In 1971, Francis Fontan outlined the Fontan procedure, the third and final stage of surgical palliation aimed at improving the survival of patients with single-ventricle congenital heart disease. Reference Rychik, Atz, Celermajer and Deal1 The Fontan procedure allows passive systemic venous return to the lungs, by bypassing the single ventricle. Reference Sunstrom, Muralidaran and Gerrah2
Palliation of a univentricular heart is normally performed as a staged reconstruction in a series of open-heart procedures to allow the heart and lungs to progressively adapt to the changes in blood flow various congenital cardiac malformations may result in a Fontan circulation after reconstruction (Fig 1a–c). The first operation is the Norwood procedure. Usually performed when the baby is around 1–2 weeks old, it commonly utilises a modified Blalock–Taussig (BT) shunt or a direct conduit from the right ventricle to the pulmonary artery (Sano technique) Reference Nayak and Booker3 . This is combined with reinforcement of the aorta, by connecting the main pulmonary artery body to the aorta, with or without a patch (Fig 1d). The second stage is the Glenn operation that occurs when the infant is 2–6 months old. At this point, the Norwood shunt has often been outgrown and can now be ligated. Reference Nayak and Booker3 In this operation, an anastomosis between the superior vena cava (SVC) and right pulmonary artery is created (Fig 1e). At age 1–5 years, the third and final stage of Fontan palliation is completed when the pulmonary arteries are of sufficient size and strength, and can allow for a low pulmonary vascular resistance (PVR). Reference Nayak and Booker3
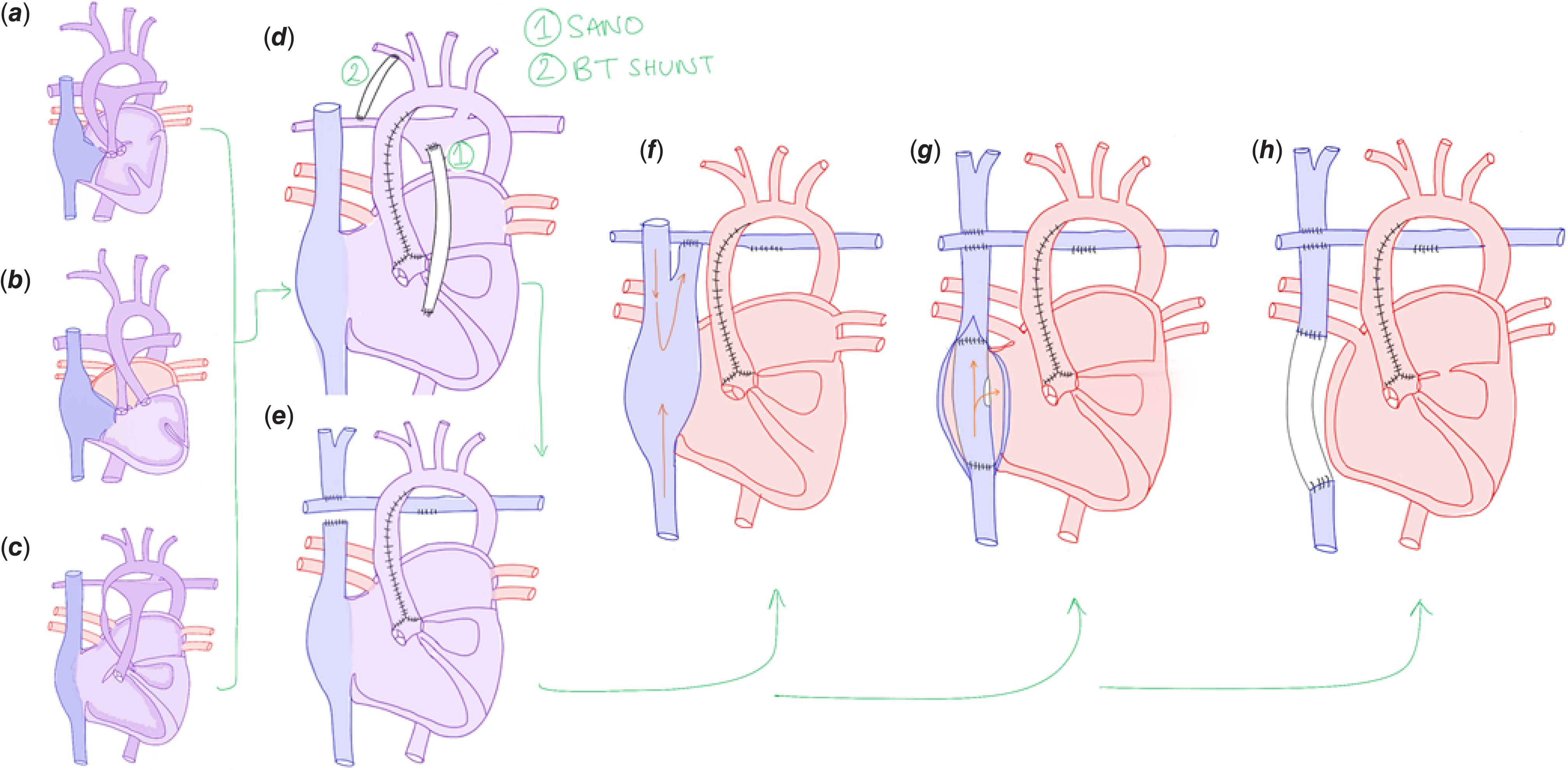
Figure 1. Diagram of anatomical considerations and procedures including variations of the Fontan. Cardiac malformations such as tricuspid atresia (a), double inlet left ventricle with L-looped ventricles and transposition of the great arteries (b), and hypoplastic left heart syndrome, (c) can be converted to a Fontan circulation, which is normally completed in stages. The first operation, the Norwood procedure (d), is usually performed at 1–2 weeks, with the aim of providing adequate pulmonary blood flow, lowering pulmonary vascular resistance, and lowering ventricular load. This involves creating an aortopulmonary connection via a Sano (d.1) or Blalock–Taussig (d.2) shunt. The Glenn procedure (e) is the second stage and involves creating an anastomosis between the superior vena cava and the right pulmonary artery. The last stage of palliation is the Fontan procedure. Three variations exist including the “classical” atriopulmonary connection (f), the lateral tunnel total cavopulmonary connection (g), and the extracardiac total cavopulmonary connection (h) where, depending on the patient, the conduit may be fenestrated and connected to the lateral atrial wall. There is ongoing discussion about the benefits of fenestration. The atriopulmonary connection is rarely performed now due to increased complications such as thrombus formation, arrhythmias, and atrial dilatation.
Three different variations of the Fontan procedure exist, evolving originally from the ventriculisation of the right atrium, otherwise known as the atriopulmonary connection (Fig 1f). This classical technique was associated with turbulent flow in the right atrium, due to the collision of streams from the superior and inferior vena cava (IVC). This decreased pulmonary perfusion and increased the incidence of late complications including thrombus formation, arrhythmias, and atrial dilation. Reference Baum, De Souza, Cronin and Maus4 The “lateral tunnel” total cavopulmonary connection (TCPC) modification was then developed (Fig 1g). Reference Baum, De Souza, Cronin and Maus4,Reference Backer, Deal, Kaushal, Russell, Tsao and Mavroudis5 This involves creating a tunnel within the right atrium using an intra-atrial patch and suturing the SVC directly to the right pulmonary artery. As a result, excessive atrial dilation is avoided, improving pulmonary flow and reducing thromboembolic risk. However, extensive atrial suture lines remain a risk for arrhythmia development. Reference Baum, De Souza, Cronin and Maus4,Reference Backer, Deal, Kaushal, Russell, Tsao and Mavroudis5
The “lateral tunnel” was then modified into the extracardiac total cavopulmonary connection (ECC) (Fig 1h), with the aim of reducing this risk. This operation bypasses the right atrium entirely through the insertion of an extracardiac conduit between the IVC and the right pulmonary artery. Reference Backer, Deal, Kaushal, Russell, Tsao and Mavroudis5 There is ongoing debate about whether the ECC or “lateral tunnel” is better, with consideration of factors such as risk of arrhythmia development, risk of thromboembolic events and optimal haemodynamics. Reference Backer, Deal, Kaushal, Russell, Tsao and Mavroudis5
Preoperative assessment
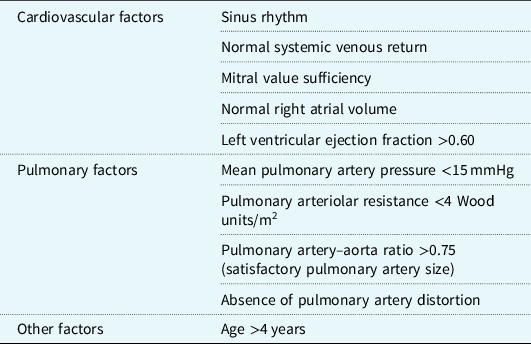
With risk minimisation at the core of preoperative assessment, in 1977, Choussat et al as cited by Stern proposed a list of “ten commandments”; Reference Choussat, Fontan, Besse, Anderson and Shinebourne6,Reference Stern7 a series of optimal characteristics of Fontan candidates (Table 1). Since 1977, these criteria have been amended and revised by individual centres with the aim of improving procedure success rates. These amendments reflect advances in preoperative assessment, intraoperative surgical techniques and postoperative management. Reference Stern7 Preoperative investigations for Fontan candidates can include echocardiography (ECHO), cardiac catheterisation (CC), and cardiac magnetic resonance (CMR). Reference Ait-Ali, De Marchi and Lombardi8
Table 1. Selection criteria for optimal surgical candidates for Fontan procedure from Choussat et al (1977) as seen in Stern (2010)Reference Choussat, Fontan, Besse, Anderson and Shinebourne6,Reference Stern7
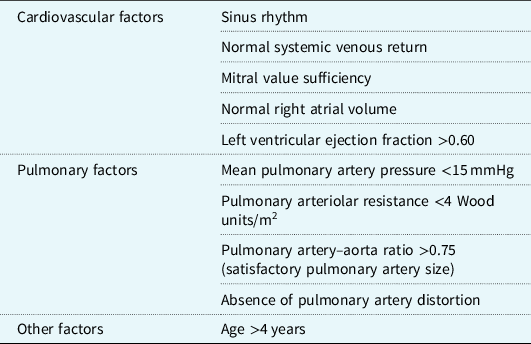
Table modified from Stern (2010).Reference Stern7
Cardiac catheterisation
Full haemodynamic cardiac catherisation enables the measurement of various cardiac and respiratory parameters. Data collected through CC includes transpulmonary gradient (TPG); end-diastolic pressure; PVR; mean pulmonary artery pressure (mPAP); and common atrial pressure. This is enabled through a set of direct calculations for blood flow and resistance as well as estimates and direct measurements. Reference Mohammad Nijres, Murphy, Diab, Awad and Abdulla9 However, due to the added risk of cancer from ionising radiation, Reference Kleinerman10 there has been a growing interest for substitution of CC with less invasive methods. Reference Mohammad Nijres, Murphy, Diab, Awad and Abdulla9,Reference Brown, Gauvreau and Powell11
Despite this, a recent cohort study aiming to compare CC with ECHO found that patients requiring systemic to pulmonary arterial or left SVC embolisation were not detected by ECHO. In a minority of patients, significant new diagnostic information was revealed through CC, which may have resulted in postponement or exclusion of a Fontan procedure. Reference Mohammad Nijres, Murphy, Diab, Awad and Abdulla9
Cardiac magnetic resonance
Whilst angiographical catherisation allows for a therapeutic approach in selected cases, its invasive nature makes it less ideal for patients without a need for its therapeutic effect. Accordingly, there is mounting interest in the less-invasive CMR. CMR may be limited by intrinsic factors such as spatial resolution, lack of patient cooperation, and contraindications in patients with metallic devices. However, it offers high diagnostic value through assessment of cardiovascular morphology, ventricular function, and blood flow, through phase contrast flow, cine imaging and magnetic resonance angiography. Reference Schicchi, Secinaro and Muscogiuri12 CMR is more commonly used for post-surgical care but it has been adapted to provide valuable insight in pre-surgical planning. Reference Fogel, Khiabani and Yoganathan13 To benefit from the radiation-free environment offered by CMR as well as the therapeutic advantage of CC, an algorithm has been proposed which seeks to eliminate the need for CC in some patients, Reference Ait-Ali, De Marchi and Lombardi8 illustrated in Figure 2.

Figure 2. A summary of algorithm for preoperative assessment of Fontan candidates reprinted from Ait-Ali et al Reference Ait-Ali, De Marchi and Lombardi8. ECHO=Echocardiogram; CMR=Cardiac magnetic resonance; CC=Cardiac Catherisation. Candidates for Fontan are assessed with a medical history, ECHO, and CMR imaging. If any of the underlying criteria (1–9) is present, the patient should be considered for CC. This minimises the exposure of all Fontan candidates to radiation in CC.
Computed tomography, angiography, and echocardiography
Another similar protocol was based on conducting non-invasive assessments including ECHO, medical history taking, and non-invasive angiography on patients for whom Fontan was previously excluded by CC was able to correctly identify all the patients who were deemed not fit for the Fontan operation by cardiac catheterisation. Reference Prakash, Khan, Hardy, Torres, Chen and Gersony14 Accordingly, a coherent and precise system should be considered before considering phasing out CC based on institutional experience and strength in imaging modalities.
Postoperative acute management
Postoperative ICU management after the Fontan procedure involves monitoring, respiratory and cardiac support, and medication infusions to aid recovery. In Fontan patients specifically, a left atrial line and pulmonary artery catheter are utilised to monitor the TPG and to enable sufficient pulmonary perfusion. Reference Jones15 Oxygen saturations should be near normal in non-fenestrated Fontan patients; hence, hypoxaemia may indicate complications caused by lung pathology preventing gas exchange, the presence of pulmonary arteriovenous or venovenous malformations or increased oxygen demand such as in sepsis or reduced cardiac output (CO). Reference Jones15
Fontan management protocols
Several protocols (Table 2 ) have been published with the aim of lowering incidence of postoperative and prolonged pleural effusions (PPE) and hospital length of stay (LOS) in post-Fontan patients. These protocols, focusing on standardised diuretic regimens, afterload reduction, anticoagulation, low-fat diets, fluid restriction, and continuous oxygen therapy, have improved outcomes for Fontan patients by reducing hospital LOS and chest tube drainage. Reference Sunstrom, Muralidaran and Gerrah2,Reference Cava, Bevandic, Steltzer and Tweddell16–Reference Lagergren, Jensen, Beaven and Goudar19 Whilst these results are promising, further multicentre randomised controlled trials are needed to provide stronger evidence for the use of a standardised approach and to determine which approach is best to manage Fontan patients in the postoperative period.
Table 2. Summary of Fontan-specific postoperative management protocols

ACEi = Angiotensin-converting enzyme inhibitor; AT3 = Antithrombin 3; ICU = Intensive care unit; IV = Intravenous; LOS = Length of stay; PICC = peripherally inserted central catheter; PO = Per oral; POD = Postoperative day; TPN = Total parenteral nutrition; LOS = Length of stay.
* Duration and discharge regimen not specified.
† Oral medications continued as outpatient for 2 weeks and weaned as per patient’s cardiologist.
‡ Titration based on patient tolerance and echocardiogram findings of systemic atrioventricular valve regurgitation.
Antibiotic prophylaxis
It is difficult to recommend standardised antibiotic prophylaxis protocols for paediatric cardiac surgery patients due to microbial sensitivity variability. Furthermore, antibiotic pharmacokinetics in paediatric patients undergoing cardiopulmonary bypass (CPB) is not fully known. Reference Jaworski, Kansy, Dzierzanowska-Fangrat and Maruszewski20 A suggested schedule has been proposed covering preoperative nasal swab screening and treatment of Staphylococcus aureus with Mupirocin, intraoperative prophylaxis with cefazolin with vancomycin added if MRSA colonisation is present, and continuation of antibiotics for 48 hours postoperatively (prolonged if delayed sternal closure or ECMO or VAD). However, more research is needed to determine and investigate an optimal protocol. Reference Jaworski, Kansy, Dzierzanowska-Fangrat and Maruszewski20
Risk factors for prolonged ICU stay
Predictors for early Fontan failure include heterotaxia, low preoperative arterial oxygen saturations, and systemic right ventricle. Reference Ovroutski, Sohn and Barikbin21 Lower oxygen saturations, higher haemoglobin, and increased pulmonary arterial pressure (PAP) are all associated with prolonged ICU LOS, Reference Sasaki, Dykes and Sosa22 suggesting that interventions to optimise the pulmonary circulation prior to surgery may improve postoperative outcomes. Postoperative complications such as PPE, chylothorax, ascites and infection, Reference Ono, Burri and Balling23 AKI, Reference Algaze, Koth, Faberowski, Hanley, Krawczeski and Axelrod24 cardiac abnormalities (such as cardiac arrest, tachyarrhythmias) or the need for unplanned intervention (such as cardiac catheterisation or reoperation), ventilatory and circulatory support, prolonged chest tube drainage, renal replacement therapy (RRT), or blood product transfusion are all associated with increased LOS. Reference Sasaki, Dykes and Sosa22 Considering these risk factors, an evidence-based protocol to safely step down Fontan patients from ICU for ward-based management would be useful.
Ventilation
Early extubation after paediatric cardiac surgery is considered safe and is associated with shorter ICU and hospital lengths of stay (LOS). Reference Alghamdi, Singh and Hamilton25–Reference Ono, Georgiev and Burri27 It is also associated with improved haemodynamics such as improved mean arterial pressure (MAP), Reference Mutsuga, Quiñonez and MacKie26,Reference Ono, Georgiev and Burri27 reduced mean pulmonary arterial pressure (mPAP) and increased CO, Reference Lofland28 lower inotrope scores, Reference Mutsuga, Quiñonez and MacKie26 lower fluid balance, and less fluid administration, Reference Mutsuga, Quiñonez and MacKie26,Reference Ono, Georgiev and Burri27 and shorter duration of mechanical ventilation and chest tube duration with no adverse effects on morbidity and mortality. Reference Alghamdi, Singh and Hamilton25,Reference Mutsuga, Quiñonez and MacKie26,Reference Ono, Georgiev and Burri27 Pain relief, whilst important for recovery, can affect ventilation. Use of excessive opioids should especially be avoided in Fontan patients due to the potential for respiratory depression, which may worsen CO due to reduced venous return and pulmonary blood flow.
Circulation
After the Fontan procedure, the patients are at risk of Low Cardiac Output Syndrome (LCOS). This may be due to multiple factors such as CPB, inadequate preload, elevated PVR, increased afterload, arrhythmias, thrombosis or obstruction in the systemic veins, or ventricular diastolic/systolic failure.
Simple, conservative measures to improve CO should be implemented such as elevating the head of the bed, bending the knees, and strict fluid management to ensure adequate central venous pressure and venous return. Medical management includes inodilators; agents that increase ventricular contractility and reduce afterload. Both dobutamine and milrinone are shown to be equally effective in reducing the risk of LCOS; however, evidence suggests milrinone has greater efficacy in afterload reduction. Reference Cavigelli-Brunner, Hug and Dave29
Whilst inotropes are useful postoperatively, they will not increase CO to the same level as biventricular patients, as the main determinant of CO in the Fontan circulation is the level of PVR, not ventricular contractility. Reference Gewillig and Brown30 Optimising perfusion pressure instead of focusing on maximising CO, by favouring low-dose dopamine agonists and lusitropic agents (milrinone) has been shown to improve mortality and reduce ventilation times in paediatric cardiac surgery patients. Reference Hosseinpour, van Steenberghe and Bernath31 Fenestration improves CO at times of high PVR by allowing diversion of blood from the “bottle-neck” of the circuit to the heart. Reference Gewillig and Brown30 More research into short-term benefits of fenestration is needed as whilst it also reduces PAP and reduces the risk of PPE, a recent meta-analysis suggests no significant difference in intrahospital mortality and LOS compared to non-fenestrated patients. Reference Bouhout, Ben-Ali, Khalaf, Raboisson and Poirier32
Although ACE inhibitors are used in some postoperative management algorithms, there is insufficient evidence supporting their use in Fontan patients with normal ventricular function in both the short and long-term. Reference Wilson, Iyengar and D’Udekem33
Pulmonary vascular resistance
Alongside milrinone, patients with high pulmonary pressures can be given inhaled Nitric Oxide (iNO), a pulmonary vasodilator. Reference Cai, Su and Shi34 Further investigation is warranted into the use of iNO with high-flow nasal oxygen therapy, as evidence shows this may reduce the duration of postoperative intubation, pleural draining, and LOS. Reference Tominaga, Iwai and Yamauchi35
Sildenafil, a PDE-5 inhibitor and Ambrisentan, a hepatically metabolised endothelin receptor antagonist, can also be used postoperatively to dilate the pulmonary vasculature. Small trials have shown improved haemodynamics, lower use of inotropes, and reduced intubation times with Sildenafil use post-Fontan procedure. Reference Tunks, Barker and Benjamin36,Reference Giordano, Palma and Poli37 Similarly, Ambrisentan administration lowers B-type natriuretic peptide (BNP) levels, Fontan pressures, and PVR. Reference Hill, Maharaj, Li, Thompson, Barker and Hornik38
Arrhythmias and thrombus formation
Whilst postoperative arrhythmias can occur after the Fontan procedure, novel surgical techniques that avoid handling and scarring of the atria reduce this risk. Early postoperatively, atrioventricular (AV) valve regurgitation is a predictor for arrhythmia and after 2 weeks postoperatively, older age at Fontan and high mPAP become predictors. Reference Sinha, Zurakowski and He39 Predictors for late arrhythmia include atriopulmonary Fontan or advanced age at operation. Reference Pundi, Pundi and Johnson40 Management includes pacing and anti-arrhythmic medications; however, the proarrhythmic effect of 1C anti-arrhythmics and amiodarone-induced thyrotoxicity should be carefully considered when prescribing these medications in Fontan patients. Reference Moore, Cordina, McGuire and Celermajer41 For patients with recurrent atrial arrhythmia, first catheter ablation, and then conversion surgery should be attempted. Up to 25% of Fontan patients require epicardial pacing to manage late arrhythmias. Reference Pundi, Pundi and Johnson40
Thrombosis risk is highest immediately after Fontan, with prolonged central venous line use, uncontrolled warfarin prophylaxis, and lower fraction of inspired oxygen (FiO2) after the procedure all increasing risk. Reference McCrindle, Manlhiot and Cochrane42 Thrombosis risk is ongoing after discharge with venous thromboembolism incidence reported as high as 22% at 2 years post-Fontan procedure. Reference Monagle, Cochrane and Roberts43 Because of this, thromboprophylaxis with warfarin or aspirin is continued long-term, with a meta-analysis showing little difference in efficacy between the two (9 and 8.6%, respectively, versus 18% in non-anticoagulated Fontan patients). Reference Alsaied, Alsidawi, Allen, Faircloth, Palumbo and Veldtman44 Further evidence is needed to evaluate the use of direct oral anticoagulants in Fontan patients.
Pleural effusions and chylothorax
Postoperative development of pleural effusions and chylothorax is a result of increased capillary permeability after CPB, systemic venous hypertension, prolonged mechanical ventilation, and alterations in fluid and electrolyte balance hormones. Intraoperative trauma to the thoracic duct and lymphatics can also contribute. Both can be diagnosed with a plain chest radiograph and pleurodesis with laboratory analysis. Management involves drainage, usually via an intercostal tube and drain, and control of fluid overload with diuretics. Reference Cava, Bevandic, Steltzer and Tweddell16,Reference Talwar, Agarwala, Mittal, Choudhary and Airan45 In patients with chylothorax with minimal drainage, conservative management with a low-fat diet and failing this, total parenteral nutrition and somatostatin analogues may be effective. Failure to resolve, or excessive drainage, may require surgical intervention. Reference Milonakis, Chatzis and Giannopoulos46
Acute kidney injury
Risk factors for AKI after the Fontan procedure include preoperative AV valve regurgitation greater than mild, preoperative PVR, bypass time, renal perfusion pressure (RPP), and peak inotrope score on postoperative day 0. Reference Esch, Salvin, Thiagarajan, Del Nido and Rajagopal47 Lower RPP is associated with a higher risk for stage 2/3 injury. Reference Algaze, Koth, Faberowski, Hanley, Krawczeski and Axelrod24 Management of AKI after paediatric cardiac surgery involves maintaining CO, avoiding potassium-based fluids and nephrotoxic drugs, maintaining fluid balance and serum electrolytes, and ensuring adequate nutrition. Reference Singh48 Renal replacement therapy (RRT) can be used if patients continue to experience worsening kidney function. Reference Gulati and Bagga49
Long-term outcomes and management
Survival outcomes
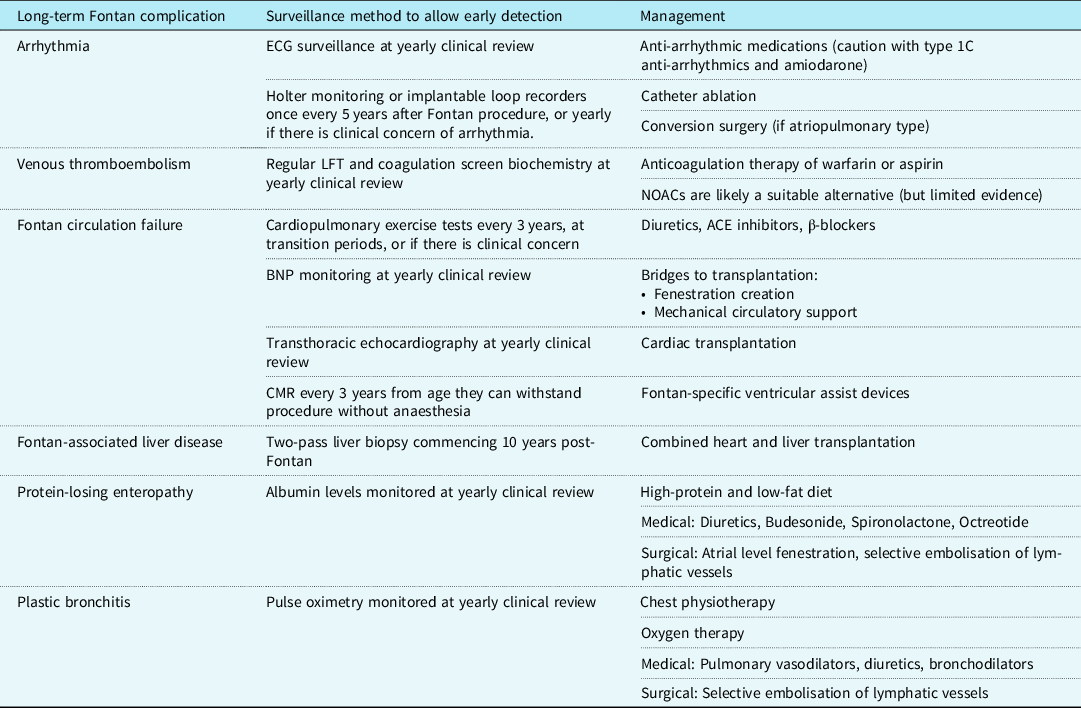
Today, short-term survival after the Fontan procedure is reported to be as high as 98%. Reference Downing, Allen and Glatz50 Medium-term outcomes are also promising, with transplant-free survival rates as high as 95% at 5 years. Reference Downing, Allen and Glatz50 The estimated survival for a Fontan patient operated on today is as high as 85% at 30 years. Reference Rychik, Atz, Celermajer and Deal1,Reference Schilling, Dalziel and Nunn51 Complications that significantly increase mortality include arrhythmia, protein-losing enteropathy (PLE), and venous thromboembolism. Reference Rychik, Atz, Celermajer and Deal1 A summary of long-term complications, recommended surveillance measures for early detection, and methods of management are summarised in Table 3.
Table 3. The surveillance and management of late complications of a Fontan circulation
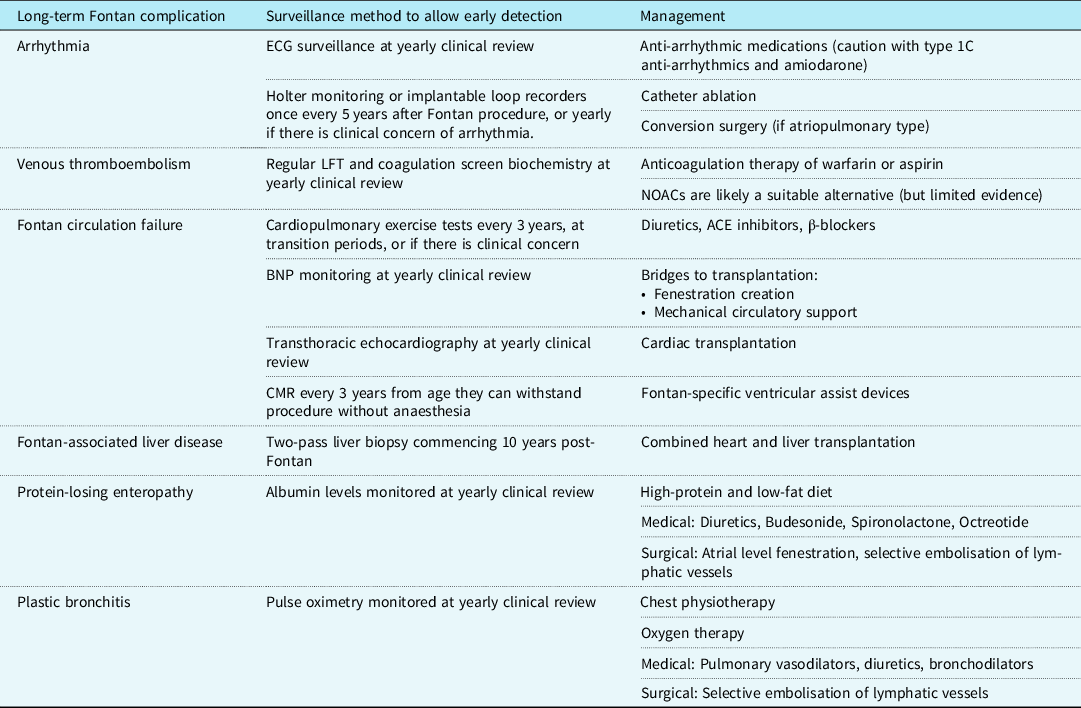
BNP = B-natriuretic peptide; CMR = Cardiac magnetic resonance; ECG = Electrocardiogram; LFT = Liver function tests; NOAC = Novel oral anticoagulants.
Table 4. List of abbreviations

Fontan circulation failure
Symptoms of heart failure are reported in up to 40% of adults with a Fontan circulation. Reference Piran, Veldtman, Siu, Webb and Liu52 There are two forms of heart failure seen; the typical ventricular pump dysfunction and the physiological consequence of a chronically reduced CO paired with persistently elevated systemic venous pressures. Heart failure signs and symptoms are a major cause of admissions in Fontan circulation patients. Reference Rychik, Atz, Celermajer and Deal1 BNP, cardiopulmonary exercise testing and echocardiography may be used to track significant changes indicating Fontan failure. Reference Atz, Zak and Mahony53
Initial management of Fontan failure involves comprehensive imaging, including the use of transthoracic echocardiography and CMR. This is particularly to rule out the presence of an obstruction, which would be managed by cardiac catheterisation. Reference Ovroutoski, Ewert, Alexi-Meskishvili, Peters, Hetzer and Berger54 Other management options include diuresis to lower filling pressures and alleviate symptoms. Standard heart failure management such as ACE inhibitors are commonly used in Fontan patients: however, their benefit requires further investigation. Reference Anderson, Breitbart and McCrindle55 There is limited evidence supporting cardiac resynchronisation therapy, which is shown to have mixed results. Reference Dubin, Janousek and Rhee56
As a bridge to transplantation for failing Fontan patients, both fenestration creation and mechanical circulatory support can be utilised. Transplantation is often the last resort for the failing Fontan resistant to other forms of management. Reference Poh, Cochrane and Galati57 Early transplant referral is beneficial for improving survival. Alongside transplant scarcity, there are no clear guidelines on transplant suitability for Fontan patients, particularly regarding the preferred timing, indications, and contraindications. Additionally, Fontan transplant candidates are more likely to be listed at the lowest urgency status compared to non-congenital heart disease candidates and have a higher cardiovascular mortality when waiting for a transplant. Reference Everitt, Donaldson and Stehlik58 Evidence suggests better survival outcomes in paediatric Fontan transplant recipients, with paediatric 1-year survival after transplant as high as 89% compared to 65% in adults. Reference Simpson, Pruitt and Kirklin59,Reference Murtuza, Hermuzi and Crossland60 Other last-resort options include ventricular assist devices, Reference Horne, Conway, Rebeyka and Buchholz61 with Fontan-specific devices being a promising field for future management of Fontan patients.
Fontan-associated liver disease
Patients with a Fontan circulation have increased risk for Fontan-associated liver disease (FALD) due to physiological consequences of a Fontan circulation, such as elevated systemic venous pressures causing hepatic congestion and reduced CO causing hepatic hypoperfusion. Incidence of severe liver fibrosis on liver biopsy is as high as 68% in post-Fontan patients. Reference Munsterman, Duijnhouwer and Kendall62 Surveillance of Fontan patients for FALD should occur around 10 years after surgery; however, the methods of this surveillance are contraversial. Recent evidence suggests that liver enzymes are rarely elevated in stable FALD and that ultrasound may fail to detect smaller lesions due to the heterogenous parenchyma in a Fontan liver. Consequently, cross-sectional imaging is increasingly used for liver lesion screening with liver biopsy and elastography included in assessment. Reference Emamaullee, Zaidi and Schiano63
Recommendations to prevent liver complications include avoidance of hepatotoxins including alcohol, amiodarone, and obesity. There is limited evidence suggesting heart transplant alone may stabilise FALD. Reference Emamaullee, Zaidi and Schiano63 Combined heart and liver transplantation (CHLT) is a rare procedure with promising supporting evidence. Both Mayo Clinic and the University of Pennsylvania have recently compared their experiences of heart transplantation and CHLT in Fontan patients; both groups found similar survival rates, but increased rates of acute rejection in the heart transplantation alone cohort. Reference Wong, Gandhi and Daly64,Reference Zhao, Wang and Kamoun65 Thus, Fontan patients with a failing Fontan circulation and evidence of FALD should be considered for CHLT, which may be more beneficial than heart transplantation alone. Reference Emamaullee, Zaidi and Schiano63
Protein-losing enteropathy
Protein-losing enteropathy (PLE) is a significant complication of Fontan palliation, occurring in up to 12% of Fontan patients. PLE is a loss of lymphatic fluid into the gastrointestinal tract as a consequence of chronically increased systemic venous pressures paired with inflammation of the gastrointestinal tract. This causes loss of high-protein lymph rich fluid, resulting in hypoalbuminaemia. Reference Rychik, Atz, Celermajer and Deal1 PLE presents with symptoms of diarrhoea, peripheral oedema, ascites, and growth failure. It usually presents in the first 10 years after the Fontan procedure. The gold standard for diagnosis is 24-hour stool collection with measurement of elevated α1 anti-trypsin levels. Routine surveillance including serum albumin levels can improve early detection of PLE. Reference Zentner, Celermajer and Gentles66 Conservative management for PLE involves dietary changes such as a high-protein and low-fat diet. However, most evidence suggests that dietary manipulation alone does not alleviate symptoms. Reference Johnson, Driscoll and O’Leary67 Medical management involves diuretics to decrease fluid overload and corticosteroid treatment with budesonide, with some centres utilising spironolactone and octreotide. Finally, a surgical intervention used to alleviate PLE symptoms includes atrial level fenestration, Reference John, Johnson, Khan, Driscoll, Warnes and Cetta68 which can be performed transcatheter. A novel catheterisation based technique involving the selective embolisation of gastrointestinal lymphatic vessels appears promising in a small number of cases; further evidence is needed to clarify its use in PLE management. Reference Itkin, Piccoli and Nadolski69
Plastic bronchitis
Plastic bronchitis (PB) is another presentation of Fontan failure, which usually occurs within the first few years after the operation in up to 5% of Fontan patients. Reference Rychik, Atz, Celermajer and Deal1,Reference Schumacher, Singh, Kuebler, Aprile, O’Brien and Blume70 Both PB and PLE have a similar aetiology, as PB is caused by lymphatic and bronchial communications leaking lymphatic fluid into the pulmonary system. Reference Dori, Keller and Rome71 Patients present with casts in the pulmonary bronchi comprised of inflammatory debris. Presenting symptoms include breathlessness and coughing fits, often associated with hypoxia. Reference Rychik, Atz, Celermajer and Deal1,Reference Avitabile, Goldberg, Dodds, Dori, Ravishankar and Rychik72 Accordingly, routine surveillance of oxygen saturations may promote early detection. Reference Zentner, Celermajer and Gentles66
Management of PB is aimed to improve symptoms and airway clearance. Oxygen provision, inhaled tissue plasminogen activator, pulmonary vasodilators, diuretics, and bronchodilators can be useful pharmacological agents to alleviate symptoms in Fontan patients. Intensive chest physiotherapy is also beneficial. Reference Avitabile, Goldberg, Dodds, Dori, Ravishankar and Rychik72 Similar to PLE, embolisation of the lymphatic system is a promising management option. Reference Dori, Keller and Rome71
Medications used in Fontan patients
The majority of Fontan patients take two or more medications long-term. Commonly utilised medications include antithrombotic agents, ACE inhibitors, cardiac glycosides, and diuretics. However, there is significant variation in medication use in Fontan patients across different centres. Reference Anderson, Breitbart and McCrindle55 Furthermore, there is limited evidence on the effectiveness of these medical therapies in Fontan patients.
ACE inhibitors and β-blockers are sometimes used in Fontan patients but lack supporting evidence. Reference Anderson, Breitbart and McCrindle55 Evidence has shown no benefit in Fontan patients using enalapril; a recent review concluded that ACE inhibitors were overprescribed in Fontan patients with limited rationale. Reference Kouatli, Garcia, Zellers, Weinstein and Mahony73,Reference Wilson, Iyengar and Winlaw74 Additionally, a large multicentre study with a small subset of patients with functionally single ventricle circulations found no benefit with carvedilol use. Reference Shaddy, Boucek and Hsu75
Hepatotoxic agents such as amiodarone, high dose acetaminophen, NSAIDs, and certain antibiotics should be avoided in Fontan patients (see Table 4 for abbreviations). Reference Emamaullee, Zaidi and Schiano63 Risk-benefit assessment before use of nephrotoxic medications such as ACE inhibitors and diuretics is beneficial, due to the association of a Fontan circulation with increased risk of long-term kidney dysfunction. Reference Zafar, Lubert and Katz76 For female patients, the use of oestrogen-based contraceptives should be avoided due to the increased risk of venous thromboembolism. Progesterone only hormonal contraceptives or barrier methods are a safer alternative. Reference Thorne, MacGregor and Nelson-Piercy77
Quality of life
The quality of life of Fontan patients is complicated by the interplay of physical, psychological, and social challenges. According to a recent meta-analysis, the health-related quality of life of a Fontan patient is lower than the general population. Reference Marshall, D’Udekem and Sholler78 Both the functional limitations of a Fontan circulation and the psychological impact of patients anticipating Fontan failure are contributors. Older age at Fontan procedure may lead to worse psychological outcomes. Reference Marshall, D’Udekem and Sholler78 More evidence assessing health-related quality of life in older Fontan patients and exploring moderating factors and variables is essential.
Conclusion
Outcomes for Fontan circulation patients have improved drastically since the operation was first pioneered, partly due to advancements in preoperative assessment, postoperative protocols, and long-term management. The long-term management of the Fontan patient involves the prevention and treatment of late complications such as Fontan circulation failure, PLE, PB, and FALD. The definitive management for late complications is transplantation; highlighting a need for improvements and standardisation in post-Fontan transplantation criteria. Further evidence assessing preoperative and postoperative protocols would be valuable for standardising the perioperative management of Fontan patients.
Acknowledgements
None.
Financial support
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Conflict of interest
None.
Ethical standards
This article does not contain any studies with human participants or animals performed by any of the authors.