INTRODUCTION
Historically, pelvic bones close to the bladder have been used as a surrogate to verify set-up accuracy and reproducibility, and planning margins in excess of 1·5 cm required to account for potential inter-fraction variability, particularly in the bladder size.Reference Henry, Stratford and McCarthy 1 The bladder and its adjacent organs at risk (OAR), specifically the rectum and small intestine, are mobile deformable structures. A change in the position, shape and size of these adjacent structures has been shown to directly influence the shape and position of the bladder within the pelvis.Reference Lutkenhaus, Visser, DeJong, Hulshof and Bel 2 – Reference Thariat, Aluwini and Pan 8 With the advent of volumetric image-guided radiotherapy (IGRT), soft tissues can now be visualised immediately before treatment delivery, and it has become widely accepted that skeletal imaging alone is inadequate for assuring accurate, conformal treatment delivery.Reference Yee, Parliament, Rathee, Ghosh, Ko and Murray 5 , Reference Thariat, Aluwini and Pan 8 – Reference Hutton, Leadbetter, Jain and Baker 11 Coupled with the facility to apply on-line corrections before treatment, volumetric IGRT has presented the potential to adopt more complex, adaptive treatment techniques.
Adaptive radiotherapy
Prospective adaptive bladder radiotherapy, commonly described as plan-of-the-day, is the technique increasingly favoured to individualise and improve patient treatment and is the focus of United Kingdom and International clinical trials.Reference Yan, Lockman and Brabbins 12 – 17 A library of plans created for each patient takes account of anticipated inter-fractional changes due to bladder filling.Reference Fouroudi, Wong and Kron 18 , Reference Lotz, Pos and Hulshof 19 Cone beam computed tomography (CBCT) is acquired immediately before treatment delivery to select the daily treatment plan whereby the planned target volume (PTV) is providing optimal bladder dose coverage. Adaptive techniques aim to facilitate increased treatment conformity and dose reduction to surrounding structures,Reference Hutton, Callender and Baker 20 with reduced dose to OAR considered a major benefit of IGRT and adaptive radiotherapy.Reference Henry, Stratford and McCarthy 1 The requirement for adaptive radiotherapy and the associated imaging is based on:
-
∙ The limitations of a ‘snap shot’ single planning scan that assumes reproducible anatomy for treatment.
-
∙ The inadequacy of bladder volume alone to reflect shape and position of the treated structure.
-
∙ The visualisation of three-dimensional images to facilitate matching.
In a recent, unpublished audit at the authors’ clinic, 20% of bladder volumes were 50 cc largerReference Hutton, Callender and Baker 20 at treatment than on the planning computed tomography (CT), which has been shown to correlate with a bladder wall displacement of up to 1 cm.Reference Lotz, van Herk, Betgen, Pos, Lebesque and Remeijer 21 , Reference Pickett, Roach and Verhey 22 This variation accounts for a significant proportion of a standard 1·5 cm elliptical margin, before any additional effect of shape deformity and intra-fraction motion are encountered.
On introducing CBCT verification, the authors implemented a number of practical approaches to achieving target consistency:
-
∙ Patients were advised and encouraged to avoid diuretics and large quantities of any fluids 30 minutes before radiotherapy.
-
∙ Patients were asked to empty their bladder immediately before entering the treatment room, even if they had recently emptied their bladder or felt that they have no fluid to empty.
-
∙ If a larger bladder volume noted on the CBCT was not adequately covered by the planned volume, patients were asked to re-empty their bladder, and the frequency of imaging increased. The importance of emptying the bladder was also reinforced.
-
∙ A re-plan was initiated for a consistently different bladder volume between planning and treatment fractions; acknowledging that the CT planning scan represents a ‘random’ situation.
The practical steps helped to achieve greater consistency of the bladder volume and provided both proactive and reactive approaches to variance.
Study rationale
Despite these practices, change of rectal volume and bladder deformation was not possible to anticipate, prompting further study.
CBCT images identified that gas in the pelvis impaired image quality and compromised the ability to visualise soft tissue interfaces (Figures 1a and 1b). This impacted on the radiographer’s ability to assess PTV coverage and potentially the time taken for practitioners to make clinical judgements. Poor image quality could lead to an under dose to part of the target volume, or the reliance on excessive planning margins.
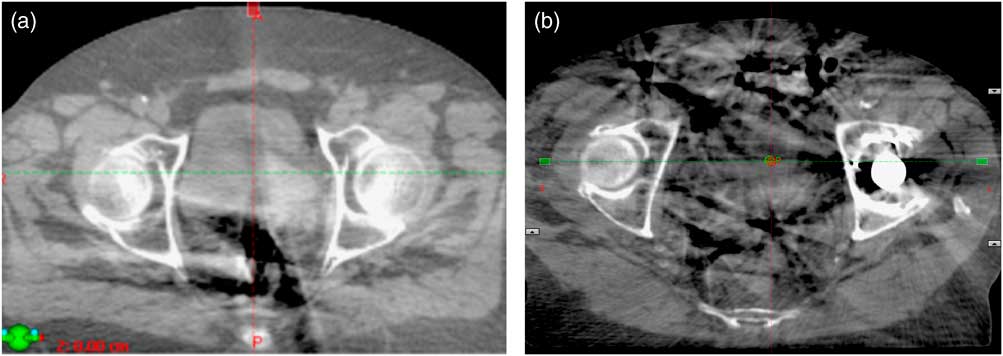
Figure 1 (a) Gas artefact obscuring the posterior aspect of the bladder. (b) Motion reconstruction artefacts from gas in the small intestine exacerbated by the left prosthetic hip.
In a single, selected, patient example, variability in rectal volume and size was observed to deform and displace the bladder (Figure 2). The CBCTs for the first three treatment fractions identified similar, significant rectal distension. A micro-enema was prescribed for the following week of treatment. The treatment position, based on bone localisation with CBCT, provided adequate dose coverage to the bladder; however, a dose–volume histogram (Figure 3), created using the CBCT data, illustrated the effect of rectal volume variation on rectal dose delivered through treatment.
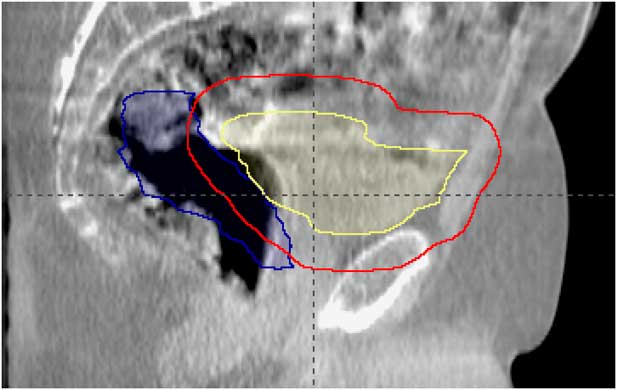
Figure 2 Bladder (yellow), rectum (blue) and planned target volume (red) contours from planning computed tomography scan displayed on fraction 1 treatment cone beam computed tomography.
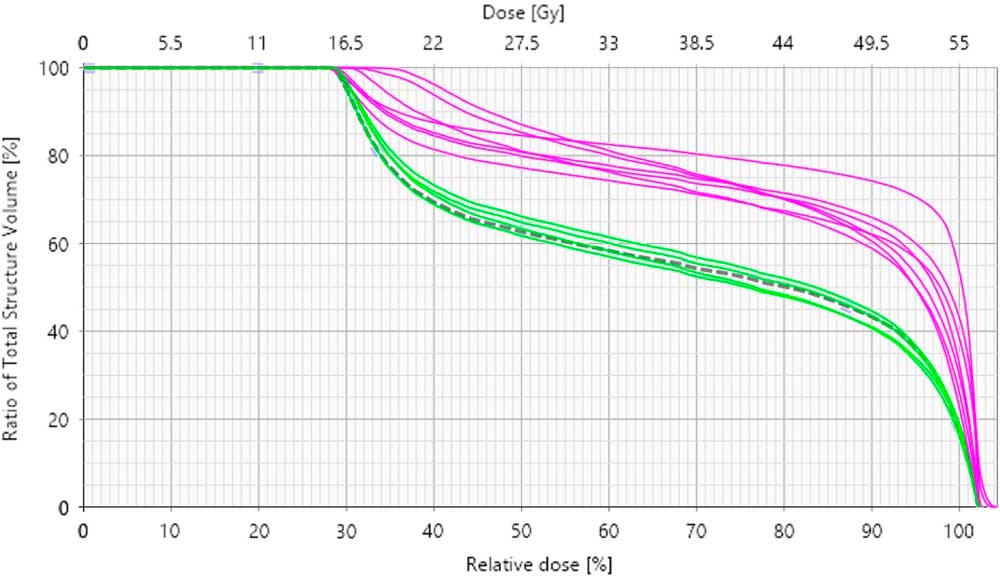
Figure 3 Dose–volume histogram (DVH) to show the rectal doses for planning and treatment for a selected patient example. Notes: The dashed line is the DVH from the planning scan. The green lines indicate the treatment fractions delivered with an enema before treatment. The purple lines illustrate the fractions delivered without a pre-treatment enema.
A literature search revealed no published data on the role of routine rectal preparation protocols for bladder cancer radiotherapy. Engagement with a radiotherapy research forum revealed no consensus on practice. Despite a paucity of evidence, rectal preparation was being used by six out of 12 radiotherapy departments that responded. Existing department protocols consisted of micro-enema (n=5) and diet (n=1). Some responding departments made a distinction between radical and palliative intent and identified IGRT resource limitations as a factor in determining their use.
The use of laxatives and micro-enemas for reducing rectal volume variations in prostate radiotherapy is well documented.Reference Stroom, Koper and Korevaar 23 – Reference Smitsmans, Pos and De Bois 25 Micro-enemas have been used as standard practice within the author’s department for 15 years, and the assessment of patient suitability is made using Patient Group Directives. Diet as a method of managing rectal variation has been investigated with mixed results.Reference Yaver, Foo and Larsen 26 – Reference Hutton, Blair, Baker and Callender 29 Looking to influence the rectal volume and the presence of gas with dietary advice proved unfeasible in our local, small group study of patients receiving bladder radiotherapy. In total, 80% of patients self-identified comorbidity that required them to follow a specific diet. The time resource of the departmental dietician to discuss dietary requirements with these patients, who were already managed by community dieticians, was significant. Patient compliance with dietary intervention was also difficult to measure. It was concluded from this limited review that generic dietary advice to influence rectal status in bladder cancer patients is largely ineffective and maybe inappropriate.Reference McNair, Wedlake, McVey, Thomas and Andreyev 27 – Reference Hutton, Blair, Baker and Callender 29
The use of a micro-enema before planning and treatment, in line with the local prostate radiotherapy protocol, was implemented and evaluated following local audit committee approval. The findings are presented.
METHOD
Patients
Within the Author’s department, ~34 patients with a node negative bladder cancer diagnosis are actioned for radiotherapy each year. This retrospective analysis includes data from patients treated over a 6-month period, from the implementation of CBCT for bladder verification (nine control patients and 13 subsequent patients using enemas) (Table 1).
Table 1 Patient Demographics

Abbreviations: CT, computed tomography.
Patient preparation
Each planning CT was acquired on a Phillips Big Bore CT (Amsterdam, the Netherlands) and reconstructed on 3 mm slices. Patients were scanned and treated supine and immobilised using an indexed knee support and foot stocks. In the control group, the patients were asked to empty their bladder immediately before planning and treatment. No guidance was given on rectal state. Based on standard departmental protocol, if a rectal diameter >5 cm was identified on the planning CT scan, the patient would be asked to empty their bowel and be re-scanned. In the intervention group, patients were asked to use a micro-enema between 20 and 30 minutes before planning and each treatment, and empty their bladder immediately before planning and treatment.
Treatment
Patients were treated on a Varian TruebeamV2.0 (CA, USA). The radical dose fractionation was 50–55 Gy in 20 fractions, and palliative dose fractionation was 36 Gy in 6 fractions over 6 weeks. Half-fan (48 cm aperture) CBCT were acquired on the first three treatment fractions and weekly for all radical treatments unless corrective action was required; and CBCT acquired for each weekly fraction for palliative treatments. A 2 mm No Action Level offline correction IGRT protocol was employed to reduce the systematic set-up error. On each imaged fraction, the acquired CBCT was bone matched using the automatic match algorithm; this was followed by manual verification of the bone match and confirmation of the bladder coverage by the PTV contour. Where bladder coverage was not achieved, a minimal manual adjustmentFootnote i was made.
Data analysis
Sample sizes were based on the timeframe for data collection. Retrospective analysis was carried out on the 97 CBCT scans acquired for the control group and 134 CBCT from the intervention group. CBCT were compared with the patient’s planning CT for bladder size, rectal status and rectal diameter. Bladder volumes were outlined and measured on planning CT and each CBCT using Varian Eclipse™ (CA, USA) contouring module. Rectal status was compared with the initial planning scan using an arbitrary scale, where 1=empty, 2=gas, 3=faeces and 4=gas and faeces as described by Smitsmans et al.Reference Smitsmans, Pos and De Bois 25 and illustrated using CBCT images from a patient within the control group (Figure 4). Anterio-posterior cross-sectional diameter of the rectum was measured at 21 mm inferior to the isocentre for each CBCT-imaged fraction and compared with the planning scan to ensure all measurements were taken inferior to the recto-sigmoid flexure. A total of 21 mm was chosen to coincide with the 3 mm reconstructed CT slices.
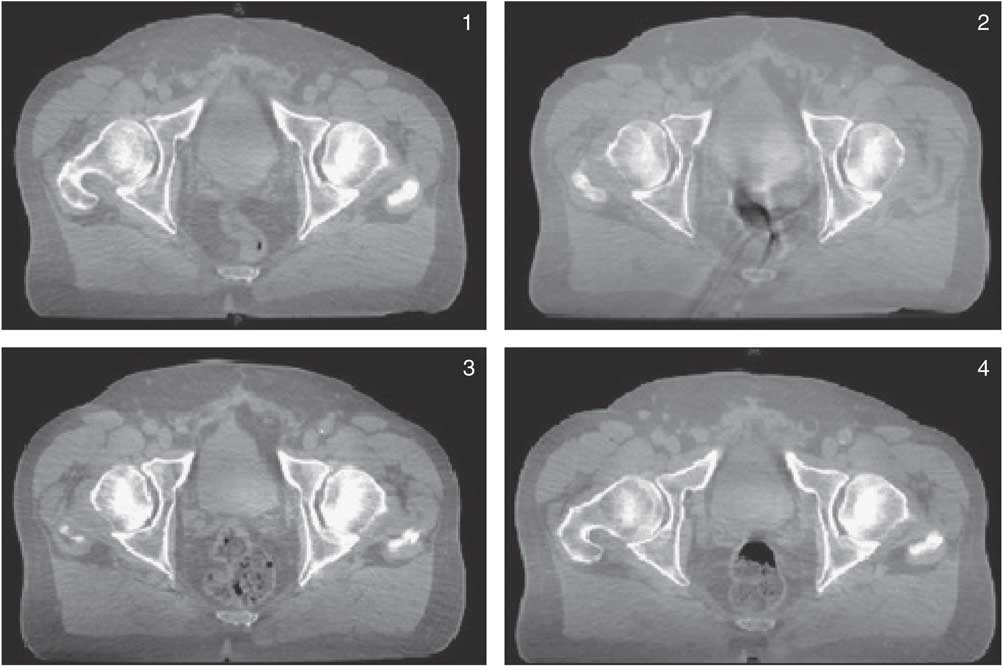
Figure 4 Grading of rectal status (1–4) using a patient example from the control cohort described in the study.
The proportion of rectal volume occupied by gas was evaluated by viewing the planning CT and CBCT images in the mid-sagittal plane. A semi-quantitative scale numbered 1–5 denoted no gas present,Reference Henry, Stratford and McCarthy 1 gas occupying 5–25%,Reference Lutkenhaus, Visser, DeJong, Hulshof and Bel 2 26–50%,Reference Pos and Remeijer 3 51–75%Reference Fokdal, Honore, Hoyer, Meldgaard, Fode and VonDer Maase 4 and >75%Reference Yee, Parliament, Rathee, Ghosh, Ko and Murray 5 of the rectum. This method is described and used by McNair et al.Reference McNair, Wedlake, McVey, Thomas and Andreyev 27 Patient compliance and tolerability of the enema was verbally assessed by the treatment delivery radiographers using a pro forma. Whenever evaluating a potential change to practice it is important to consider any resource implications. The time for acquisition and evaluation of the CBCT datasets was collected retrospectively from Varian’s record and verify system, ARIA (CA, USA).
Results
Table 2 displays the statistical analysis of the bladder and rectal volume variation. The returned values from the Shapiro–Wilk test (for control and intervention groups was <0·001) confirmed that data were not normally distributed, therefore a non-parametric test, Mann–Whitney was performed.
Table 2 Independent samples test

Bladder
Data from both the control group and the enema intervention group (Figures 5a and 5b) indicate that for the sample, the use of an enema did not correlate with variations in bladder size between planning and treatment. As a group, bladder volume variation was greater in the intervention group although this is not statistically significant. Variations appear to be patient specific. Patients who received chemotherapy requiring pre-hydration before a radiotherapy treatment fraction were not identified within the results. For these patients, protocol is to acquire a CBCT before radiotherapy on that day due to anticipated residual bladder volume.

Figure 5 Bladder volume variations for patients treated with enema preparation (a) and without enema preparation (b).
Rectum
The micro-enema was well tolerated with no reported adverse effects for the intervention group.
An empty rectum (status 1) was achieved in 70% of imaged fractions delivered using an enema compared with 33% of fractions for the control group. The intervention was also successful in reducing the presence of faeces or faeces and gas (Table 3). The control group had 46% of fractions delivered with the presence of faeces or faeces and gas, compared with 10% of fractions for the intervention group. The enema had no effect on the presence of gas without faeces recorded as 21 and 20% for control and intervention group, respectively.
Table 3 Rectal status observed on cone beam computed tomography for control and intervention group

A significant effect of the micro-enema (p<0·001) was seen in the quantity of gas (Table 4). The intervention group saw the majority of fractions delivered (65%) with <5% gas, and a further 29% of fractions delivered with 5–25% gas. This contrasts with the control group where 32% of fractions were delivered with no gas or <5% and the majority of fractions (55%) were delivered with 5–25% of gas.
Table 4 Presence of gas: control versus intervention group

Variation in rectal diameter between planning and any treatment fraction for the control group ranged between +4·5 and −3·0 cm. The intervention group had a significantly smaller rectal diameter variation between planning and CBCT’s (p<0·001), with mean variation of 1·39 cm and 0·50 cm (95% CI, confidence 1·10–1·68 cm and 0·40–0·60 cm for the control and intervention groups, respectively) (Figure 6).

Figure 6 Rectal diameter variation between planning and treatment fractions for the control group and intervention group.
Image analysis timings
Timings for the evaluation of CBCT were retrospectively collected from the Aria Record and Verification system. The time taken for image analysis and decisionmaking was calculated as time from CBCT completion to first beam on. Mean image evaluation time for the control group was 4 minutes 6 seconds (min=1 minute 47 seconds, max=7 minutes 44 seconds, SD 1·31) compared with 5 minutes 20 seconds (min=2 minutes 12 seconds, max=15 minutes 9 seconds, SD 3·07) for the intervention group.
DISCUSSION
In this cohort study, we found a significant reduction in rectal volume variability resulting from gas and faeces following the introduction of micro-enema before planning and treatment (p=<0·001). The control group result for rectal reproducibility is concordant with a previous audit that showed that 60% of fractions were delivered with a different rectal volume to that of the planning scan.Reference Hutton, Callender and Baker 20 The study is limited by a small sample size, consecutive patient groups and non-matched treatment schedules (palliative and radical).
The issue of inter-fraction bladder variation during radiotherapy for bladder cancer is well documented.Reference Yee, Parliament, Rathee, Ghosh, Ko and Murray 5 The most pronounced displacement is witnessed in the anterior-posterior and craniocaudal directions.Reference Fokdal, Honore, Hoyer, Meldgaard, Fode and VonDer Maase 4 , Reference Hutton, Callender and Baker 20 , Reference Lotz, van Herk, Betgen, Pos, Lebesque and Remeijer 21 These displacements are often due to variation in bladder filling, rectum or bowel contentReference Lotz, van Herk, Betgen, Pos, Lebesque and Remeijer 21 and may exceed the tolerance of the CTV–PTV. Pos et al.Reference Pos, Koedooder, Hulshof, Van Tienhoven and Gonzalez 6 reported that 65% of patients had a part of the CTV outside the PTV at least once during treatment. All imaged fractions within the study were delivered to ensure that the bladder was treated within standard PTV margins, however, displacement within the PTV alters the dose received by surrounding OAR.Reference Lutkenhaus, Visser, DeJong, Hulshof and Bel 2 , Reference Foroudi, Wong and Haworth 7 , Reference Hutton, Leadbetter, Jain and Baker 11 Managing the size, shape and position of the rectum is likely to reduce the magnitude and frequency of bladder displacement, an important factors in the accurate and effective delivery of a radiotherapy prescription to the bladder.
In addition, gas pockets create motion reconstruction artefacts. Smitmans et al.Reference Smitsmans, Pos and De Bois 25 reported correlation between the presence of faeces and gas in the rectum, improved image quality, and the success of automatic registration in a cohort of prostate patients. Reducing the artefacts can enhance the quality of the acquired image, which improves the accuracy of CBCT assessment. Efficiency of image analysis can also be considered a factor in accuracy in the context of intra-fraction motion. The introduction of an enema had no impact on the time taken for image analysis in the study groups. This may have been influenced by a number of factors. The control group image analysis and decision to treat was performed by three therapeutic radiographers who developed the technique at the trust. The intervention group image analysis was undertaken by a wider team that included an element of online training. This additional training element and the inclusion of staff with less experience in the practice of CBCT matching and decisionmaking may have impacted timings.
Variation in rectal diameter is significant in the context of CTV–PTV margin of 1·0–1·5 cm and the position of the posterior bladder wall. The variation was significantly reduced with the use of a micro-enema. The outliers observed in the intervention group identify that patient reported compliance with enema use does not consistently correlate with effective implementation in all cases.
CBCT contouring and dose evaluation is useful to quantify the quality or appropriateness of a planned treatment measured against pre-defined criteria and dose constraints. The value of meeting established dose constraints on the plan is significantly reduced if the organs under consideration undergo significant inter-fractional variation. It is therefore essential, even when adopting adaptive treatment methods, that inter-fractional changes are limited as far as reasonably practicable.
It is important to acknowledge the difference in patient demographic between prostate and bladder cancer, and therefore to appreciate the potential limitations in implementing the same rectal preparation. The use of micro-enemas was guided by the same directives that apply for prostate patients, eliminating patients with an inflammatory bowel disease.
CONCLUSION
The use of a micro-enema before planning scan and each fraction was well tolerated. Although it is important to note that tolerance is subjective and the small numbers accessed in this study. The micro-enema proved effective in managing and reducing inter-fraction variation in terms of rectal volume. The introduction of a micro-enema did not reduce the presence of transient gas although it did reduce the quantity—with subsequent reduction in motion reconstruction artefacts and the associated blurring of soft tissue interfaces. The use of a micro-enema is a simple, effective method to improve the reproducibility of bladder radiotherapy treatment delivery by controlling rectal volume, influence image quality and accuracy of image matching.
Acknowledgement
None.













