Introduction
The order Ascocerida Kuhn, Reference Kuhn1949, comprises relatively small cephalopods that occur in the upper Middle Ordovician to uppermost Silurian strata (Flower, Reference Flower1941). Ordovician ascocerids occur in Laurentia (e.g., Sweet, Reference Sweet1958; Kesling, Reference Kesling1961, among others, see references in Aubrechtová and Meidla, Reference Aubrechtová and Meidla2016), Baltica (Strand, Reference Strand1933; Sweet, Reference Sweet1958; Kröger, Reference Kröger2007, Reference Kröger2013), and Avalonia (Evans, Reference Evans1993). In the Silurian, the ascocerids had a wide geographical distribution (Holland, Reference Holland1999), having been reported from more regions in Baltica and from Perunica (references cited in Aubrechtová and Meidla, Reference Aubrechtová and Meidla2016). The ascocerids achieved a relatively high diversity and abundance in specific regions such as Bohemia and Gotland, where they are especially well preserved, as documented in the classic studies by Barrande (Reference Barrande1877) and Lindström (Reference Lindström1890). However, they are usually rare or very rare and their diversity in a time slice is rather low or moderate (e.g., less than 10% of the cephalopod fauna in the upper Silurian of the Prague Basin, Manda and Frýda, Reference Manda and Frýda2010).
Until now, no ascocerids have been reported from southern latitudes of Gondwana, and the group has been considered to be mainly restricted to warm water carbonate platforms (e.g., Mutvei, Reference Mutvei2012; Kröger, Reference Kröger2013; Aubrechtová and Meidla, Reference Aubrechtová and Meidla2016). Exceptions are Bohemia, where ascocerids occur in a temperate carbonate platform (Manda and Kříž, Reference Manda and Kříž2006), and Avalonia, which was a tropical but siliciclastic shelf (Hewitt and Watkins, Reference Hewitt and Watkins1980).
The conch of ascocerids consists of two growth stages, which differ in shell morphotype: the first consists of a slender cyrtocone, whereas the last formed one is a brevicone. Both parts were separated by shell truncation, being the cyrtocone decollated. This radical change in conch morphology, which includes also differences in the suture line as well as in the siphuncle structure, is of particular taxonomic significance (Furnish and Glenister, Reference Furnish and Glenister1964). The two stages are rarely found joined together in the fossil record. Evidence of attachment of the ontogenetic stages was found in two species of Ascoceras Barrande (Lindström in Holland, Reference Holland1999).
In addition, in many examples in the Swedish material, although not attached, both deciduous and mature stages of the shell are present with matching ornament. The presence of the shell truncation implies a shift in the mode of life from the slender cyrtocone with straight sutures to the relatively streamlined mature form, with its series of camerae above the extended body chamber providing effective buoyancy (Kesling, Reference Kesling1961; Furnish and Glenister, Reference Furnish and Glenister1964).
Working with a small and deficiently preserved collection, we studied three specimens assigned to ascocerids, and two more doubtfully considered as belonging to the same group. Surprisingly, they were preserved with the juvenile and mature parts of the conch joined together, indicating a probable subadult ontogenetic stage. They were collected, along with some undetermined orthoceratoids, in the Itacurubí Group of Paraguay. This is a lower Paleozoic clastic succession deposited on the western margin of the large Paraná Basin (Fig. 1) that includes, in ascending order, the Eusebio Ayala, Vargas Peña, and Cariy formations (Harrington, Reference Harrington1972). Our collection comes from the two older units (Fig. 2).

Figure 1 (1) Paleogeographic map of Gondwana, location map of the Paraná Basin during Late Ordovician–early Silurian times, (2, 3) geological map of the studied area (3) with fossil localities indicated.
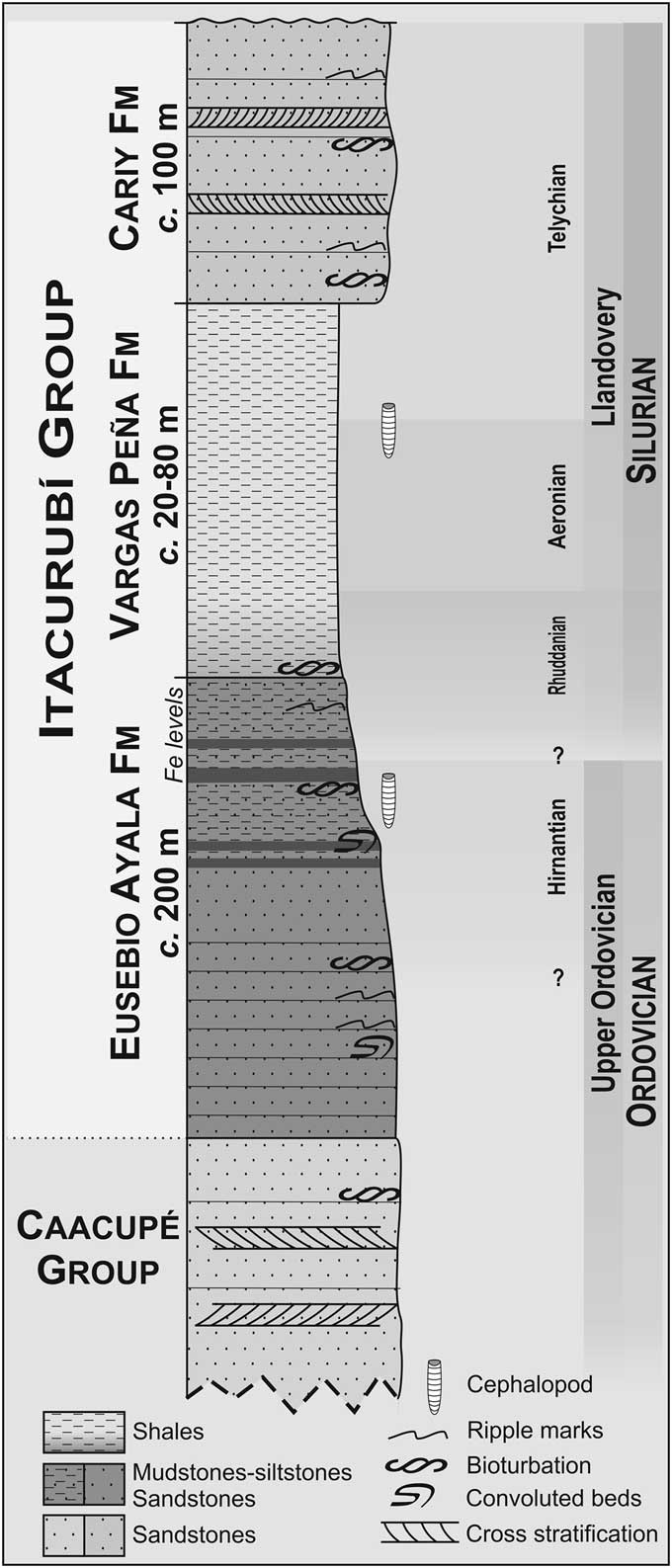
Figure 2 Stratigraphic scheme of the Itacurubí Group with facies and cephalopod occurrences indicated.
Numerous taxa have been reported from these strata (mostly from the Vargas Peña and Cariy formations), including brachiopods (e.g., Harrington, Reference Harrington1950; Benedetto, Reference Benedetto2002; Benedetto et al., Reference Benedetto, Halpern and Galeano Inchausti2013); graptolites and trilobites (e.g., Harrington, Reference Harrington1950; Baldis and Hansen, Reference Baldis and Hansen1980; Boucot et al., Reference Boucot, de Melo, Santos Neto and Wolff1991; Uriz et al., Reference Uriz, Alfaro and Galeano Inchausti2008; Cingolani et al., Reference Cingolani, Uriz, Alafaro, Tortello, Bidone and Galeano Inchausti2011; Tortello et al., Reference Tortello, Uriz, Alfaro, Cingolani, Bidone and Galeano Inchausti2012; Alfaro et al., Reference Alfaro, Uriz, Cingolani, Tortello, Bidone and Galeano Inchausti2013), trace fossils (e.g., Galeano Inchausti and Poiré, Reference Galeano Inchausti and Poiré2006), chitinozoans (e.g., Wood and Miller, Reference Wood and Miller1991), miospores (e.g., Steemans and Pereira, Reference Steemans and Pereira2002), tentaculitids (Godoy Ciguel, Reference Godoy Ciguel1988), conularids (Babcock et al., Reference Babcock, Gray, Boucot, Himes and Siegele1990), tabulate corals, bivalves, gastropods, and hyolithids (Harrington, Reference Harrington1950). However, the presence of cephalopods was reported and illustrated briefly as Orthocerida by Dyck (Reference Dyck1991), who suggested with doubts the presence of the families Orthoceratidae, Geisonoceratidae, and Dawsonoceratidae. This is the first report of the presence of ascocerids in southern Gondwana.
Stratigraphy and geological setting
The large Paraná Basin (Fig. 1) extends through southern Brazil, Paraguay, Uruguay, and northern Argentina (Milani and de Wit, Reference Milani and de Wit2007). In Paraguay, the lower Paleozoic rocks crop out irregularly along the western margin of the Ypacaraí graben (Degraff et al., Reference Degraff, Franco and Orué1981), 35 km east of the city of Asunción, in a series of isolated clay quarries, and around the town of Eusebio Ayala. Surveyed sections include the Santa Elena and the San Fernando quarries, both located a few kilometers northwest of the town of Itaguá (Fig. 1). The Itacurubí Group overlies conformably the nonfossiliferous Caacupé Group (Harrington, Reference Harrington1950) of inferred Ordovician age, which consists of conglomerates and sandstones, probably estuarine or fluviodeltaic in origin (Benedetto et al., Reference Benedetto, Halpern and Galeano Inchausti2013). The base of the Eusebio Ayala Formation (200 m thick) marks the onset of a transgressive event and consists of reddish micaceous sandstone grading upward to fine-grained fossiliferous sandstone and siltstone (Fig. 2). Ripple surfaces are common on the top of the sandstone beds that also display internally wavy and lenticular lamination (Benedetto et al., Reference Benedetto, Halpern and Galeano Inchausti2013). Traces of Skolithos are very abundant, suggesting lower intertidal–shallow subtidal depositional settings (Ciguel et al., Reference Ciguel, Rosler and Clerici1987). The overall upward-fining trend of the Eusebio Ayala and Vargas Peña formations records the transition from shoreface to platform deposits, and the pale grey fossiliferous claystones of the Vargas Peña Formation represent the maximum flooding. The lithology of the Cariy Formation indicates a shoreline progradation culminating the Itacurubí Group succession (Benedetto et al., Reference Benedetto, Halpern and Galeano Inchausti2013). The age of the two stratigraphically younger formations is considered early Silurian based on the monograptid graptolites and palynomorph assemblages. The Eusebio Ayala Formation is believed to be at least partially of Hirnantian age, based on the recent discovery of a graptolite association including Normalograptus persculptus Salter in Huxley and Etheridge, Reference Huxley and Etheridge1865 (Alfaro et al., Reference Alfaro, Uriz, Cingolani, Bidone and Galeano Inchausti2010, Reference Alfaro, Uriz, Cingolani, Tortello, Bidone and Galeano Inchausti2013; Cingolani et al., Reference Cingolani, Uriz, Alafaro, Tortello, Bidone and Galeano Inchausti2011), and the reassignation of some brachiopods to the species Arenorthis paranaensis Benedetto, Halper, and Galeano Inchausti, 2013 and Plectothyrella? itacurubiensis Benedetto, Halper, and Galeano Inchausti, 2013.
The Itacurubí Group is locally covered by either Cretaceous–Tertiary rocks (Asunción Group) or Quaternary alluvial deposits. Benedetto et al. (Reference Benedetto, Halpern and Galeano Inchausti2013) noticed that even though there are no recognized glacial sediments exposed in the studied area, a ~50 m thick succession of tillites underlying sandstones and shales bearing Late Ordovician–early Silurian palynomorphs has been reported from drill cores (Figueredo, Reference Figueredo1995). This succession is probably stratigraphically older than the Eusebio Ayala Formation.
Materials and methods
Drawing on material collected during a field trip in 2008 by N.J.U., M.B.A., and J.C.G.I., we distinguished at least three specimens that can be certainly assigned to Ascocerida and at least two more that are considered with doubts to belong to the same group. It is probable that the poor preservation of most specimens is related to their unusually thin septa (Furnish and Glenister, Reference Furnish and Glenister1964). Studied specimens were collected from the Eusebio Ayala Formation (Hirnantian?–Llandovery stages) in the Santa Elena Quarry (MLP 35794, 35796, 35797) and from the Vargas Peña Formation (Llandovery Stage) in the San Fernando Quarry (MLP 35795, 35798). The material from the Eusebio Ayala Formation is preserved three-dimensionally, although MLP 35796 is somewhat flattened. By contrast, the material from the Vargas Peña Formation is highly compressed and without visible sutures or evidence of the siphuncle. Specimen MLP 35794 (Fig. 3) is an internal mold with both the inflated and the cyrtoconic parts attached. During its manipulation, however, the specimen fell apart, revealing the siphuncular foramen and the shape of the shell cross section. Specimen MLP 35796 (Fig. 4.3–4.5) is poorly preserved, but it also has joined cyrtocone and ascocerid parts of the shell. It is composed of the internal mold of the brevicone and the beginning of the cyrtocone on one surface of the rock (which is very friable) and the external mold of the brevicone with a portion of the cyrtocone preserved as an impression with cavities left by siphuncular segments on its counterpart. To see more details, the internal mold of the brevicone was prepared with a needle, and the matrix was nearly entirely removed, freeing both the internal and the external molds. Unfortunately, the internal mold was broken during manipulation due to its high fragility, before it was photographed. Specimen MLP 35795 (Fig. 4.1, 4.2) is highly compressed and consists of two parts (that fit along the plane of stratification). In one of them the specimen is slightly convex, whereas the other is slightly concave, representing the external mold. This specimen has both the breviconic and the cyrtoconic parts adjacent in the sample, although they are slightly displaced. The rest of the cephalopod specimens are not described here because they do not resemble ascocerids (e.g., because of their shell shape, one of the specimens could be interpreted as a juvenile part, but it shows several closely spaced ‘sutures’ that do not correspond to those known in ascocerids). Specimens MLP 35797 and 35798 are assigned to ascocerids with doubts. The first is composed of three parts: two external molds within the rock and a free internal mold of a very incomplete phragmocone that partially fits with them (Fig. 4.6, 4.7). Specimen MLP 35798 is preserved in the same way as MLP 35795, flattened and without details.
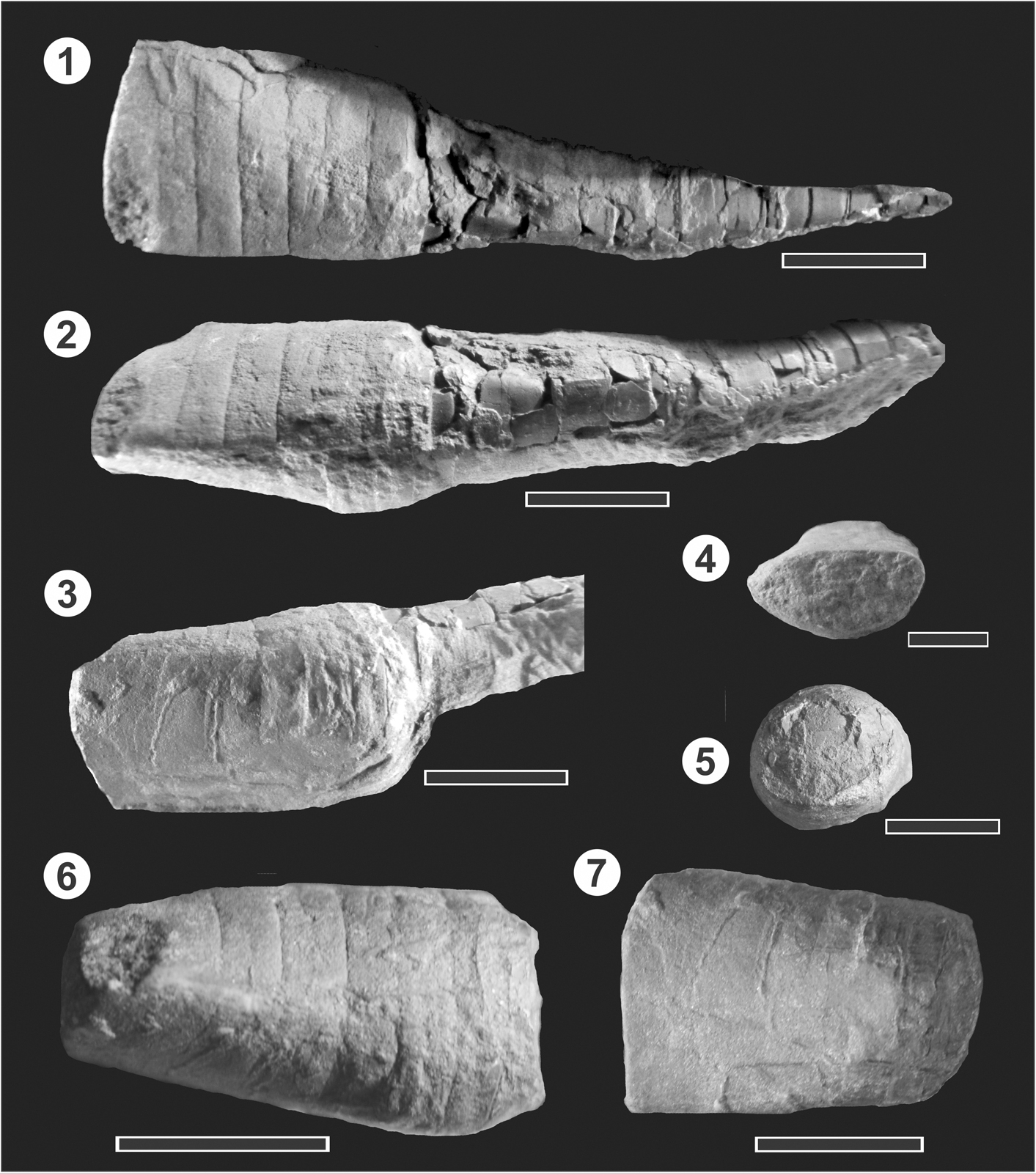
Figure 3 (1–7) ?Probillingsitinae Flower, Reference Flower1941 gen. et sp. indet. from the Paraná Basin. MLP 35794 from the Eusebio Ayala Formation, Santa Elena Quarry, Hirnantian?–early Llandovery. (1) Dorsal view of the complete specimen before breakage. Note straight sutures in both parts of the conch; (2) left lateral view of the complete specimen; (3) lateroventral view of the brevicone, showing the loss of the sutures and the lines that make the interpretation difficult; (4) anterior view of the brevicone, with the depressed form partially due to compaction; (5) posterior view of the brevicone after breakage, showing the siphuncle foramina and the subcircular section (orientation in the lamina with venter upward); (6) left lateral view of the brevicone showing the sutures and the adapically inflated shape; (7) ventral view of the brevicone. Note the loss of the suture lines and the poor preservation of the right lateral side (that was partially covered with sediment). Scale bars = 1 cm.
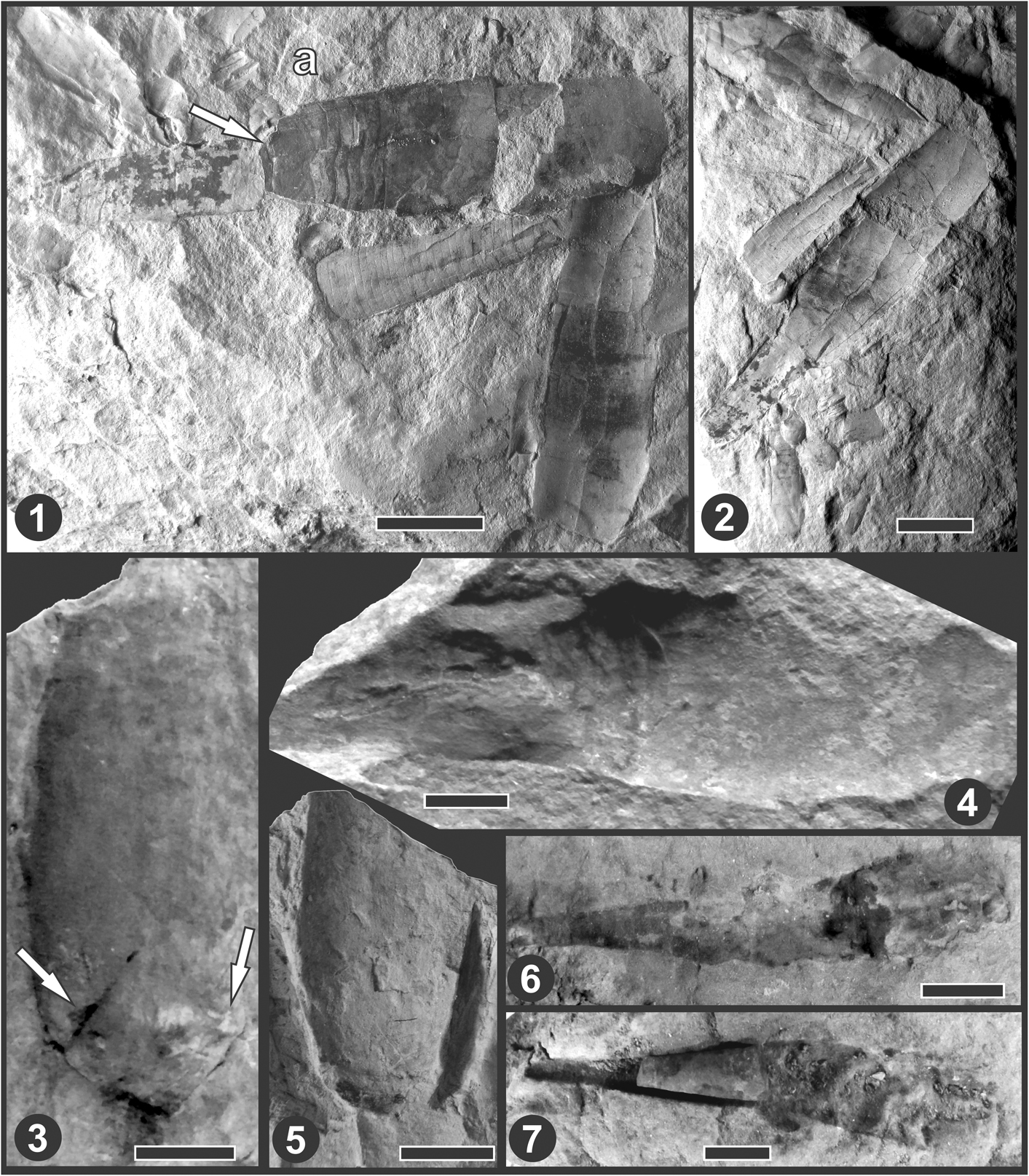
Figure 4 (1–7) ?Probillingsitinae Flower, Reference Flower1941 gen. et sp. indet. from the Paraná Basin. (1, 2) MLP 35795 from the Vargas Peña Formation, San Fernando Quarry, Llandovery Stage; (1) rock with specimens in a positive, showing 35795 consisting in both parts of the conch slightly displaced. Arrow points to the proposed septum of truncation; (2) counterpart of (1); (3–5) MLP 35796 from the Eusebio Ayala Formation, Santa Elena Quarry, Hirnantian?–early Llandovery stages; (3) internal mold of the brevicone with the beginning of the cyrtocone attached before removing the matrix and breakage; arrows point to possible sutures, posterior one could correspond to the septum of truncation, the other could be related to a sigmoid suture; (4) external mold of the brevicone showing the probable septum of truncation and part of the cyrtocone attached, with siphuncle visible as cavities of the segments; (5) external mold of the brevicone of the other side (after removing the internal mold) showing the mark of the possible septum of truncation and other lines and fractures that make the interpretation difficult; (6, 7) MLP 35797 from the Eusebio Ayala Formation, Santa Elena Quarry, Hirnantian?–early Llandovery stages; (6) external mold of a probable isolated cyrtocone, representing the juvenile portion of an ascocerid; (7) counterpart of (6) with part of the internal mold hosted with the external mold. Note the straight sutures. Scale bars = 1 cm.
Some specimens were prepared manually using needles under a binocular microscope. They were photographed with a Canon Power Shot SX 20 digital camera. All measurements were taken using digital calipers with a resolution of 0.1 mm.
Repository and institutional abbreviation
The material from Paraguay is housed under the prefix MLP in the paleontological collections of the Museo de Ciencias Naturales de La Plata (Buenos Aires, Argentina).
Systematic paleontology
Class Cephalopoda Cuvier, Reference Cuvier1797
Subclass Multiceratoidea Mutvei, Reference Mutvei2013
Superorder Nautilosiphonata Mutvei, Reference Mutvei2015
Order Ascocerida Kuhn, Reference Kuhn1949
Family Ascoceratidae Barrande, Reference Barrande1867
Subfamily ?Probillingsitinae Flower, Reference Flower1941
Genus indet. species indet.
Description
The best preserved specimen (MLP 35794) is an internal mold 60.3 mm long, consisting of the cyrtocone attached to the brevicone, both parts incomplete (Fig. 3). The cyrtocone is 39 mm long and the brevicone is 21.3 mm long. The brevicone has a slightly convex dorsum and an adapically inflated venter that becomes gradually lower toward the anterior part (Fig. 3.2, 3.6). Adorally, the cross section is rather depressed, in part because it was flattened during diagenesis (Fig. 3.4); it is 15.8 mm in width and 7.15 mm in height. Adapically, the cross section is nearly circular (Fig. 3.5), with 12.2 mm in width and 10.7 mm in height, and the siphuncle foramina is 1 mm in diameter (10% of that of the shell), being slightly displaced to the ventral side. The apical angle of the brevicone (measured with lateral diameters) is 9.6º. Seven straight suture lines are visible along the dorsum (Fig. 3.1), with a distance between them of ~2–2.5 mm. They are not always visible on the sides and the venter, especially those adorally located (Fig. 3.2, 3.3, 3.7). A number of fractures or undetermined lines are present, which interfere with the sutures (Fig. 3.7), making interpretation difficult. It is not clear whether they were straight all along the margins and have been obscured during diagenesis, or whether the shape of the sutures was, for example, sinuous on the venter, as they are not visible there, and they cannot be followed along their trajectories. The adapical-most suture seems to be entire, and maybe one or two more also; however, the other sutures (adorally) are not visible. Ventral sutures are at similar distances as the dorsal ones at equivalent positions. The cyrtocone is exogastric and lacks the apical-most part most probably due to breakage (Fig. 3.1, 3.2). There are 12 straight suture lines along its length, and the camerae length is between 2.5 and 4 mm, although not regularly. The anterior cross section is 12.7 mm in width and 9 mm in height. The adapical cross section is ~6.5 mm in height, but it is not possible to estimate the width because it is covered by the rock and badly preserved. It is not possible to observe the siphuncular structure, just the septal foramina of the last formed septum. The apical angle measured considering the dorsoventral diameter (height) is 3.7º.
The specimen MLP 35795 is very flattened and poorly preserved (Fig. 4.1, 4.2) but also has both parts of the conch joined together. The brevicone is 38 mm long, with an adoral width of 11 mm and an adapical width of ~9 mm, at the position of the probable septum of truncation (see arrow), which can be seen as a thick suture or rim. The cyrtocone is 22.5 mm long, with an adoral width of ca. 7.5 mm and an adapical width of ca. 3 mm. The mature brevicone is adorally broken and displaced transversally by 1.5 mm. At a position 6 mm from the junction between both parts there are six transverse and sinuous lines, 1 mm apart, that cross the surface (it is not possible to know whether it is the dorsal or the ventral side, or even the lateral). We interpret these lines not as suture lines but as ornamentation because they are closely space and their irregularity is notable (Fig. 4.1). There are no visible suture lines on the cyrtocone either, although some lines are distinguished that could also be ornamentation. The fact that the proportions of both parts of the conch are similar to those of MLP 35794 reinforces our interpretation of the specimen as an ascocerid.
The specimen MLP 35796 is also poorly preserved (Fig. 4.3–4.5). The internal mold of the brevicone is flattened and attached to a small portion of the cyrtocone, interpreted as the filling of the adoral segments of the siphuncle. In the counterpart, there is the external mold of the brevicone and part of the internal mold of the cyrtocone, diagonally broken, and with the cavities left by the siphuncle, although very poorly preserved and hardly visible (Fig. 4.4). There seem to be at least eight segments of the siphuncle, with an apparent suborthochoanitic structure, but the adapical three are slightly displaced. The adapical end of the cyrtocone is not preserved because of breakage. The diameter of the siphuncle at a shell width of 14 mm is 2 mm at the connecting rings level and 1.5 mm at the septa foramina. The brevicone is 43 mm long, ~18 mm wide adorally, and ~14 mm wide adapically (Fig. 4.3, 4.5). There are no visible sutures and no ornamentation, with the exception of an adapical line that was interpreted as the basal septum (Fig. 4.3, right arrow). In addition, adorally of said line, there is another line that resembles a fracture that crosses the surface with a central inflection toward the peristome (Fig. 4.3, left arrow). Its interpretation is difficult, but we consider it could be related to some sinuous suture. As mentioned, the internal mold of the brevicone was uncovered by removing the sediment with a needle, and the opposite surface could be seen. Unfortunately, it broke due to its fragility before photos could be taken. However, there were no obvious suture lines in that face, but some undetermined lines were identified. In the surface that is seen in Figure 4.4, there are also some undetermined lines, especially on lateral sides. The interpretation is that it could be the ventral side and those lines may represent part of the sutures that have inflections toward the venter. Figure 4.5 is the external mold of the opposite side of the brevicone, in which both mentioned lines and others difficult to interpret can be seen.
Finally, there are two more specimens that are interpreted as ascocerids with doubts. The specimen MLP 35797 (Fig. 4.6, 4.7) is preserved as two external molds and one internal mold of part of the cyrtocone, with straight sutures. The part preserved as internal mold is shorter than the external molds (Fig. 4.6, 4.7). The maximum length of the external mold is 45 mm, whereas the fragment preserved as an internal mold is 14 mm long. The apical diameter of the internal mold is 3 mm and the adoral diameter is 6 mm. In the external, mold the adoral diameter is 8 mm. The apical angle would be rather high, 9º, although the bad preservation could affect the measurements. The small fragment of the phragmocone has at least 8 camerae, each 1.5 mm long. The siphuncle was not observed. The cross section is subcircular.
The specimen MLP 35798 is preserved similarly to MLP 35795, flattened and without details. There seems to be a change in the angle of expansion interpreted as the junction between the brevicone and the cyrtocone. Otherwise, the proportions correspond to those of the other specimens. It was not illustrated due to its very poor preservation.
Materials
Five specimens, MLP 35794, 35796, and 35797 from the Santa Elena Quarry (Hirnantian?–early Llandovery stages) and MLP 35795 and 35798 from the San Fernando Quarry (Llandovery Stage), Paraná Basin, Paraguay.
Remarks
The described specimens have been assigned to Ascocerida because of the recognition of both parts of the ascocerid conch: the slender cyrtocone, interpreted as the deciduous conch, and the brevicone, which would be the mature part. The differences in the preservation modes and visible features make comparisons difficult, preventing interpretation of all specimens as a single taxon. The same limitations apply to comparisons with species from other regions. The presence in most cases of the two parts of the conch is assumed to be related to a subadult stage of development of the respective specimens. Regarding the classification into the subfamily Probillingsitinae, the impossibility of seeing the entire course of sutures to test the presence of lacunose septa imposes doubts about this. The straight and regularly spaced sutures in the dorsal side of MLP 35794 suggest lacunose septa are improbable. The specimen MLP 35796 is dubious regarding this. We do not exclude the possibility of having more than one genus. The change in the angle of expansion suggests that our material does not coincide with Hebetoceratidae Flower, Reference Flower1941 or Choanoceratidae Miller, Reference Miller1932. The poor to very poor preservation of the majority of the material prevents us from determining the specimens at a generic level.
The cephalopod fauna from the Upper Ordovician of County Tyrone (North Ireland, peri-Laurentia for Late Ordovician–early Silurian times) includes some ascocerids that Evans (Reference Evans1993) described as undetermined Billingsites Hyatt, 1884 and Probillingsites Foerste, Reference Foerste1928. In the remarks of Probillingsites, the author proposed that his specimen could be intermediate between Probillingsites and Schuchertoceras Miller, Reference Miller1932 because of the presence of not only more than one normal septum but also two ascoceroid septa. We think that our specimens could also represent intermediate forms between the relatively primitive ascoceroids and the more complex ones. Interestingly, ascocerids described by Evans (Reference Evans1993) occur in coeval (or slightly older) strata as specimens studied here. The relatively high number of sutures seen in MLP 35794, being straight and regularly space dorsally but uncertain laterally and ventrally, is a rare feature difficult to interpret. The subadult ontogenetic stage could also be influencing the particular patterns observable. The septa in the mature form could be incompletely developed. The fact that the septa of ascocerids were very thin is probably the first cause of the state of preservation where the sutures are badly preserved.
Discussion
Paleoecology
The truncation process is the natural removal, in life, of the apical portion of the shell (Furnish and Glenister, Reference Furnish and Glenister1964). The cyrtoconic or juvenile part of the ascocerids has been considered by some authors to have undergone periodic truncation (e.g., Lindström, Reference Lindström1890; Kesling, Reference Kesling1961; Flower, Reference Flower1963; Furnish and Glenister, Reference Furnish and Glenister1964; Aubrechtová and Meidla, Reference Aubrechtová and Meidla2016). Kesling (Reference Kesling1961) exposed a very detailed description of the steps from the hatchling of the organism to the complete adulthood, enumerating the different stages and their respective buoyancy conditions, to explain the mode of life of Billingsites Hyatt, Reference Wood and Miller1884 (Kesling, Reference Kesling1961, fig. 6).
Dzik (Reference Dzik1984) refuted the suggestion of ontogenetic truncation, pointing out that postmortem truncation near the base of the body chamber would have been facilitated by the shape of the shell. Holland (Reference Holland1999) mentioned that if the cyrtoconic stage had remained attached to the mature shell, it would have been a disadvantage for forward movement and a serious impediment to backward propulsion. Thus, he accepted the fact of natural truncation at the mature stage, with the apex of the shell appropriately sealed off. However, Holland (Reference Holland1999) is against the idea of a repeated truncation of the cyrtoconic portion of the shell. Once the mature shell had developed, the cyrtocone would have been redundant and there would be little advantage in the loss of only part of it. He considered far more likely that cases of partially truncated cyrtocones were the result of breakage. Kröger (Reference Kröger2007) considered, as well, the possibility of the truncation as a single event in Parvihebetoceras Kröger, Reference Kröger2007.
A single-event truncation and the loss of the apical part of the phragmocone were documented also in Silurian brevicone oncocerids (Stridsberg, Reference Stridsberg1985), while periodic truncation was recorded only in the lower Paleozoic straight-shelled orthocerid Sphooceras Flower, Reference Flower1962 (Turek and Manda, Reference Turek and Manda2012). We agree with Holland (Reference Holland1999) and consider that truncation probably occurred once through the life of the individual ascocerids, when the mature shell was completely developed. We interpret our material as representing a subadult stage of development, during which both parts of the conch were still joined together. At least three specimens of our collection have both parts of the conch attached. In addition, the cyrtoconic portions are, if not nearly complete, long enough to be interpreted as postmortem cases of breakage and not partially truncated shells (see Figs. 3.1, 3.2, 4.1, 4.4). If periodic truncation would have been the rule, the near completeness of these specimens may be highly improbable. Therefore, we considered our specimens as evidence to support the ‘single-event truncation’ in ascocerids.
Westermann (Reference Westermann1999) has not only assumed as a fact the process of truncation in ascocerids, and even that it occurred just once when the maturity was achieved, but furthermore, like Kesling (Reference Kesling1961), analyzed the different buoyancy-related scenarios in which truncation occurred, considering as a possibility that the truncation was a voluntary act, comparable to that in some septate gastropods that truncate their shells during a controlled behavior (cf. Denton in Westermann, Reference Westermann1999).
The scenario proposed by Westermann (Reference Westermann1999) is one in which, once the adult body chamber was complete, the subadult cephalopod migrated to shallow waters where it began to produce the anticlastic septa and the expanded siphuncle. The new mature chambers would remain flooded until truncation, which occurred after the sealing of the siphuncle, making the animal negatively buoyant. After that, a rapid emptying of the new chambers (potentially during several days) allowed recovering the neutral buoyancy and changing the attitude from subvertical to horizontal. We propose that our specimens were in the stage previous to, or during, the formation of the ascoceroid septa, already in shallow waters, when they died and were buried before truncation occurred.
The mode of life of ascocerids has been discussed extensively. A strong consensus exists in that the adult form (ascoceroid) was an efficient horizontal swimmer, with the dorsally located camerae providing effective buoyancy (e.g., Furnish and Glenister, Reference Furnish and Glenister1964; Crick, Reference Crick1988; Holland, Reference Holland1999; Westermann, Reference Westermann1999). Mutvei (Reference Mutvei2012) recently discussed the mode of life of ascocerids based on Choanoceras Lindström, Reference Lindström1890, comparing it with two discosorid genera and concluding that the orders are phylogenetically related. On the basis of the siphuncular structure and the muscle attachment scars, he proposed that adult ascocerids were specialized for vertical (probably diurnal) migrations by feeding on zooplankton, and their vertical migrations were mostly propelled by using buoyancy regulation.
This agrees with Kröger (Reference Kröger2003), who considers two types of buoyancy regulation: Nautilus style and Sepia style. Adult ascocerids had the Sepia style, with the existence of a siphuncle as a thin surface at the ventral, open, side of the chambers (Kröger, Reference Kröger2003, p. 40), which allowed for rapid buoyancy changes. The cyrtocone, or juvenile, part of the conch had a typical Nautilus type siphuncle, implying that no rapid buoyancy changes were possible. According to the nomenclature of Kröger (Reference Kröger2008) for morphological traits, the juvenile could be termed ‘augustocone,’ and the mature form could be termed ‘euorthocone.’ The first term refers to slender, often small conchs with a narrow siphuncle, often having wide septal spacing and lacking cameral and endosiphuncular deposits. They are interpreted as cephalopods with low energy needs and adapted to environments with low food availability that lived as vertical migrants in the free water column (Kröger, Reference Kröger2008). The second type comprises those with a comparatively high angle of expansion, wide siphuncle that may be expanded within the chambers, and massive cameral and endosiphuncular deposits. Although ascocerids lacked deposits, the relative position of the phragmocone and body chamber acted as a control of the attitude of the animal. Euorthocones are interpreted as cephalopods with a comparatively energy-intensive lifestyle; they were potentially also active vertical migrants. The comparatively large siphuncular surfaces are evidence for an ability to actively regulate buoyancy. Following Kröger (Reference Kröger2008), buoyancy regulation in this type of cephalopod was much more cost intensive than that in angustocones, demanding more active searching for high-energy food. A demersal lifestyle of euorthocones is inferred from this evidence (Kröger, Reference Kröger2008). Therefore, adult ascocerids may have been demersal horizontal swimmers with capacity for diurnal vertical migrations. Westermann (Reference Westermann1999) considered the juvenile ascocerids to have had a subvertical orientation, very sluggish forward and downward swimming in deep waters (i.e., mesopelagic), and therefore to have been planktonic or demersal. After truncation, the ascocerid individuals became efficient subhorizontal swimmers. Due to the thin and sigmoid septa, and the siphuncle with expanded segments, they would have been extremely weak against water pressure, therefore living in very shallow waters (in particular, he points to a reef environment). On the contrary, Mutvei (Reference Mutvei2013) considered ascocerids to have lived in relatively deep waters, based on the Choanoceras siphuncle, which has thick connecting rings, and assumed the records in shallow depositional environments to be reworked. Considering the high fragility of the ascocerid shells, it is more likely that the real habitat of the adult ascocerid was a shallow one.
Following Westermann (Reference Westermann1999), when the subadult cephalopod migrated to shallow waters and began to produce anticlastic septa, as the new mature chambers remained flooded until truncation occurred, the attitude of the animal would be subvertical. Our specimens represent, therefore, a very particular and short-lived stage in the life cycle of ascocerid cephalopods, as demonstrated by the scarcity of those stages in the known global fossil record until today.
The material here described comes from two different formations and environmental settings. As depicted in the ‘Stratigraphy and geological setting’ section, the Eusebio Ayala Formation is interpreted as a lower intertidal–shallow subtidal depositional setting (Ciguel et al., Reference Ciguel, Rosler and Clerici1987), whereas the Vargas Peña Formation represents the maximum flooding stage. The complete specimen (MLP 35795) deposited in the Vargas Peña Formation, preserved as a flattened mold, could have suffered some transportation from shallower waters or could represent the stage in which the individual had formed the body chamber of the mature stage but still was in deep waters, previous to the migration to shallower environments where it would form the sigmoid septa and the truncation would occur. However, we have no data to decide between these options.
The two complete specimens (MLP 35794, 35796) collected in the Eusebio Ayala Formation are most likely autochthonous fauna in a very shallow environment. They represent the stage during the formation of ascoceroid septa, before truncation. It is worth noting that a reef environment, as proposed by Westermann (Reference Westermann1999) as natural for ascocerids, is absent in the Itacurubí Group of the Southern Paraná Basin. In fact, the accompanying fauna is more typical of cold climates, according to the paleogeographic position of the studied area.
Paleobiogeography
As previously mentioned, until now, all ascocerid faunas had been reported from low to mid (e.g., Perunica) paleolatitudes (see Aubrechtová and Meidla, Reference Aubrechtová and Meidla2016). In fact, water temperature was considered to have played a role as a dispersion barrier for ascocerids (Kröger, Reference Kröger2013). Our records demonstrate that this was not the case, since during the Late Ordovician–early Silurian times, the southern Paraná Basin was situated in high paleolatitudes, at around 60ºS (Fig. 5).
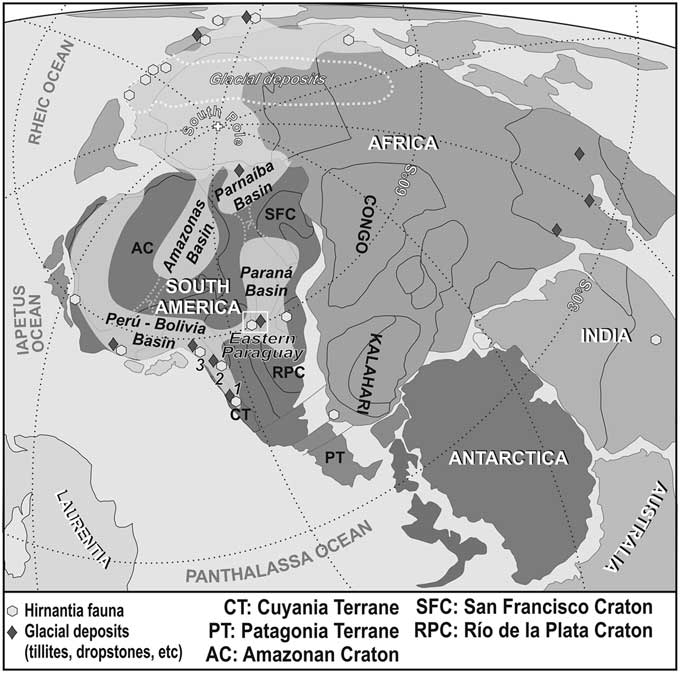
Figure 5 Gondwanan perspective of the paleogeography of the Late Ordovician-early Silurian times (modified from Cocks, Reference Cocks2001). Note the location of the studied eastern Paraguay sector of the Paraná Basin. 1= Argentine Precordillera; 2 = northwestern Argentina; 3 = southern Bolivia.
Brachiopod faunas associated with our ascocerid material show faunal affinities with North African brachiopods (Benedetto et al., Reference Benedetto, Halpern and Galeano Inchausti2013). However, no cephalopod record is known throughout the Upper Ordovician–Silurian interval of North Africa. In addition, although not formally studied yet, considering the available bibliography and scattered collections at different institutions (personal observation), Argentine Late Ordovician–early Silurian cephalopod collections lack ascocerids (e.g., Cecioni, Reference Cecioni1953, Reference Cecioni1965; Aceñolaza and Beresi, Reference Aceñolaza and Beresi2002).
Adult ascocerid animals could not migrate through shelves because of their demersal mode of life combined with the need for shallow water due to the fragility of their septa (Westermann, Reference Westermann1999). Embryonic conchs of ascocerids are poorly known, with only a single apex described by Lindström (Reference Lindström1890) of Parascoceras Miller, Reference Miller1932 and another one of a probable Parvihebetoceras (Kröger, Reference Kröger2007). Considering the size and form of the ascocerid embryonic conchs (see Furnish and Glenister, Reference Furnish and Glenister1964, fig. 192, 1a, 1b; Kröger, Reference Kröger2007, fig. 4c), it is probable that the early posthatching was planktonic, as was the juvenile stage (following Westermann [Reference Westermann1999] and Kröger [Reference Kröger2008]). In that case, the ascocerids from Paraguay may have been transported by currents during the planktonic stage of their ontogeny (e.g., Manda, Reference Manda2008), from low to high paleolatitudes. At the end of the Ordovician, and for the first time in the Phanerozoic, when the postglacial high stand developed, an extensive marine flooding through the intracratonic basins occurred. The immigration of ascocerids to the Paraná Basin could have been related to this episode, but the migratory route is less clear due to the absence of records of the group in adjacent basins that would allow following the migration pathway.
It is worth noting that with increasing knowledge about lower and middle Paleozoic southern Gondwana cephalopod faunas, current hypotheses regarding nonammonoid cephalopod distribution patterns have started to be reviewed (Cichowolski et al., Reference Cichowolski, Rustán and Uriz2018). Recently, Cichowolski and Rustán (Reference Cichowolski and Rustán2017) reported the presence of bactritids from the Malvinokaffric Realm in Argentina. This Devonian paleobiogeographic unit was considered to be characterized, along with other features, by a noticeable scarcity of cephalopods (Boucot and Racheboeuf, Reference Boucot and Racheboeuf1993). The bactritids were along a rather diverse association including orthocerids, oncocerids, pseudorthocerids, and, especially important here, lamellorthoceratids. This last group was thought, like the ascocerids, to be restricted to warm-water paleoenvironments (e.g., Kröger, Reference Kröger2008). Therefore, the absence of some groups in high latitudes, as well as the supposed rarity of cephalopods within the Malvinokaffric Realm, is more probably related to the poor knowledge about the fossiliferous record from these regions than to a real pattern distribution. For the case of ascocerids reported here, more material should improve our understanding of their immigration to distant realms by the end of Ordovician times. This would allow us to discuss whether they could have developed a stable population, or whether our specimens represent just a fortuitous and isolated arrival to these latitudes where they could not establish and expand through time.
Acknowledgments
Financial support was provided through projects PIP-CONICET-647 and UNLP 11/547. The Ministerio de Obras Públicas y Comunicaciones, Subsecretaría de Minas y Energía, República del Paraguay, provided invaluable logistical assistance during our field work. A. Siccardi (Universidad Nacional de La Plata, Argentina) is acknowledged for taking new photos of some of the specimens. A. Folguera (University of Buenos Aires) helped with the English language. MC is grateful to D. Evans (Natural England, UK) for useful discussions about ascocerid systematics. M. Aubrechtová (Charles University, Prague), an anonymous reviewer, and the editors M. Yacobucci and J. Jin are warmly thanked for their comments and suggestions that greatly improved an early version of this manuscript. This is the contribution R-245 to the Instituto de Estudios Andinos ‘Don Pablo Groeber’ (IDEAN-CONICET).







