Introduction
Emerging infectious diseases (EID) are those caused by the spread of parasites/pathogens within new hosts and geographical areas (Daszak et al., Reference Daszak, Cunningham and Hyatt2000) and those which incidence has increased in recent times or that threatens to increase in the near future (Friend et al., Reference Friend, McLean and Joshua Dein2001). Current and projected habitat modification and overexploitation can increase the frequency of EID affecting humans, livestock and wild animals (Lafferty, Reference Lafferty2003; Sehgal, Reference Sehgal2010; Tompkins et al., Reference Tompkins2015), and these factors together are a major threat to the conservation of biodiversity (Smith et al., Reference Smith, Acevedo-Whitehouse and Pedersen2009). There are several examples of population decimations following the spread of infectious diseases within wild animals (van Riper et al., Reference van Riper1986; Robinson et al., Reference Robinson2010; Preece et al., Reference Preece2017), and these negative effects can be favoured in human-modified landscapes (Mennerat et al., Reference Mennerat2010; Brearley et al., Reference Brearley2013; Becker et al., Reference Becker, Streicker and Altizer2015; Tompkins et al., Reference Tompkins2015). However, detecting novel pathogens in natural populations before the onset of mortality episodes is challenging (Preece et al., Reference Preece2017).
Domestic animals are important sources of novel pathogens for naïve populations of wild animals, and disease spillover is facilitated due to the encroachment of livestock into natural areas (Daszak et al., Reference Daszak, Cunningham and Hyatt2000; Deem et al., Reference Deem2012). Livestock activities usually maintain high densities of domestic animals (Lafferty and Gerber, Reference Lafferty and Gerber2002), and such areas can be important food and shelter sources for opportunistic free-living wild animals, where they gather at high densities (Gottdenker et al., Reference Gottdenker2005; Carrete et al., Reference Carrete2009; Tompkins et al., Reference Tompkins2015). Moreover, these areas can increase the abundance of important disease vectors by providing artificial water bodies for the development of bloodsucking dipterans (Norris, Reference Norris2004; Reiter and LaPointe, Reference Reiter and LaPointe2007). Synanthropic and opportunistic wild animals can use areas with different levels of habitat integrity and they can act as a bridge, carrying pathogens from degraded areas where livestock is raised to preserved forests with a higher diversity of habitat-specialists animals (Karlsson et al., Reference Karlsson2015; Ferreira et al., Reference Ferreira2017). These epidemiological aspects make the interface between natural and anthropogenic-modified areas important hotspots for the emergence of novel host-pathogen interactions (Smith et al., Reference Smith, Acevedo-Whitehouse and Pedersen2009; Karlsson et al., Reference Karlsson2015). The expansion of agricultural frontiers is reshaping these interactions (Sehgal, Reference Sehgal2015), and one prediction, for instance, is that habitat modifications will change the distribution of avifauna populations, increasing the chances of disease emergence in wild birds (Friend et al., Reference Friend, McLean and Joshua Dein2001; Fuller et al., Reference Fuller2012).
Haemosporidians are widely distributed protozoan parasites, and two main genera infecting birds are Plasmodium, transmitted by dipterans of the family Culicidae, and Haemoproteus, which vectors are members of the families Ceratopogonidae and Hippoboscidae (Valkiūnas, Reference Valkiūnas2005). Haemosporidian infections on avian hosts have been linked to reductions on the reproductive success and longevity in chronic infections (Knowles et al., Reference Knowles, Palinauskas and Sheldon2010; Asghar et al., Reference Asghar2015) and is also related to mortality outbreaks in populations with no evolutionary association with these parasites (Pacheco et al., Reference Pacheco2011b; LaPointe et al., Reference LaPointe, Atkinson and Samuel2012; Vanstreels et al., Reference Vanstreels2015). Avian haemosporidians can infect a wide number of bird species, and this host breadth can be labile across different geographical areas and temporal scales (Ellis et al., Reference Ellis2015), with elevated chances of novel host–parasite interactions occurring at areas under anthropic alterations (Santiago-Alarcon et al., Reference Santiago-Alarcon, Palinauskas and Schaefer2012a).
Plasmodium gallinaceum and P. juxtanucleare, two species that naturally infect domestic chickens (Gallus gallus domesticus), have originated in the Asian continent, but only the second has global distribution (Valkiūnas, Reference Valkiūnas2005). Plasmodium juxtanucleare was described for the first time in 1941 in Brazil (Versiani and Gomes, Reference Versiani and Gomes1941) and since then it has been reported in Asia (Bennett and Warren, Reference Bennett and Warren1966; Chen et al., Reference Chen2015) and in many parts of the globe, including Africa (Earle et al., Reference Earle1991; Poulsen et al., Reference Poulsen2000) and other countries in the American continent, such as Mexico and Uruguay (Garnham, Reference Garnham1966). This parasite is widely distributed in Brazil, and prevalence varies from 4 to 100% in backyard chicken (Paraense, Reference Paraense1949; Krettli, Reference Krettli1972; Mota et al., Reference Mota2000; Prezoto et al., Reference Prezoto2001). The chronic phase of the infection is usually benign (Bennett and Warren, Reference Bennett and Warren1966; Silveira et al., Reference Silveira, DaMatta and Dagosto2009), but anaemia, prostration and clinical signs such as incoordination and paralysis due to neurological lesions are common at the acute phase of infection, with some cases evolving to death under experimental conditions (Krettli, Reference Krettli1972; Silveira et al., Reference Silveira2013).
Plasmodium juxtanucleare has been detected in several hosts of the family Phasianidae (order Galliformes), but experimentally infected Columbiformes (Columba livia), Anseriformes (Anas platyrhynchos) and Passeriformes (Passer domesticus and Serinus canaria) were shown to be refractive (Valkiūnas, Reference Valkiūnas2005). This parasite was associated to an outbreak that killed five black-footed penguins (Spheniscus demersus, Sphenisciformes) under rehabilitation in South Africa (Grim et al., Reference Grim2003), and caused the mortality of a captive white eared-pheasant (Crossoptilon crossoptilon; Galliformes) in Japan (Murata et al., Reference Murata2008). Even though P. juxtanucleare infects non-domestic birds, there are no reports of this parasite infecting free-living bird communities in any part of the globe.
We captured wild birds at the border between a protected preserved area and modified landscapes to investigate the distribution of avian haemosporidians in different bird communities. Robust morphological and molecular analyses demonstrated that wild passerines are competent natural hosts of P. juxtanucleare. We detected the same parasite lineage in a backyard chicken sampled in the surroundings where infected passerines were captured, showing that avian malaria spillover to wild birds is possible. Determining whether this spillover could lead to an EID should be considered a priority for wildlife conservation in the Cerrado biome, one of the most threatened biodiversity hotspot in South America.
Material and methods
Study site and bird sampling
We conducted wild bird sampling within the limits of the Chapada dos Guimarães National Park (CGNP), and at its influence area in Chapada dos Guimarães municipality, which is covered by the Cerrado biome in the Brazilian central plateau in the State of Mato Grosso. Cerrado is a biodiversity hotspot (Myers et al., Reference Myers2000), but is also one of the most endangered eco-regions on Earth, with land conversion for agriculture constituting the main cause of habitat loss (Beuchle et al., Reference Beuchle2015).
Wild birds were sampled in five field campaigns of five days each from April 2013 until May 2014. We operated 10 mist-nets (10 m long × 3 m high, with 20 mm mesh size) for 5 h starting at sunrise, and nets were checked every 30 min. In April 2013, we captured 16 passerines inside the limits of CGNP (15°19′5.08″ S, 55°53′2.18″ W), a protected conservation site of 32 600 ha created in 1989 which is currently inside a strict nature reserve of 251 848 ha. In September 2013, and in February 2014, we captured 42 passerines in a gallery forest not protected by Brazilian laws (15°29′55.80″ S, 56°10′30.30″ W). However, this area is partially preserved and has integrity levels and forest structure compared with the CGNP. In August 2013 and in May 2014, we captured 10 passerines in a secondary forest contiguous with a pasture area belonging to a small farm (15°21′30.34″ S, 55°27′23.99″ W). This sampling point is situated between the strict nature reserve and an area of sustainable use of natural resources encompassing 39 500 ha (Fig. 1). In December 2014, we sampled 30 backyard chickens raised in a small farm, located 50 m distant from the area where we mist-netted the birds. Flock size in this farm commonly fluctuates between 40 and 80 chickens, where they breed locally for meat and egg production. Individual chickens are kept until egg production ceases, and roosters are managed for more than 1 year. Sampled birds did not present physical alterations and estimated age ranged between three to 6 months. We could not sample younger chicks and older females.

Fig. 1. Map of Chapada dos Guimarães National Park (dark grey area) in Mato Grosso, Brazil, showing sampling areas. A medium grey area indicates the distribution of the Cerrado biome. The white solid line represents the strict nature reserve and the white dashed line represents the area of sustainable use of natural resources.
Captured passerines and chickens were physically restrained and we obtained blood samples through brachial venipuncture. We prepared two blood smears per passerine and the material was air dried and fixed with absolute methanol for 1 min within 12 h of preparation. We did not obtain blood smears from sampled chickens. We stored the remaining volume of blood samples in absolute ethanol at room temperature for a maximum of five days and the material was ultimately kept at −20 °C until DNA was extracted. Before release, passerines were tagged with individual aluminium leg-rings.
Blood smear analysis
Blood smears were stained with 10% Giemsa solution for 40 min within two weeks of preparation. An Olympus CX31 light microscope equipped with an Olympus Q-Color5 imaging system (Olympus, Tokyo, Japan) together with Q-Capture Pro7 imaging software (QImaging, Surrey, Canada) were used to examine passerine blood smears and to capture images. We analysed blood films only from birds positive at the screening PCR. At least 200 microscopic fields under 1000 × magnification were examined for the detection of parasites which were identified following Valkiūnas (Reference Valkiūnas2005). Parasitaemia was estimated based on the actual counting of infected erythrocytes in a total of 10 000 observed erythrocytes.
Molecular analyses
Approximately 10 µL of blood was transferred to 1.5 mL microtubes and samples were dried at 37 °C for subsequent DNA extraction, for which we used a conventional phenol–chloroform method with isopropanol precipitation (Sambrook and Russell, Reference Sambrook and Russell2001). The genomic DNA pellet was resuspended in 50 µL of ultrapure water and quantified using a NanoDrop 2000 (Thermo Scientific, Waltham, United States). Between 50 and 100 ng of the extracted DNA was used for a screening PCR that amplifies a 154-nucleotide segment (excluding primers) of ribosomal RNA coding sequence within the mitochondrial DNA (mtDNA) of Plasmodium and Haemoproteus in a single reaction. We used the primers 343F (5′-GCTCACGCATCGCTTCT-3′) and 496R (5′-GACCGGTCATTTTCTTTG-3′) designed by Fallon et al. (Reference Fallon2003) under PCR conditions and amplification analysis described by Roos et al. (Reference Roos2015).
DNAs from positive individuals were submitted to a nested-PCR targeting the amplification of a 478 bp region of the cytochrome b (cyt b) gene. For the first reaction, we used primers HaemNFI (5′-AGACATGAAATATTATGGITAAG-3′) and HaemNR3 (5′-GAAATAAGATAAGAAATACCATTC-3′) (Hellgren et al., Reference Hellgren, Waldenström and Bensch2004) with 50–100 ng of genomic DNA. A 1-μL aliquot of this PCR product was then used as a template for the second reaction with the primers HaemF (5′-CTTATGGTGTCGATATATGCATG-3′) and HaemR2 (5′-CGCTTATCTGGAGATTGTAATGGTT-3′) (Bensch et al., Reference Bensch2000). Both reactions contained 1 × buffer, 4 mm of MgCl2, 0.3 mm of each dNTP, 1 unit of Taq (Phoneutria, Belo Horizonte, Brazil), 0.4 mm of each primer, and nuclease-free water in 25 µL reaction volumes. DNA extracted from blood samples of chickens experimentally infected with P. gallinaceum and ultrapure water was used as positive and negative controls, respectively. These nested-PCRs followed the protocol by Hellgren et al. (Reference Hellgren, Waldenström and Bensch2004).
Products from all positive nested-PCRs were purified with Polyethylene Glycol 8000 (Sambrook and Russell, Reference Sambrook and Russell2001) and bi-directionally sequenced with dye-terminator fluorescent labelling in an ABI Prism 3100 sequencer (Applied Biosystems, Foster City, USA). DNA sequences were aligned, checked for the presence of mixed infections (the presence of double peaks in the eletrochromatograms), edited using ChromasPro 2.0.6 (Technelysium Pty Ltd, Helensvale, Australia), and compared with data available in the public databases GenBank and MalAvi (Bensch et al., Reference Bensch, Hellgren and Pérez-Tris2009). Detected cyt b sequences were deposited in GenBank under accession numbers MG598389–MG598398 and MG598406.
In addition to the cyt b gene, seven almost complete mitochondrial genomes (mtDNA) of P. juxtanucleare were amplified, cloned and sequenced from the following positive bird samples: pearly-vented tody-tyrant (Hemitriccus margaritaceiventer, n = 2), a red-crested finch (Coryphospingus cucullatus), eastern slaty thrush (Turdus subalaris, n = 2), a short-crested flycatcher (Myiarchus ferox) and a plain-crested elaenia (Elaenia cristata). In order to avoid potential mixed infections, PCR products were amplified and cloned as followed: the primers forward 5′-GAGGATTCTCTCCACACTTCAATTCGTACTTC and reverse 5′CAGGAAAATWATAGACCGAACCTTGGACTC were used to amplify 5904 base pairs of mtDNA genome with TaKaRa LA TaqTM Polymerase (TaKaRa Mirus Bio Inc., Shiga, Japan) as described by Pacheco et al. (Reference Pacheco2011a). Wherever the parasitaemia was low, a nested PCR was performed using the internal oligos forward 5′-TTTCATCCTTAAATCTCGTAAC-3′/reverse 5′-GACCGAACCTTGGACTCTT-3′. PCR amplifications for both PCR (outer and inner) were carried out in a 50 µL volume using 20 ng of total genomic DNA. The PCR conditions were: a partial denaturation at 94 °C for 1 min and 30 cycles of 30 s at 94 °C and 7 min at 68 °C, followed by a final extension of 10 min at 72 °C. Following manufactory directions, two independent PCR products (bands of approximately 6 kb) were excised from the gel, purified using QIAquick® Gel extraction kit (Qiagen, GmbH, Hilden, Germany) and cloned in the pGEM®-T Easy Vector systems (Promega, Madison, USA). For at least three clones, both strands were sequenced using an Applied Biosystems 3730 capillary sequencer. There were no inconsistencies among the clones. The mtDNA genome sequences were submitted to GenBank under accession numbers MG598399–MG598405.
Phylogenetic analyses
Nucleotide alignment was produced by using ClustalX v2.0.12 and Muscle as implemented in SeaView v4.3.5 (Gouy et al., Reference Gouy, Guindon and Gascuel2010) with manual editing. The alignment was constructed with a total of 17 mtDNA genome sequences (5356 bp excluding gaps) belonging to three genera (Leucocytozoon, Haemoproteus and Plasmodium). Then, the alignment was divided into six partitions corresponding to the three non-coding regions between the ORFs (fragmented SSU rRNA and LSU rRNA) and the three coding genes, keeping their order in the mtDNA genome (non-coding, cox3, non-coding, cox1, non-coding, cytb, non-coding). Then, the phylogenetic relationship was inferred based on the alignment using the Bayesian methods implemented in MrBayes v3.2.6 with the default priors (Ronquist and Huelsenbeck, Reference Ronquist and Huelsenbeck2003). A general time reversible model with gamma-distributed substitution rates and a proportion of invariant sites (GTR + Γ + I) was used for each partition; which was the model with the lowest Bayesian Information Criterion (BIC) scores for both alignments and each partition as estimated by MEGA v7.0.14 (Kumar et al., Reference Kumar, Stecher and Tamura2016). Bayesian support for the nodes was inferred in MrBayes by sampling every 500 generations from two independent chains lasting 104 Markov Chain Monte Carlo (MCMC) steps. As a ‘burn-in’, 50% of the sample was then discarded once convergence was reached. The chains were assumed to have converged once the value of the potential scale reduction factor (PSRF) was between 1.00 and 1.02 and the average standard deviation of the posterior probability was below 0.01 (Ronquist and Huelsenbeck, Reference Ronquist and Huelsenbeck2003). In addition, the effective sample sizes (ESSs) for all the parameters were checked by using Tracer v1.6 (Rambaut et al., Reference Rambaut2015). ESSs are recommended to be >200. In our case, all ESS values were higher than 750 (ranging from 769 to 5245), confirming a good mixing and convergence of the chains.
Results
Haemosporidian screening
We analysed blood samples from 68 wild birds, comprising 31 species from 13 families (Supplementary Table 1). Although our sample sizes did not allow estimating parasite prevalence in particular host species, the screening PCR targeting the rRNA from Plasmodium/Haemoproteus showed that 19.1% of the samples were positive. One out of 16 birds captured in the protected area of CGNP was positive (6.3%), five out of 42 birds sampled in the gallery forest were positives (12%), and seven out of 10 birds sampled in the transition between a secondary forests and pasture areas were positives (70%).
Molecular detection of P. juxtanucleare and other haemosporidian parasites
We sequenced a 478 bp fragment of the parasite's cyt b from 10 positive samples, as we could not obtain high-quality sequences from one bird sampled in the protected area and from two birds sampled in the gallery forest, even though these samples were positive in the nested-PCR. All seven samples from the secondary forest near pasture areas were successfully sequenced and they had 100% identity with a P. juxtanucleare strain isolated from chickens in southeastern Brazil (GenBank accession number KC142195) (Silveira et al., Reference Silveira2013). Infected birds species were pearly-vented tody-tyrant (H. margaritaceiventer, n = 2), a red-crested finch (C. cucullatus), eastern slaty thrush (T. subalaris, n = 2), a short-crested flycatcher (M. ferox) and a plain-crested elaenia (E. cristata). This parasite lineage had also been detected in Malaysia (unpublished work, KT290910) and in Thailand (Tattiyapong et al., Reference Tattiyapong2016), and has a single nucleotide polymorphism in relation to the P. juxtanucleare associated to the death of one captive white eared-pheasant (C. crossoptilon) in Japan (AB302893) (Murata et al., Reference Murata2008).
Sequenced parasites not related to P. juxtanucleare obtained from birds captured at the gallery forest were an already described Plasmodium sp. lineage (BAFLA04; acc. no. JX029861) in an eastern slaty thrush (T. subalaris) and newly described lineages related to Haemoproteus sp. (acc. no. MG598390) and to Plasmodium sp. (acc. no. MG598391) detected in Monasa nigrifrons, order Galbuliformes (Supplementary Table 1).
After detecting P. juxtanucleare in passerines, we sampled and tested 30 backyard chickens maintained at 50 m from the area where these passerines were captured. The screening PCR revealed one positive sample (3.3%), which was confirmed as P. juxtanucleare by sequencing 478 base pairs of the cyt b, with 100% similarity with the parasite detected in the passerines.
Then, we sequenced the almost complete mitochondrial genome (17 sequences and 5356 bp excluding gaps) from parasites detected in all seven passerines infected by P. juxtanucleare. Results were identical between samples and these parasites had polymorphisms at four sites when compared with a P. juxtanucleare isolated from chickens in Japan (Omori et al., Reference Omori2007), the single isolate with the complete sequence at the mitochondrial level. Only one synonymous substitution occurred in cyt b gene, two substitutions occurred in two large subunit ribosomal RNAs and one substitution occurred in the RNA11 region (Fig. 2).
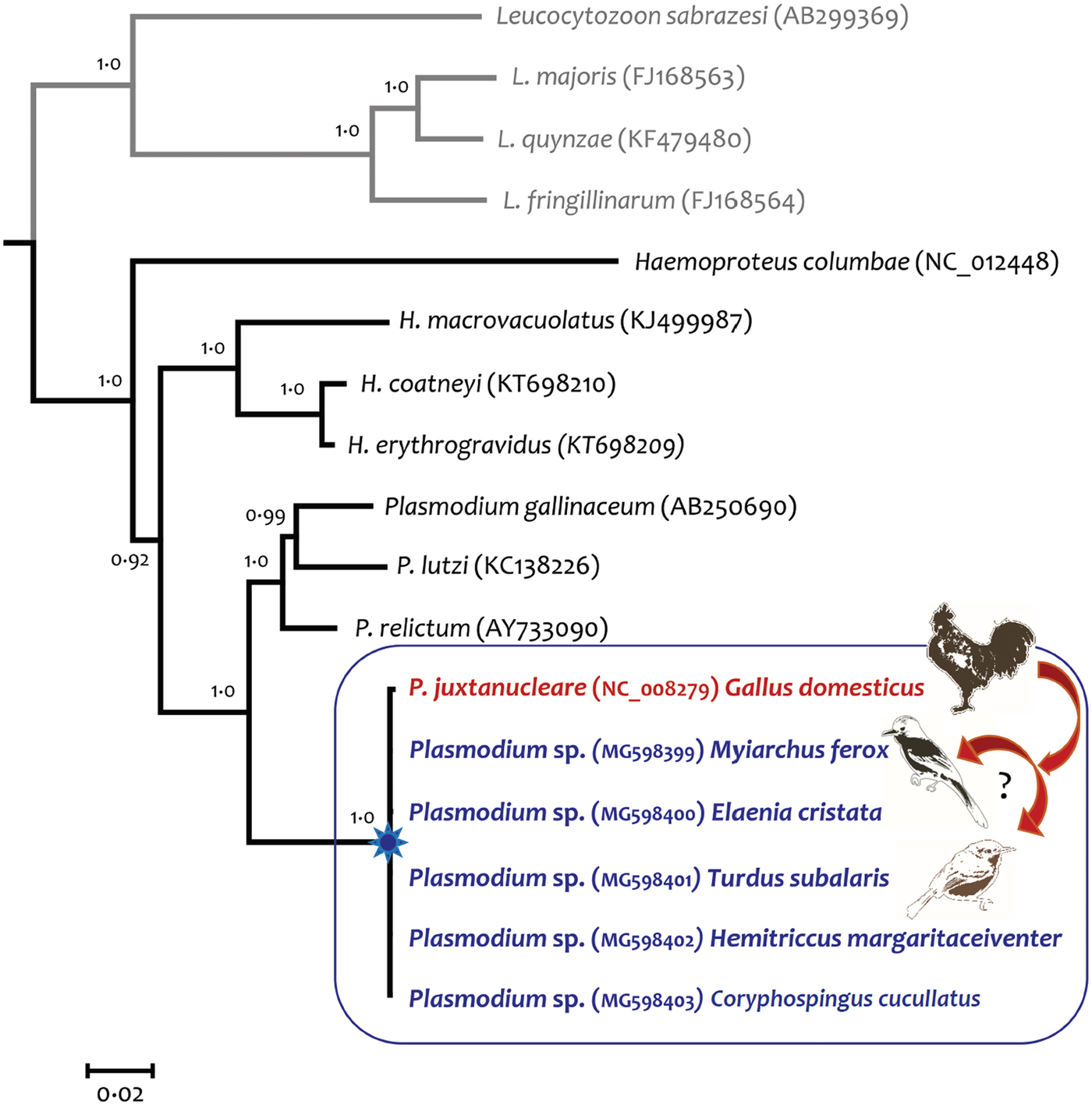
Fig. 2. Bayesian phylogenetic tree showing the relationships between Plasmodium juxtanucleare detected in passerines and in domestic chicken from Japan. Bayesian inference was conducted based on the almost complete mitochondrial genome (17 sequences and 5356 bp excluding gaps) of avian haemosporidians. Values above branches are posterior probabilities and the Leucocytozoon genus was used as an outgroup. All sequences obtained from passerine birds were identical but differed at four sites from the lineage isolated from Gallus gallus domesticus.
Morphological confirmation of complete development of P. juxtanucleare in passerines
Parasite identity was morphologically confirmed as P. juxtanucleare for all seven positive samples infected with this parasite in the sequencing results (Fig. 3). Parasitaemia ranged from 0.12–0.23%, and trophozoites, meronts, macro and microgametocytes were visualized in all blood smears (Table 1), except in a T. subalaris, in which sample we did not find gametocytes despite detecting a 0.19% parasitaemia. Features used to identify this parasite included trophozoites of generally small size, with scanty cytoplasm and usually adhering to the erythrocyte nucleus; meronts with scanty cytoplasm adhering to the subpolar portion of the nucleus, with three or four merozoites; macro and microgametocytes usually roundish, but that sometimes display oval to irregular shape, with mature forms never exceeding the size of the erythrocyte nucleus. Mature macro and microgametocytes possess a maximum of three roundish pigment granules clumped at the edge of the parasites. The overall proportion of infected wild birds with P. juxtanucleare was 10.3%.
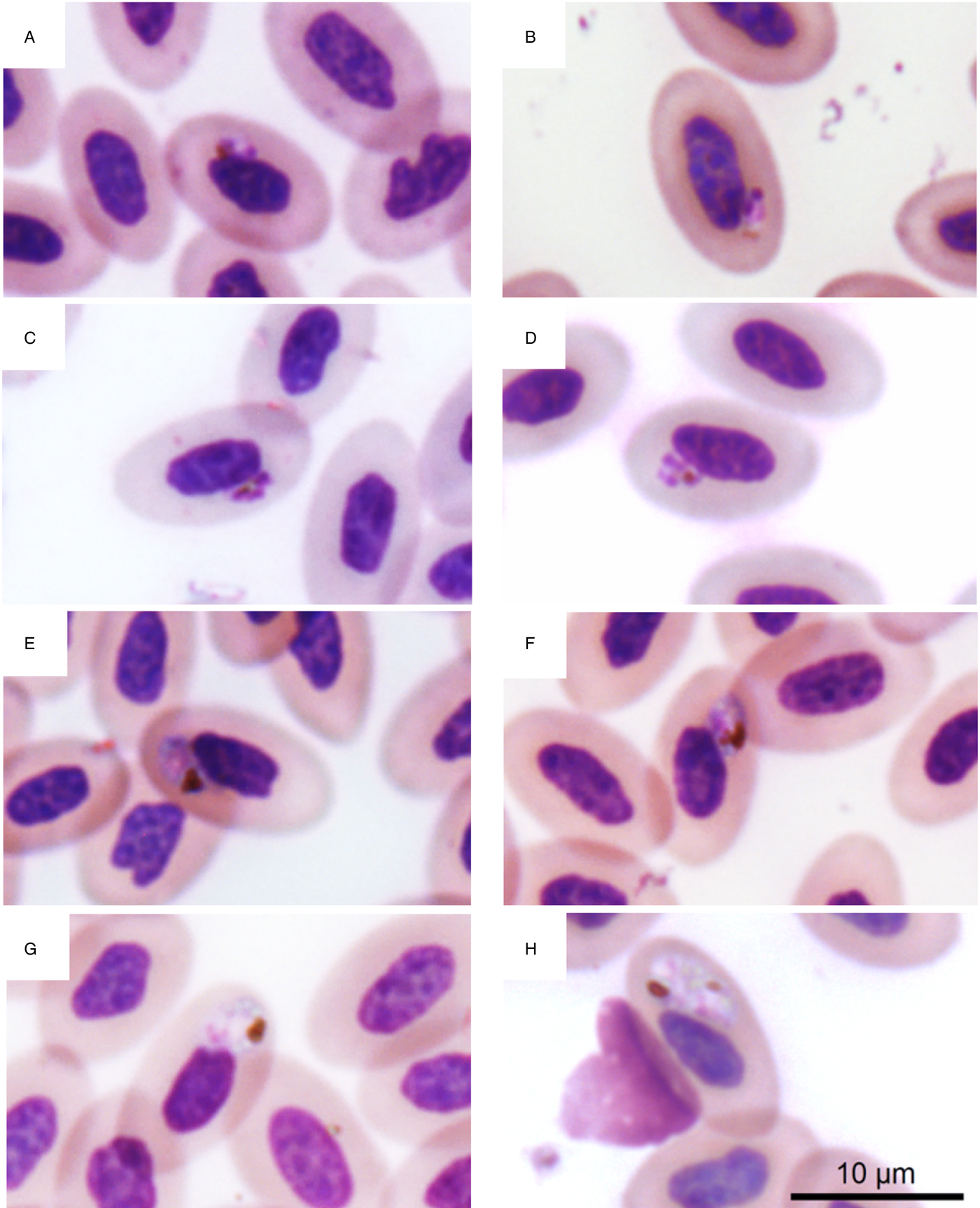
Fig. 3. Blood stages of Plasmodium juxtanucleare from passerines captured in the interface between preserved and modified landscapes. (A, B) trophozoites; (C, D), meronts with three and four merozoites, respectively; (E, F), macrogametocytes; (G, H), microgametocytes.
Table 1. Parasite forms and parasitaemia of Plasmodium juxtanucleare detected in wild birds sampled in the Brazilian Cerrado

Troph., trophozoites; Meron., meronts, Macrog. macrogametocytes; Microg., microgametocytes.
Parasitemia estimated in 20 000 erythrocytes counted at 1000 × magnification under optical microscope.
Discussion
We demonstrated, using molecular and microscopy analyses, that P. juxtanucleare, a parasite typically found in domestic fowl, naturally infects free-living passerines in Brazil. We confirmed the presence of mature gametocytes in the blood of five bird species from four different families, revealing these passerines as competent hosts of P. juxtanucleare. This same parasite lineage was also detected in a backyard chicken managed close to the area where infected passerines were captured, revealing that wild and domestic birds share the same haemosporidian at the limits of a strict nature reserve. Our study shows a new parasite spillover from domestic livestock to wild birds in a highly threatened ecosystem from a megadiverse country. Plasmodium juxtanucleare can be highly pathogenic for non-domestic birds under captive conditions (Grim et al., Reference Grim2003; Murata et al., Reference Murata2008), thus these novel host–parasite associations may pose a real threat to the avifauna in Brazil.
Only birds captured in a secondary forest at the boundaries of a farm managing chickens were positive for P. juxtanucleare. Previous studies demonstrated that wild birds captured closer to poultry farms are more likely to be infected by general haemosporidians even though P. juxtanucleare was not detected (Gonzalez-Quevedo et al., Reference Gonzalez-Quevedo, Davies and Richardson2014; Padilla et al., Reference Padilla2017). Here, we show that the infection by P. juxtanucleare also seems to be related to the proximity to poultry farms, and this is expected because chickens are a major reservoir for this parasite. Farms managing backyard chickens can act as ecological traps for wild birds (Carrete et al., Reference Carrete2009; Becker et al., Reference Becker, Streicker and Altizer2015), where they gather in high densities (Gottdenker et al., Reference Gottdenker2005) and may present an increased likelihood of being infected by a parasite that we demonstrate to be more generalist than previously thought. Seven out of eight passerines captured in this area in May 2014 were positive for P. juxtanucleare, suggesting that such environmental conditions created by livestock production might favour parasite transmission.
Determining whether this parasite will become a wildlife health problem requires additional investigations, as new host–parasite interactions do not necessarily lead to EIDs (Ebert and Herre, Reference Ebert and Herre1996; Pacheco et al., Reference Pacheco2013; Hillman et al., Reference Hillman, Lymbery and Thompson2015; Tompkins et al., Reference Tompkins2015). Passerines found infected in our study had parasitaemias similar to levels at which chicks experimentally infected with P. juxtanucleare presented physiological alterations such as anaemia (Silveira et al., Reference Silveira2013). This indicates that wild birds can survive the infection by a pathogenic parasite and regain locomotor activity at natural conditions, what had been demonstrated in experimental conditions (Mukhin et al., Reference Mukhin2016). Consequently, passerines can disperse P. juxtanucleare across different biomes and countries in South America due to their wide geographic range (Sick, Reference Sick1997). The eastern slaty thrush (T. subalaris), for example, is a migratory species wintering at central Brazil (our area of study) that use the southernmost region of the country as their breeding sites (Vogel, Reference Vogel2014). Long and short-distance migrants disperse avian haemosporidians within the American continent, sharing a high proportion of parasites with resident species in both wintering and breeding grounds (Roos et al., Reference Roos2015; Ricklefs et al., Reference Ricklefs2017), and the eastern slaty thrush may be important at dispersing this parasite in a broad geographical range. Additionally, P. juxtanucleare-infected bird species use habitat at different integrity levels, such as advanced secondary forests and preserved Cerrado, as well as they are found in peri-urban areas around the protected area of the park (Lopes et al., Reference Lopes2009), what may facilitate the dissemination of P. juxtanucleare within bird communities that do not get into close contact with domestic chickens.
Wild birds infected with P. juxtanucleare presented circulating gametocytes, meaning that they can be the source of infection for vectors, which could subsequently infect other wild birds without the need to feed on domestic chickens. We cannot determine if parasite transmission within wild birds is dependent on the presence of domestic chickens or if the transmission is sustained within passerines. However, nine individuals from four species found infected with P. juxtanucleare near to the farm were not infected by this parasite when captured in the Gallery forest, indicating that the conditions created at backyard chicken production are important for parasite persistence among passerines.
The detection of gametocytes in passerines indicates that P. juxtanucleare can be transmitted from wild to domestic birds as well. This parasite seems to be established within these populations and transmission is likely to occur in both directions. Local asynchronous breeding in the farm analysed here provide susceptible hosts year round, favouring a consistent transmission cycle that may persist over time. Consequences of this infection are usually mild for domestic chickens (Krettli, Reference Krettli1972; Silveira et al., Reference Silveira, DaMatta and Dagosto2009), although high mortality rates (Versiani and Gomes, Reference Versiani and Gomes1943; Garnham, Reference Garnham1966) and reduction in egg production may occur (Massard, Reference Massard1982), showing that P. juxtanucleare epizootic cycle might affect food production and income in small farms.
Mosquitoes from the genus Culex are major vectors of P. juxtanucleare in Asia (Bennett et al., Reference Bennett, Warren and Cheong1966; Chen et al., Reference Chen2015), and C. saltanensis is the only confirmed vector for this parasite in Brazil (Lourenço-de-Oliveira and de Castro, Reference Lourenço-de-Oliveira and de Castro1991). This mosquito species is highly ornithophilic, and inhabit secondary forests and areas under anthropic modifications (Lourenço-de-Oliveira et al., Reference Lourenço-de-Oliveira1986; Consoli and de Oliveira, Reference Consoli and de Oliveira1994). This habitat flexibility can facilitate parasite transmission between habitat generalists and forest specialist birds, favouring host switching even in the absence of infected domestic chickens. Haemosporidian vectors can have broad host preferences in forests under the anthropic influence, favouring parasite shifts between different groups of hosts (Santiago-Alarcon et al., Reference Santiago-Alarcon2012b). Culex saltanensis is also found in Argentina, Panama, and Venezuela (Laurito et al., Reference Laurito, Visintin and Almirón2008), suggesting that transmission of P. juxtanucleare can take place in different areas in South America. However, additional investigations are required to confirm whether C. saltanensis is the vector of this parasite in our study area or if other mosquito species can transmit this parasite as well.
Whole mitochondrial sequencing revealed that P. juxtanucleare infecting passerines in Brazil are very similar to the ones from Asia (Omori et al., Reference Omori2007), the region where this parasite is likely to have been originated. Previous observations suggested that American strains of P. juxtanucleare are more pathogenic (Valkiūnas, Reference Valkiūnas2005), but we lack genetic information to address whether this high virulence is a phenotype linked to particular genetic characteristics of those parasite populations (e.g. unknown virulent factors), circumstances surrounding host exposure, or to other host-related factors.
The novel interaction between birds and parasites reported here have unpredictable conservational consequences. Understanding the epidemiology and transmission cycle of P. juxtanucleare within and between passerines and domestic chickens can provide valuable information to assess disease risk in wild bird populations due to the spillover of this pathogen. Reducing cross-species transmission of pathogens by limiting contact with domesticated animals may significantly reduce the risk of pathogen transmission to wildlife (Pedersen et al., Reference Pedersen2007). However, this can be unachievable in the avian malaria system, as free-living backyard chicken production is widespread in rural areas worldwide. This control is also difficult given that this is a mosquito-borne disease, and vectors can move freely between areas at different levels of habitat integrity (Consoli and de Oliveira, Reference Consoli and de Oliveira1994; Ferreira et al., Reference Ferreira2016), having access to birds that do not get into close contact with domestic fowl.
Common pathogens in poultry production such as Avian paramyxovirus 1 (Garcia et al., Reference Garcia2013) and Mycoplasma gallisepticum (Luttrell et al., Reference Luttrell2001) had been demonstrated to spill over to wild birds, causing clinical disease and mortality episodes. Here, we describe a new pathogen to be considered in future studies assessing diseases transmission risk between wild and domestic birds, such as in programmes for the reintroduction of endangered species (Deem et al., Reference Deem2012) or in wild animals translocations (Ewen et al., Reference Ewen2012; Sainsbury and Vaughan-Higgins, Reference Sainsbury and Vaughan-Higgins2012). Screening for P. juxtanucleare would aim to avoid the introduction of this parasite into new geographical areas or would avoid the introduction of birds in locations where P. juxtanucleare is present in wild birds or in backyard chickens.
Although previous investigations in Brazil did not detect P. juxtanucleare in wild birds captured in urban parks and in the interface between urbanized and preserved areas in the Cerrado biome (Belo et al., Reference Belo2011; Fecchio et al., Reference Fecchio2013), future studies should monitor whether our findings indicate a common or rather a transient and geographically isolated avian malaria spillover. On the other hand, P. juxtanucleare was detected in 2013 by employing partial cyt b sequencing in samples from wild passerines in the Brazilian Pantanal, a wetland-type biome, showing that this spillover may not be restricted to the Brazilian Cerrado (Richard C. Pacheco, unpublished observations). Continuous surveillance with capture-mark-recapture of birds together with haemosporidian screening can detect a possible fluctuation in population densities due to parasite infection (Podmokła et al., Reference Podmokła2017) and may detect the impact of P. juxtanucleare at host community levels.
Experimental studies should be conducted to assess at which levels P. juxtanucleare is pathogenic for native avifauna throughout the globe, to predict current and future impacts of a pathogen spillover that may occur in a broad range. Furthermore, future studies should elucidate which factors may have driven this spillover to be detected only 70 years after the first description of P. juxtanucleare, to understand whether this is due to recent or local modifications in host-vector-parasite relationships or if it was simply due to failures to detect this parasite in the wild.
In conclusion, P. juxtanucleare spillover can be considered another detrimental impact derived from land conversion into agriculture areas in the Brazilian Cerrado, where an epizootic cycle seems to be established among and between domestic and wild birds. With this in mind, the distribution of P. juxtanucleare among free-living birds should be evaluated at large scale given the global distribution of this parasite in domestic livestock. Our study emphasizes that it is important to combine molecular and morphological analyses in blood hematozoa studies since we could only confirm host competence for a novel pathogen relationship after detecting mature gametocytes in blood smears from free-living passerines. Conducting epidemiological surveillance in transition areas between protected areas and livestock production can demonstrate the emergence of pathogens that can threaten wildlife conservation.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S003118201800077X
Acknowledgments
The authors thank the Program for Technological Development in Tools for Health-PDTISFIOCRUZ for use of its facilities. We are grateful to Gabriel M. de La Torre for helping to design the map of our sampling area. We thank Ariana Cristina Pacheco for the silhouettes design and the DNA laboratory at the School of Life Sciences (Arizona State University) for their technical support. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. This manuscript was greatly improved by the insightful comments from four anonymous reviewers.
Financial support
This work was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG). F. C. F. J. and P. S. were supported by the National Postdoctoral Program/CAPES (PNPD/CAPES).
Conflicts of interest
None.
Ethical standards
This study was approved by the Ethics Committee on Animal Research of the Federal University of Mato Grosso (Protocol #23108.033602/12-0) and by Instituto Chico Mendes de Conservação da Biodiversidade (SISBIO 57491).







