The ductus arteriosus is a physiological foetal construction that connects pulmonary artery to the descending aorta and closes spontaneously after birth. If the ductus is left open, it would cause many complications such as left ventricular failure, endarteritis and arrhythmias. Reference Fernando, Koranne, Loyalka, Kar and Gregoric1 In addition to medical approaches for managing complications, trans-catheter closure of patent ductus arteriosus (PDA) is the favourite therapeutic alternative over surgical ligation in infants, children and adults. But when it comes to large PDAs, some issues including selecting proper patients and finding a suitable device are raised. For this reason, recently, the Amplatzer Duct Occluder (ADO) has been developed based on shape, size and orientation of ductus. Reference Brickner, Hillis and Lange2,Reference Meadows and Landzberg3
In this report, we have described the physiological and haemodynamic features of five patients who were referred to our clinic with large symptomatic PDA, for whom trans-catheter closure of PDA was performed using Amplatzer muscular ventricular septal defect (mVSD) device.
Cases
From 23 January, 2010 to 31 July, 2018, five patients were referred to our clinicFootnote a with large PDA and pulmonary arterial hypertension for additional evaluation and choosing appropriate treatment. All of them were symptomatic and had complaint from dyspnoea at exertion. In physical examination, differential cyanosis were not seen in any of them, and electrocardiogram (ECG) showed evidence of biventricular hypertrophy. After further evaluation with echocardiography and CT angiography, we performed diagnostic catheterization. With regard to elevated pulmonary artery pressure, we did vasodilator test by administering oxygen. After 20-minute of oxygen therapy, pulmonary artery pressure (PAP) decreased more than 25% from base in all patients, indicating positive response to vasodilator test. Due to the fact that the pulmonary pressure was sub-systemic in all patients and vasodilator test was positive, after team consultation, we decided to close the PDA with trans-catheter method for all of the mentioned patients.
Procedural technique
The size and shape of ductus were evaluated by CT angiography and aortography in left anterior oblique (LAO) and right anterior oblique (RAO) views. In two cases, PDA was conical shape (Fig 1a) and in three remaining patients, it was tubular (Fig 1b). We chose double disk muscular Occluder device because it fits more for the physiology of pulmonary hypertension.

Figure 1. Conical (1A) and tubular (2A) shapes of PDA in our patients (arrowed).
After local anaesthesia, we started the procedure via femoral approach. First, the right vein and arterial access was obtained and a 5F Occutech sheath was placed. After that, Heparin was injected intravenously based on the patient’s weight. Pulmonary artery; left and right heart pressure chambers were recorded and indicated no obstructions. Then, based on findings of CT angiography and aortography, the device was selected and placed through the femoral vein and the desired sheath. After making sure of the proper device position, sheath was removed and the tubular end of the device was opened in the duct. At the end of the procedure, aortic root injection in the left lateral view was performed to check the proper position of the device and the amount of residual shunt, which showed a small residual shunt in one case. Follow up echocardiography was done immediately and 24 hours after closure for detection of possible complications.
Ethical considerations
The patients were informed about the procedure and we answered any question related to their procedure. Informed written consent for the procedure and for reporting the case was taken from each patient separately.
Results
Our patients aged from 21 to 44 years. One of them was male. Pulmonary artery pressure and systemic pulse pressure decreased after procedure in all cases. Twenty-four hour, post procedural echocardiography showed the devices were in the proper position in four cases. The device was embolized into the right pulmonary artery in one patient. To retrieve the device, the patient underwent surgery. We also observed a small residual shunt in another patient the day after intervention whose symptoms improved following a conservative strategy.
Demographic, echocardiographic and haemodynamic data of all patients are summarized in Table 1.
Table 1. Clinical and haemodynamic data of the reported patients

DAO = descending aorta; LPA = left pulmonary artery; LVEDVI = left ventricular end diastolic ventricular index; LVEF = left ventricular ejection fraction; mPAP = mean PAP; PAP = pulmonary artery pressure; RAP = right atrial pressure; RV = right ventricle; RVP = right ventricular pressure; SVC = superior vena cava.
Discussion: morphology and clinical presentation
The clinical presentation of the PDA is different. Some patients are diagnosed randomly with medical examinations, but sometimes the patient may present with symptoms of chronic heart failure. In addition, reduced functional capacity is one of the symptoms mentioned by some patients. Reference Schneider and Moore4
PDA in adults is usually classified in three categories according to its size and hemodynamic effects: small PDA without significant haemodynamic effect; moderate-to-large PDA with left heart volume overload but without augmented pulmonary vascular resistance; and large PDA with increased pulmonary vascular resistance symptoms. Reference Meadows and Landzberg3
Diagnostic methods
Primary diagnostic tool for detection of PDA is chest X-ray (CXR) which may show specific radiological features known as the ‘railroad track’. Furthermore, chest radiography might show some complications of the disease for example heart failure or cardiomegaly but none of them are specific and CXR may be normal in spite of having PDA. Reference Schneider and Moore4
Another diagnostic method for evaluation of PDA is echocardiography which is used for classification of PDA and evaluation of other associated anomalies Reference Arlettaz5 but in cases of increased pulmonary vascular resistance and pulmonary hypertension, diagnosis of even large type PDA is very difficult. Reference Schneider and Moore4
Multi-planar CT is another tool which provides good information about size, morphology and presence of calcified tunnel which could be helpful for choosing therapeutic option. Reference Morgan-Hughes, Marshall and Roobottom6 When deciding on treatment strategy, factors such as PDA anatomy and proximity to the trachea (which is easily detect by this method) are important for choosing patients for trans-catheter closure. Reference Goitein, Fuhrman and Lacomis7 In addition, we can choose proper device based on CT characteristics of PDA.
Cardiac catheterization is another diagnostic method, which is now the preferred therapeutic method in most centres. This method makes it possible to have complete evaluation of patients, assessment of pulmonary pressure and vascular resistance and various vasodilator tests which may be beneficial in determining advisability of ductus closure. In addition, catheterization provides enough insight on exact anatomy of ductus and choosing appropriate device in terms of type and size. Reference Schneider and Moore4
Treatment options
PDA is usually detected and treated in childhood, and spontaneous closure is rare in adults and for this reason, it is recommended to close PDA immediately after diagnosis even in asymptomatic patients because of the numerous complications that will cause as the result of ductus which remain open. Reference Schneider and Moore4 But it is important to keep in mind that in patients with irreversible pulmonary hypertension, neither surgery nor a trans-catheter closure is recommended because it increases mortality. Reference Cassidy, Cassidy and Blackshear8,Reference Kalavrouziotis, Kourtesis, Paphitis and Azariades9
Generally, in patients with PDA, trans-catheter closure of PDA is the preferred therapeutic technique and has many benefits, such as shorter hospital stays and surgical avoidance and is associated with fewer complications compared with surgery. Reference Dries, Luc, Geert, Bert and De Wolf10 Furthermore, in patients with large PDA and pulmonary arterial hypertension, successful PDA closure applying different devices such as Amplatzer Duct Occluder or even Amplatzer Muscular Ventricular Septal Defect Occluder has been reported. Reference García-Montes, Camacho-Castro and Sandoval-Jones11 However, in patients whose anatomical characteristics of the ductus are not acceptable (for example duct with large diameter or short length), the results of using these devices may not be satisfactory.
Catheter intervention
For the first time, trans-catheter closure of PDA was explained by Portsmann et al Reference Porstmann, Wierny and Warnke12 who used a conical Ivalon plug in 1967, and then followed by Rashkind and Cuaso. Reference Fernando, Koranne, Loyalka, Kar and Gregoric1 This technique has been well developed over the years and Krichenko et al Reference Krichenko, Benson, Burrows, Möes, McLaughlin and Freedom13 divided the isolated PDAs into five groups according to anatomical and topological variations, which can affect the technique of catheter occlusion. This classification is based on angiographic appearances in which the narrowest segment of the ductus arteriosus is used as a landmark (Table 2).
Table 2. Krichenko classification of PDA based on shape and orientation
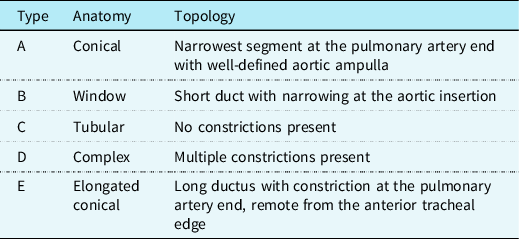
Adapted from Fernando et al. Reference Fernando, Koranne, Loyalka, Kar and Gregoric1
Today, various devices have been used commercially for trans-catheter closure of PDA, but only five of them have been approved by the Food and Drug Administration: The Gianturco coil (1992); the Cook detachable coil (1994); the Flipper detachable coil (1996); the GianturcoGrifka sac (1996); and the Amplatzer Duct occluder (ADO) I (1996). Reference Rao14 Another off-label device is ADO II (2009) that has two retaining discs on either side to prevent embolization of the device. In addition, it has a flexible waist that makes it fit well with the lumen and alignment of the duct and prevents disc protrusion and obstruction of pulmonary artery or aorta. Reference Thanopoulos, Eleftherakis, Tzannos and Stefanadis15 Furthermore, due to the maximum waist diameter of this device (12 mm), we cannot use it to close large PDA, but in clinical setting, feasibility and efficacy (96–100%) of the ADO II for using in PDA with a minimum diameter >2 mm have been well-known. Reference Thanopoulos, Eleftherakis, Tzannos and Stefanadis15,Reference Dua, Chessa and Piazza16 In some situations, Amplatzer vascular plugs and septal Occluders in an off-label have been used for PDA closure due to the large difference in the size and shape. Reference Meadows and Landzberg3
Compared with the ADO II, Amplatzer mVSD Occluder device has a different structure that allows it to accommodate a thicker part of the septum. Moreover, it makes a better approximation to the PDA wall due to the larger waist and two concentric discs. The higher waist-to-disc ratio (0.44 for the smallest to 0.69 for the largest compared with 0.33 for the smallest to 0.5 for the largest in ADO II) and larger maximum waist diameter (22 mm in the mVSD Occluder compared with 12 mm in ADO II) of this device, makes it suitable for closure of large PDA such as our patients. This device can cause residual shunting when used in patients similar to our cases with short length and large diameter. In addition, studies have shown that the presence of double discs reduces the chances of complications such as migration of device, and the presence of such a structure in this device makes it very effective in patients with high pulmonary pressures.
An important issue to consider is choosing a device with a suitable waist size for trans-catheter closure of PDA that lead to complete closure of the duct. Studies have shown that 50% of the compressive effects of the device are due to the waist and only 25% of each of the disc. Another point which should be in mind is about retention disk which is designed in the Amplatzer mVSD Occluder device in such a way that is accompanied by an angle and concavity that causes the device to be completely fixed at the end of the aorta. In addition, García-Montes suggested Amplatzer mVSD as the first choice device in patients with large type C (≥8 mm) or some cases with type A (who have diameter of aortic ampulla more than 1.5 times of the pulmonary end). Reference García-Montes, Camacho-Castro and Sandoval-Jones11 In our experience with these five cases, one of the most influential reasons for success in implantation of device is the position of retention aortic disc in duct. If the retention disc is dislodged to duct, the risk of embolization will be very high, as we had it in our last case (Fig 2a–d).

Figure 2. Abnormal position of retention disc in duct (2C) in conical PDA (2B).
Conclusion
Today, due to the advances made in the development of new devices, the trans-catheter closure of PDA has gained a special place in the treatment of patients and has become as a preferred alternative for surgery. For patients with inappropriate anatomy and special PDA shape, different methods are used in different centres. Recently, applying some special devices such as Amplatzer device of VSD has been proposed and has been successful. The special shape of these devices, which makes them better placed in the duct, can be a guide for developing newer devices to be used for interventional closure of large PDA in adults.
Acknowledgments
We express our gratitude of our kind patients who agreed to report their case. We also thank all kind nurses of Rajaei educational and research center.
Conflicts of interest
None to be declared.
Funding
We did not receive any funding for this study.
Ethical standards
The patients were informed about the procedure and we answered any questions related to their procedure. Informed written consent for the procedure and for reporting the case was taken from each patient separately.







