phylogenetic results
The final 28S alignment consisted of 1325 nucleotide positions; no regions of alignment ambiguity were detected. Bayesian inference and maximum likelihood analyses produced trees with identical topologies (Fig. 1). Species of six genera, including representatives of all three of the currently recognized subfamilies were included, and all currently recognized morphological groupings formed highly-supported clades in the phylogenetic analyses.

Fig. 1. Phylogenetic relationships of the Gyliauchenidae based upon Bayesian inference (BI) and maximum likelihood (ML) analyses of the 28S rRNA dataset. BI posterior probabilities are shown above the node and ML bootstrap support is shown below. Support values less than 50 not shown.
The results of our molecular phylogenetic analyses were much the same as the most recent for the Gyliauchenidae (Bray et al., Reference Bray, Waeschenbach, Cribb, Weedall, Dyal and Littlewood2009). Robphildollfusium fractum (Rudolphi, 1819), the sole representative of the Robphildollfusiinae Paggi & Orecchia, 1963 in our analyses, was found sister to species of the Petalocotylinae Ozaki, 1934. Robphildollfusium fractum + species of Petacotylinae were sister to the remaining genera, which are all members of the Gyliaucheninae Fukui, 1929. Species of Paragyliauchen Yamaguti, 1934 were sister to the remaining genera of the Gyliaucheninae represented in our analyses, which formed two clades, one comprising species of Ptychogyliauchen Hall & Cribb, 2004 and another further subdivided into three small clades representative of Flagellotrema Ozaki, 1936, Affecauda Hall & Chambers, 1999 and the gyliauchenids collected from K. cornelii in Western Australia. The molecular data, combined with morphological evidence (see below), warrants the erection of a new genus and the description of a new species.
Taxonomy
Family Gyliauchenidae Fukui, 1929
Subfamily Gyliaucheninae Fukui, 1929
Genus Endochortophagus gen. nov. Figures 2 and 3

Fig. 2. Endochortophagus protoporus gen. nov., sp. nov. (A) Holotype, dorso-ventral view. (B) Paratype, lateral view. (C) Ovarian complex; dark circle is outline of ovary situated ventrally. (D) Terminal genitalia. Scale bars: A, B, 1000 µm; C, D, 500 µm.

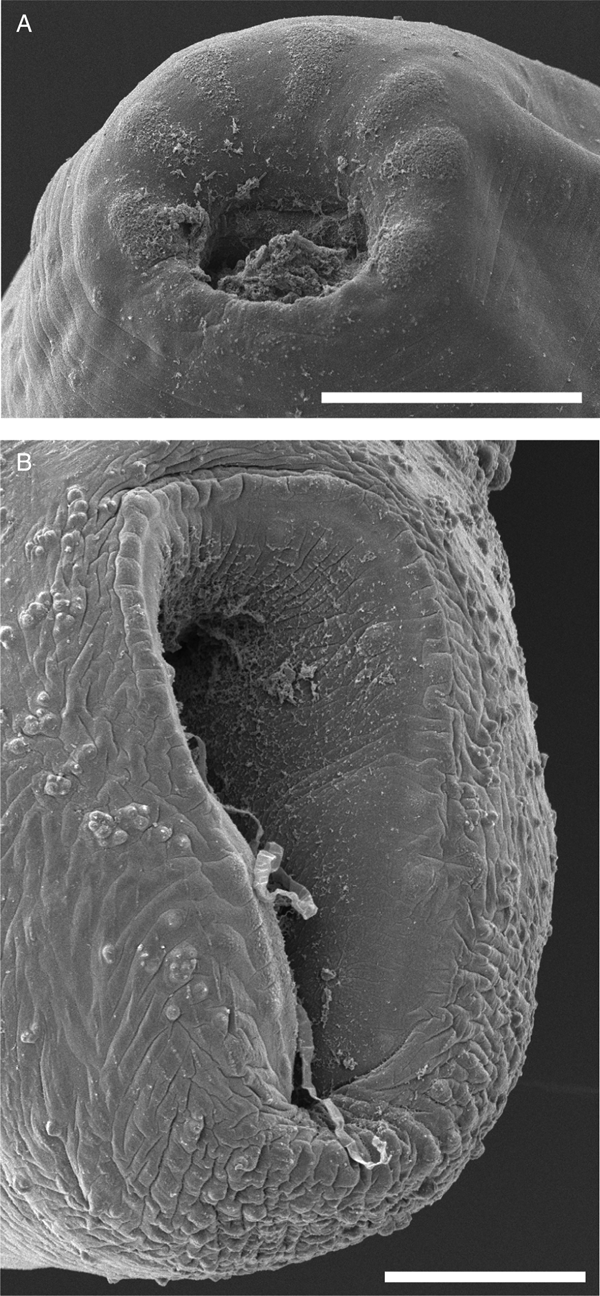
Fig. 3. Scanning electron micrographs of E. protoporus gen. nov., sp. nov. (A) Anterior extremity, showing seven indistinct lobes with coarse tegument surrounding mouth. (B) Posterior extremity and ventral sucker. Scale bars: 100 µm.
Zoobank Life Science Identifier: urn:lsid:zoobank.org:act:0EC999B9-B0A7-415E-A77E-3FFD83E04A8C
Type and only known species: Endochortophagus protoporus sp. nov
Diagnosis. Body elongate, fusiform. Tegument smooth. Pharynx spheroid to dolioform. Ventral sucker ellipsoidal, near posterior extremity. Oesophagus muscular, with one or two loops, with direction of looping variable, length representing less than 50% of body length. Oesophageal bulb muscular, ellipsoidal, smaller than pharynx. Caeca two, blind, terminate in mid-body. Testes two, diagonal or tandem, in posterior third of body. Seminal vesicle external, distinct. Pars prostatica partially external to cirrus sac. Cirrus sac robust, in mid-body. Genital atrium indistinct. Ovary subglobular, smaller than testes. Uterus convoluted, heavily coiled. Eggs thin-shelled, unembryonated in utero. Vitellarium follicular, profuse; fields extend from pharynx to posterior testis. Excretory vesicle elongate, claviform, reaching to about level of posterior margin of posterior testis, but dependent on preparation/orientation of specimens, subterminal. Excretory pore opening on a small, conspicuous, subterminal papilla. In intestine of Indo-West Pacific herbivorous teleosts (Kyphosidae).
Etymology. The name of the new genus is constructed from Latinized Greek: ‘endo’ = within, and ‘chortophagus’ = vegetarian/vegetable eater, and is treated as masculine. The name is in reference to the algal cells often visible in the guts of these worms, which suggests that they may be herbivores living within herbivores.
Remarks. There are many unpublished sequences of gyliauchenids on GenBank that refer to taxa which are presently nomina nuda. The diversity among these unverified and otherwise unpublished sequences suggests that the genus-level classification of the Gyliauchenidae is far from settled. Analysis of these sequences however, shows that none of these unpublished taxa are especially close to E. protoporus; certainly none has been detected from a kyphosid.
The results of our molecular phylogenetic analyses (Fig. 1) place E. protoporus sp. nov. (see description below) as sister to Affecauda annulata Hall & Chambers, 1999. However, following the key to the Gyliauchenidae provided by Hall and Cribb (Reference Hall, Cribb, Jones, Bray and Gibson2005a) and the subsequently updated diagnoses of Flagellotrema Ozaki, 1936, Ichthyotrema Caballero & Bravo-Hollis, 1952 and Telotrema Ozaki, 1933 (Hall and Cribb, Reference Hall and Cribb2005b, Reference Hall and Cribb2007, Reference Hall and Cribb2008), E. protoporus is the most similar morphologically to species of Flagellotrema Ozaki, 1936. We note that in our phylogenetic analyses (Fig. 1) the branch lengths separating species of Flagellotrema and Ptychogyliauchen Hall and Cribb, Reference Hall and Cribb2004 are longer than those separating E. protoporus from A. annulata, but there is sufficient morphological evidence to recognize these species in separate genera.
Although E. protoporus is quite similar morphologically to species of Flagellotrema, it can be distinguished from species of that genus by having a short, relatively straight ejaculatory duct rather than one which is long and convoluted, a genital atrium without cilia-like lining, a uterus which may reach past the ovary rather than being entirely pre-ovarian, and having vitelline follicles reaching to the posterior testis, rather than being restricted entirely to the pre-testicular region. Species of Affecauda differ from E. protoporus primarily in having far more slender bodies, tegument with annulation, rather than being smooth, a large convoluted genital atrium rather than one which is short and unspecialized, and having vitelline follicles restricted to the pre-testicular region rather than extending to the posterior testis. Endochortophagus protoporus is readily distinguished from species of the remaining genera of the Gyliauchenidae using the key provided by Hall and Cribb (Reference Hall, Cribb, Jones, Bray and Gibson2005a).
Endochortophagus protoporus sp. nov. Figures 2 and 3.
Zoobank Life Science Identifier: urn:lsid:zoobank.org:act:7AA3D447-F945-4A40-B228-BBF89BEBCFF9.
Type-host: Kyphosus cornelii (Whitley, 1944) Western buffalo bream (Perciformes: Kyphosidae).
Type-locality: off Pt. Peron, WA, Australia (32°15′S, 115°41′E).
Other localities: off Garden Island, WA, Australia (32°13′S, 115°40′E).
Site in host: hindgut fermentation chamber (see Fig. 4).

Fig. 4. Diagram of (A) the alimentary tract of Kyphosus cornelii and (B) a representation of the distal portion of the intestine of other Kyphosus species collected by the authors. (1) Section of K. cornelii intestine in which the enenterid Koseiria allanwilliamsi is found. (2) Section of K. cornelii intestine (subsection of hindgut fermentation chamber) in which E. protoporus gen. nov., sp. nov. is found.
Prevalence: six of seven (86%).
Type material: Holotype (WAM V9322), 27 paratypes, including three hologenophores (WAM V9323–9349).
Representative DNA sequences: three identical sequence replicates each of COI mtDNA, ITS2 rDNA and 28S rDNA (one of each submitted to GenBank, COI: MK396256, ITS2: MK396258, 28S: MK396257).
Etymology: ‘protoporus’ is a Latinized Greek word meaning pioneer. The name is chosen because the new species is the first gyliauchenid known from a species of the teleost fish family Kyphosidae.
Description (Figs 2 and 3), measurements given in Table 2.
Table 2. Morphometric data for Endochortophagus protoporus gen. nov., sp. nov. expressed in micrometres or as percentages

n, number measured; B, body; L, length; W, width; D, depth; Ph, pharynx; Oe, oesophagus; Oeb, Oesophageal bulb; VS, ventral sucker; Ae, anterior extremity; Pe, posterior extremity; C, caeca; ab, at caecal bifurcation; Pcs, post-caecal space; At, anterior testi; Pt, posterior testi; Pts, post-testicular space; Ov, ovary; Sr, seminal receptacle; Cs, cirrus sac; PP, pars prostatica; V, vitellarium; Voc, vitellarium occupies; Ev, excretory vesicle; EP, excretory papilla.
A dash (–) indicates distance between two features.
Based on 28 specimens, five dorso-ventral wholemounts, 20 lateral wholemounts and three lateral hologenophores. Body elongate, fusiform, often strongly bent at midbody. Tegument smooth. Ventral sucker ellipsoidal, longitudinally oblate, near posterior extremity, ventro-subterminal; aperture longitudinal, attenuating posteriorly. Mouth surrounded by seven indistinct lobes with coarse tegument (Fig. 3). Pharynx spheroid to dolioform, ventro-subterminal; ventral portion with anteriorly protruding ‘lip’. Oesophagus muscular, with one or two loops; direction of looping variable; prominent gland cells surround caeca along entire length. Oesophageal bulb in anterior third of body, muscular, ellipsoidal, smaller than pharynx. Intestine bifurcates immediately posterior to oesophageal bulb; caeca two, blind; gastrodermis heavily developed. Algal cells commonly visible in oesophagus and intestine.
Testes two, subglobular, diagonal or tandem, in posterior third of body. Seminal vesicle swollen, arises near anterior testis, passes anteriorly among uterine coils, uniting with pars prostatica. Pars prostatica amphoriform, penetrates cirrus sac; dense field of prostate gland cells surround pars prostatica and external to cirrus sac. Cirrus sac conspicuous, ellipsoidal to reniform, muscular, medial in midbody, ventral to caeca; ejaculatory duct broad or narrow, canalicular, relatively straight, passes directly to genital atrium. Genital atrium short, indistinct, unspecialized. Genital pore ovoid, medial, at level of caecal termination.
Ovary subglobular with position variable though generally intertesticular and contiguous with both testes. Seminal receptacle large, swollen, lachrymiform, antero-dorsal to ovary. Mehlis’ gland and oötype dorsal or anterior to ovary. Uterus long, convoluted, heavily coiled, medial portions broad, typically pre-ovarian, rarely proximal portions coil just posterior to ovary, joins genital atrium ventral to cirrus sac; opening simple. Vitellarium follicular, profuse, fields confluent, extend from pharynx to posterior testis; ventral field forms X shape, with anterior arms extending along either side of oesophagus, posterior arms diverging at caecal bifurcation; sinistral and dextral fields pass posteriorly from pharynx, diverge around caeca, re-join posterior to cirrus sac; dorsal field continuous, reaches from pharynx to posterior third of body. Vitelline reservoir dorsal to ovary; two main collecting ducts pass anteriorly, becoming indistinguishable from vitellarium anterior to cirrus sac. Eggs numerous, unembryonated in utero.
Remarks. This is the first gyliauchenid known from a kyphosid, but is otherwise morphologically unremarkable relative to the rest of the Gyliauchenidae. The worms were found only in a restricted region of the so called ‘caecal pouch’ or ‘hindgut fermentation chamber’ (see Rimmer and Wiebe, Reference Rimmer and Wiebe1987; Clements and Choat, Reference Clements and Choat1997) (Fig. 4), with the majority of specimens being collected from the lateral lobes of this structure. The enenterid Koseiria allanwilliamsi Bray and Cribb, Reference Bray and Cribb2002 was also found in K. cornelii in this study, from the same individuals infected with E. protoporus. However, they were found only in the mid-intestine (Fig. 4), demonstrating clear niche partitioning between E. protoporus and K. allanwilliamsi.
Discussion
The discovery of a gyliauchenid in a kyphosid was unexpected, but not unlikely. The most important host groups for species of the Gyliauchenidae are the rabbitfishes (Siganidae), parrotfishes (Scaridae) and surgeonfishes (Acanthuridae) (see Hall and Cribb, Reference Hall, Cribb, Jones, Bray and Gibson2005a), and these three groups often represent the largest component of herbivore biomass in reef ecosystems (Cheal et al., Reference Cheal, Emslie, Miller and Sweatman2012). Correspondingly, the gyliauchenid life cycle has adapted to exploit these herbivorous fishes through the omission of a second intermediate host, with metacercariae encysting in the environment (Al-Jahdali and Hassanine, Reference Al-Jahdali and Hassanine2012). However, species of the genus Kyphosus are also significant herbivores in terms of biomass on tropical and temperate reefs (Knudsen and Clements, Reference Knudsen and Clements2013, Reference Knudsen and Clements2016), and are thus likely to routinely consume gyliauchenid metacercariae. It seems likely then, that repeated encounter over time would create the opportunity for colonization. The present discovery was unexpected however, because there is a clear pattern in which gyliauchenids utilize all the major herbivore groups in the Indo-West Pacific except for species of the Kyphosidae, which are instead utilized by species of the sister family to the Gyliauchenidae, the Enenteridae (Bray and Cribb, Reference Bray and Cribb2001, Reference Bray and Cribb2002, Reference Bray and Cribb2012; Bray et al., Reference Bray, Waeschenbach, Cribb, Weedall, Dyal and Littlewood2009). Enenterids are restricted almost entirely to kyphosid fishes, and until now there was no host overlap between enenterids and gyliauchenids (Bray and Cribb, Reference Bray and Cribb2001, Reference Bray and Cribb2002; Bray et al., Reference Bray, Waeschenbach, Cribb, Weedall, Dyal and Littlewood2009). The finding of E. protoporus from K. cornelii is a significant exception that deserves consideration.
How it is that a gyliauchenid has colonized a kyphosid in Western Australia, but not in other Indo-West Pacific localities, is unclear. Colonization of a new host requires that the infective stage of a parasite pass successfully through two filters: encounter and compatibility (Combes, Reference Combes2001). In Western Australia, K. cornelii and K. sydneyanus occur sympatrically, but partition food resources, with K. cornelii feeding almost exclusively on benthic red algae and K. sydneyanus feeding almost exclusively on benthic brown algae (Rimmer and Wiebe, Reference Rimmer and Wiebe1987). We examined specimens of K. gladius and K. sydneyanus from the same localities as K. cornelii (including on the same day), and gyliauchenids were not found in either of these species. Routine encounter between gyliauchenid metacercariae and many kyphosid species throughout the Indo-West Pacific seems highly likely. However it is conceivable that gyliauchenid cercariae have some level of specificity for the type of algae on which they encyst. If the cercariae of E. protoporus encyst only on red algae, then encounter between E. protoporus metacercariae and other kyphosid species might be rare.
Although a level of specificity for aquatic vegetation may explain the lack of gyliauchenids in K. sydneyanus and K. gladius in Western Australia, this idea is not convincing in other parts of the Indo-West Pacific. On the Great Barrier Reef for example, Kyphosus cinerascens feeds on the same algae species as Acanthurus lineatus, Acanthurus nigricans, Naso tuberosus and Zebrasoma scopas, and the diet of Kyphosus vaigiensis is comparable with that of Naso unicornis (Choat et al., Reference Choat, Clements and Robbins2002). We have collected gyliauchenids from all of these acanthurid fishes on the Great Barrier Reef (unpublished data), but never from any kyphosids. If an explanation based on dietary distinction is not compelling in the tropics, then it may also be unconvincing for southwestern Australia. If it is likely that gyliauchenids routinely pass the encounter filter, but have not colonized other kyphosids of the Indo-West Pacific, compatibility may be a better explanation.
The site of infection in K. cornelii, the hindgut fermentation chamber, is unusual among kyphosids, in having two blind, lateral lobes, rather than being just an enlargement of the posterior portion of the intestine (Rimmer and Wiebe, Reference Rimmer and Wiebe1987; Clements and Choat, Reference Clements and Choat1997). This distinct morphological difference seems an important clue. Kyphosus azureus (Jenkins & Evermann), which occurs only along the Pacific coast of North America (Knudsen and Clements, Reference Knudsen and Clements2013, Reference Knudsen and Clements2016), has a similar hindgut fermentation chamber morphology, having a single blind lobe (Sturm and Horn, Reference Sturm and Horn1998; Fidopiastis et al., Reference Fidopiastis, Bezdek, Horn and Kandel2006), but apparently no trematodes have been reported from this species. Understanding the function of these unique intestinal morphologies may explain why K. cornelii is the only kyphosid known to host gyliauchenids, but at present, it is unclear what physiological purpose the specialized hindgut configurations in K. azureus and K. cornelii serves (Clements and Choat, Reference Clements and Choat1995; Mountfort et al., Reference Mountfort, Campbell and Clements2002; Fidopiastis et al., Reference Fidopiastis, Bezdek, Horn and Kandel2006). This difference may be due to diet and/or differences in the biochemical processes required for fermentation of different algal types.
There is little reported difference between acanthurids and kyphosids in the chemical composition, amount and proportion of short-chain fatty acids (SCFAs) produced as a result of microbial fermentation in the hindguts of these fishes (Rimmer and Wiebe, Reference Rimmer and Wiebe1987; Clements and Choat, Reference Clements and Choat1995; Choat and Clements, Reference Choat and Clements1998; Choat et al., Reference Choat, Clements and Robbins2002, Reference Choat, Robbins and Clements2004). However, little is known about the biodiversity and richness of the microbial communities which inhabit the intestinal tracts of marine herbivorous fishes. Although it is not entirely clear from where gyliauchenids derive their nutrition, they are thought to have a fluidic diet and to have adapted to feeding on the host's luminal content rather than the mucosa (Jones et al., Reference Jones, Hughes-Stamm, East and Cribb2000). Furthermore, species of both the Enenteridae and the Gyliauchenidae consume algal cells, either purposefully or incidentally, as the cells are often apparent in mounted specimens. We have also observed enenterids expelling large numbers of live ciliate protists through the anus when they are removed from the host and placed in saline. In their study of microbial fermentation in K. cornelii and K. sydneyanus, Rimmer and Wiebe (Reference Rimmer and Wiebe1987) found that the greatest number and diversity of microorganisms occurred in the hindgut fermentation chamber, and that the proportions of bacteria and protists differed between the two fish species. Perhaps gyliauchenids and enenterids feed on some of the fermenting endosymbionts producing SCFAs, or ingest them incidentally while consuming a nutritive slurry containing the SCFAs. Conceivably, the gyliauchenids and enenterids diverged based upon physiological differences in the bacteria and protist endosymbiont communities associated with different host lineages.
At present there seems little evidence to explain the observed host-specificity patterns, but further insights might be gained through detailed study of the interactions between digeneans and other endosymbionts of herbivorous marine fishes. Kyphosus cornelii also hosts a species of enenterid, K. allanwilliamsi Bray and Cribb, Reference Bray and Cribb2002, and thus represents the first known host in which both an enenterid and gyliauchenid occur. Further study of K. cornelii and its endosymbiotic community may reveal factors which have led to the host-partitioning pattern observed between the Enenteridae and Gyliauchenidae.
Endochortophagus protoporus is the southernmost gyliauchenid yet reported. The range of E. protoporus may extend further south than the type locality, to Cape Leeuwin, Western Australia (34°22′S, 115°6′E), which is the southern range of the host, K. cornelii (Knudsen and Clements, Reference Knudsen and Clements2013). At a minimum, the new record extends the range of gyliauchenids approximately 700 km further south, as the previous southernmost record was Petalocotyle adenometra from Amity Pt. in Queensland, Australia (27°23′S, 153°26′E). Gyliauchenids appear to range approximately as far north of the equator as south, with the northernmost record being near Tokyo, Japan (~35°N; Goto and Matsudaira, Reference Goto and Matsudaira1918). The exception to this pattern is the two species of the unusual Gyliauchenidae genus Robphildollfusium, which parasitize non-typical hosts, omnivorous fishes of the family Sparidae, in the Mediterranean and Atlantic. Species of Robphildollfusium have been reported as far north as the Adriatic Sea (~45°N). The centre of richness and diversity for the major host groups of the Gyliauchenidae is the tropical Indo-West Pacific (Nelson, Reference Nelson2006), but clearly a few gyliauchenids have managed to expand into subtropical zones [such as southwestern Australia where acanthurids, siganids and scarids are mostly absent (Gomon et al., Reference Gomon, Bray and Kuiter2008)] through the colonization of other herbivorous host groups.
Author ORCIDs
Daniel C. Huston, 0000-0002-1015-4703
Acknowledgements
The authors thank Nick Wee for his assistance during field collections in Western Australia, and Michelle Achlatis for his assistance with Greek. We thank our anonymous reviewers for helpful comments which improved the manuscript. The authors acknowledge the facilities, and the scientific and technical assistance, of the Australian Microscopy & Microanalysis Research Facility at the Centre for Microscopy and Microanalysis, The University of Queensland.
Financial support
This project was funded by a grant to DCH from the Holsworth Wildlife Research Endowment – Equity Trustees Charitable Foundation & the Ecological Society of Australia.
Conflict of interest
None.
Ethical approval
This study was conducted in compliance with all institutional, national and international guidelines on the care and use of animals.