Introduction
How likely is it that intelligent life will evolve on a planet? Is it inevitable and can we draw any meaningful conclusions from terrestrial life? In answering this question many authors make direct comparisons with the pace and direction of terrestrial evolution or draw upon the probability of each step in our evolution, based upon the time it took to happen here (Szathmary & Smith Reference Szathmary and Smith1995; Hanson Reference Hanson1998; Watson Reference Watson2008; Calcott & Sterelny Reference Calcott and Sterelny2011; Spiegel & Turner Reference Spiegel and Turner2011; Morris Reference Morris2015; Stevenson Reference Stevenson2017; Schopf Reference Schopf1995; Carter Reference Carter, Bertola and Curi1993). Is the rarity of an event an indication of its inherent low probability, or is it a consequence of the success of the organism that achieved it (Allen & Vermaas Reference Allen and Vermaas2010)?
Consequently, our attempts to answer the so-called Fermi Paradox (Brin Reference Brin1983; Loeb et al. Reference Loeb, Batista and Sloan2016) are at best limited or at worst fatally flawed.
In this paper, we attempt to circumvent these problems by examining not life itself, but the nature of the geography of the planet upon which life evolves. Rather than question the probability of an event in relation to its biological likelihood, we look at the information density of the environment and draw upon recent observations that suggest evolutionary speciation is driven not by biological events such as reproductive isolation, but rather by the availability of niches (Rabosky & Matute Reference Rabosky and Matute2013; Price et al. Reference Price2014). This produces a model that ties the geology of the Cambrian era to the explosive diversification of eukaryote life at the start of this era (Peters & Gaines Reference Peters and Gaines2012).
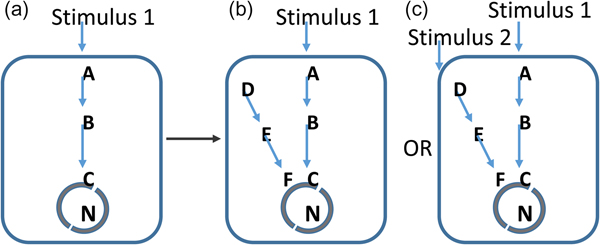
We hypothesize that, the pace of evolution's major innovations is governed by the growth in the information content of the environment – in effect, its Shannon entropy, illustrated in Fig. 1 (Shannon Reference Shannon1948a, Reference Shannonb; Yockey Reference Yockey2005). Organisms that can respond to this growth information content have a clear selective advantage over those which cannot. In this context, information content equates to environmental complexity and includes every factor that can supply measurable information to an organism. Therefore, it includes not only abiotic factors, but also those biotic factors, such as competition, predation and mutualism to which the organism can respond. In this model, biological transitions are irrelevant as a measure of evolutionary pace. The timing of these is misleading, because all you are observing is a record of those events that survive rather than the rate at which they occur. In effect most of the record of innovation at the genetic or biochemical level will be lost if the environment in which the organism exists is not permissive to the change.
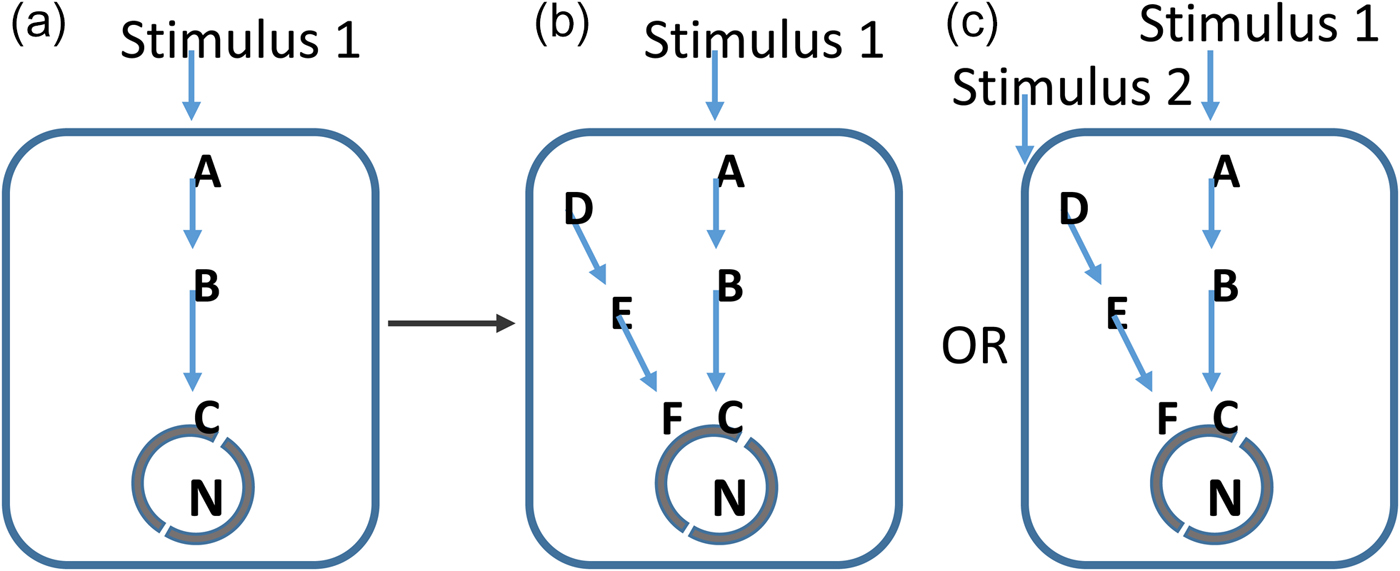
Fig. 1. Evolutionary permissivity. From the perspective of evolution, a mutation in a pre-existing network (a) could give rise to a new network path (b). However, without a clear stimulus to detect with this, the network path will be energetically costly to the organism and will be selected against. In c, an increasingly complex environment provides detectable stimuli and here the same biological change is advantageous. In this model, the pace of evolutionary innovation is set by increases in the information content of the environment. Geological evolution provides these necessary stimuli.
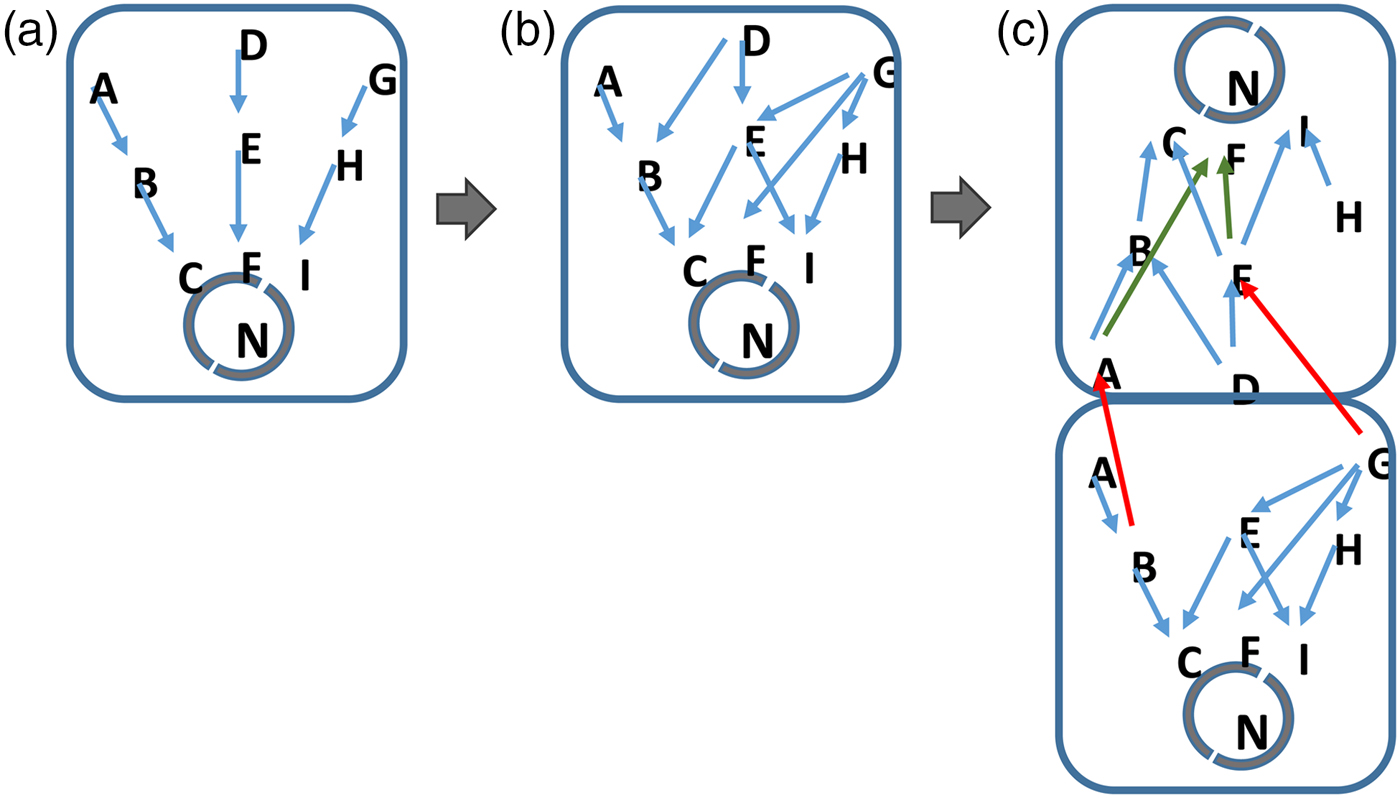
Finally, multicellularity and differentiation of cellular organisms emerges as the complexity of information exceeds the realistic ‘bandwidth’ of the network components in the cell (Fig. 2). Here, the signalling and gene networks in unicellular organisms can diversify so that two or more cells can use pre-existing components to respond to different environmental and cellular signals. By differentiating, cells of different types process different subsets of the available data. Again, this process is driven by the selection of organisms that best respond to the data that are present in their environment and perceivable by them.
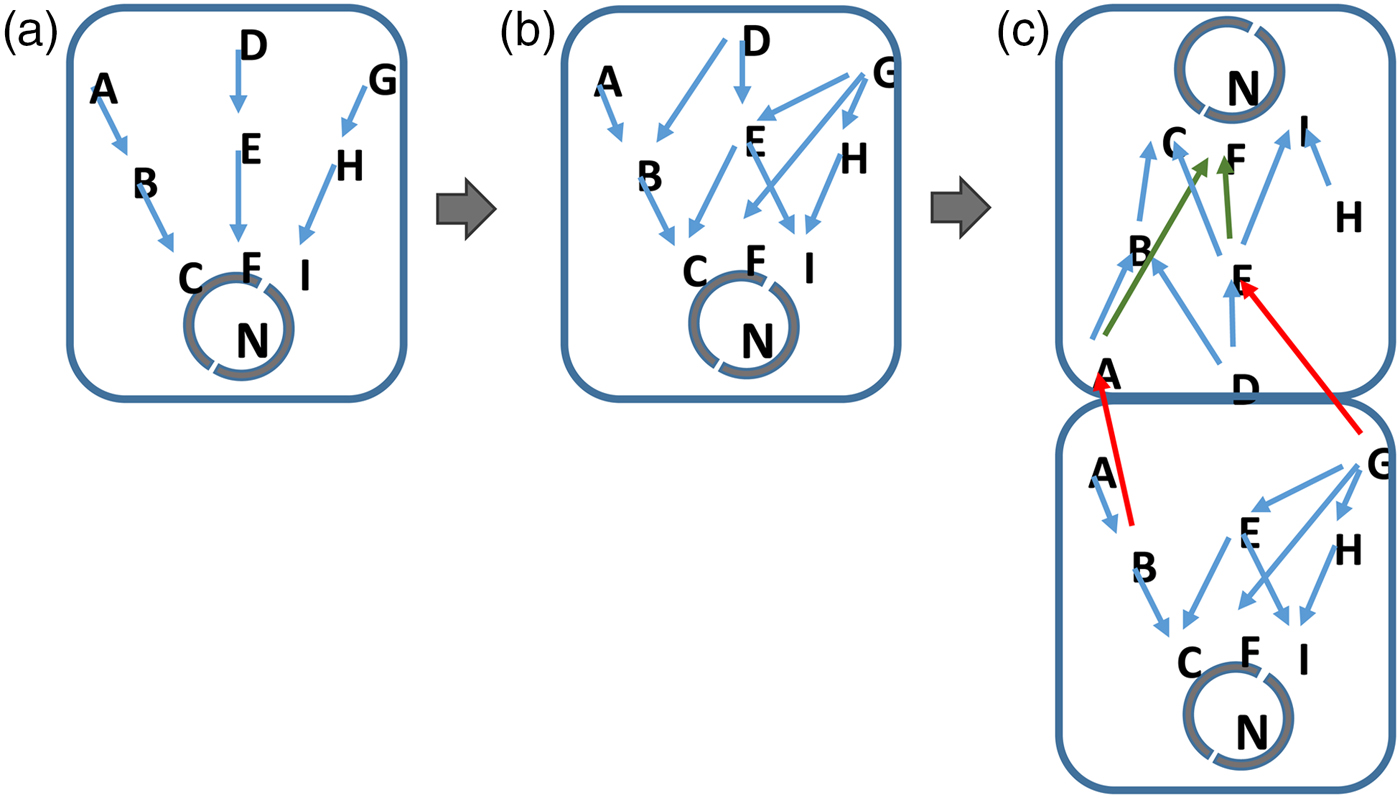
Fig. 2. Information complexity and the inevitable development of life. Cells evolve in step with the increase in complexity of their environment. More signalling pathways for example, emerge in response to an increasingly rich environment (a to b). In (c), the signalling networks in cells become saturated with information. Where the cell cannot realistically or energetically develop further components, a unicellular organism will be at an advantage if it develops additional types of cell, each of which processes a restricted subset of the available environmental information. In this situation, cells do not need to develop entirely new network components but allow different cells within their colony or structure to handle separate subsets of data on their pre-existing networks (c). Such a mechanism will be energetically more favourable to the cells within the organism as a whole. A jack-of-all trades becomes a master of several.
This hypothesis is supported by recent observations by Trevor Price and colleagues who examined speciation in Himalayan song birds (Price et al. Reference Price2014). Their work demonstrated that speciation was dependent on the number of available niches above any other factor. Evolutionary rate was irrespective of the type of niche. This suggests that evolutionary pace is driven by organisms evolving into their environments, rather than something intrinsic to the organism such as the rate of mutation. Competition between species with overlapping niches eventually slowed the rate of evolution in these niche-filling species. Price et al. clearly demonstrate that speciation is not driven by reproductive isolation as is often thought to be the case. This agrees with earlier studies by Rabosky & Matute (Reference Rabosky and Matute2013). Moreover, it concurs with evidence that the Cambrian explosion occurred in an era of expanding niche availability (Peters & Gaines Reference Peters and Gaines2012). As the amount of environmental information is proportional to the number and types of niche one expects the complexity of life to increase in step with the complexity of the environment.
Validating evidence: information density and planetary evolution
Here, we view biological evolution entirely within the fold of planetary evolution (nature ref). Young terrestrial planets will have a lower information density than more mature planets like the present day Earth.
Consider that the early Earth was likely largely or wholly covered with water and bereft of continental landmasses (Rosing et al. Reference Rosing, Bird, Sleep and Bjerrum2010). This is likely also true for a large number of terrestrial planets with low vertical relief in their crust and a substantial supply of volatile materials. During this period the only crust will be dense, mafic (ferromagnesian-rich) in composition. Mantle processes then give rise to further differentiation of the crust and the formation of low-density granitic material. This produces crustal material of greater thickness and buoyancy. Such crust rises higher from the mantle and can produce subaerial land. On Earth this process took at least 300 million years to produce continental rocks of sufficient volume to be recorded in zircons. During this period of low environmental complexity, life likely originated in or around deep oceanic hydrothermal vents on the otherwise cold, dark acidic ocean floor.
On Earth, plate tectonics or related processes then produced voluminous continental rocks and the first continents by 4 billion years ago. Such crust produces a variety of different landscapes, including shorelines, mountains, freshwater lakes and river systems. In our model, the growth of information content in the terrestrial environment drives the development of biological complexity. Increasingly complex environments provide a corresponding increase in environmental data that can select for changes within cellular systems. Organism that innovate new sensory systems will be able to perceive and respond to a greater wealth of information and thus have a selective advantage over those that do not (Fig. 2).
Differentiation of cellular function is driven by the carrying capacity of the networks within the cell – in effect their bandwidth. Where the information density exceeds the cellular bandwidth, cells will be unable to process the additional information. Producing cells with varying characteristics will allow each to process different subsets of environmental information. While biological change produces these subsets of cells from clonal progenitors, only a growth in environmental complexity allows them to persist by selecting for them.
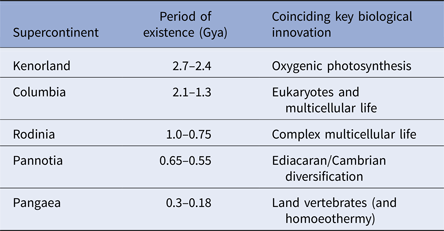
During the last four billion years there are at least three identifiable periods where continents assembled into larger aggregations called supercontinents. These produce environments that are particularly information-rich (Table 1). While the landscapes themselves may be complex, supercontinents also alter the distribution of planetary angular momentum. Where continents aggregate near polar regions, the spin of the planet is destabilized. Episodes of true-polar wander ensue and these tend to redistribute continental masses nearer to the equator (Mitchell et al. Reference Mitchell, Kilian and Evans2012). Such events also, clearly impact on the distribution of climatic belts with respect to the underlying landmasses and these will also alter environmental information leading to changes in environmental data.
Table 1. Supercontinents and life
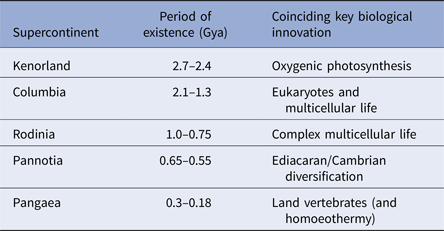
The correlation between the formation, persistence and dissolution of supercontinents and key innovations in biology. Multicellular life emerges shortly after the great oxidation event at 2.45 billion years ago and coincides the oxygen spike that followed it. When Columbia formed oxygen levels declined and eukaryotes emerged. Eukaryotes proliferate into all of their present groups (fungi, animals, plants and protists) during the subsequent reign of Rodinia. Contemporary forms of animal life radiate during the Ediacaran and Cambrian explosion as Pannotia assembles and partly fragments (650–550 million years ago) – the Cambrian explosion occurring 7 million years into the break up. Finally, homoeothermy emerges shortly after the formation of Pangaea. This is likely a response of living organisms to life on land where environmental temperature changes diurnally – and may be an inevitable consequence of living in such habitats.
Geophysical evidence suggests that major periods of supercontinent formation and residence occurred at 2.7–2.45 billion years ago; 2.0–1.3 billion years ago; 1–0.75 billion years ago; 600–550 million years ago and finally 350–180 million years ago (Meert & Torsvik Reference Meert and Torsvik2003; Zhao et al. Reference Zhao, Sun, Wilde and Li2003; Meert Reference Meert2012; Supercontinents: a retrospective essay 2014). The emergence of the first of these supercontinents was coincidental with the development or proliferation of oxygenic photosynthesis (Dismukes et al. Reference Dismukes, Klimov, Baranov, Kozlov, DasGupta and Tyryshkin2001; Allen & Vermaas Reference Allen and Vermaas2010). The second supercontinent coincides with the emergence of eukaryotes (Schirrmeistera et al. Reference Schirrmeistera, de Vosb, Antonellic and Bagheria2013); the third with the proliferation of eukaryotes into their modern lineages (Koonin Reference Koonin2010). The fourth supercontinent coincides with the development of complex multicellular organisms (Hedges et al. Reference Hedges, Blair, Venturi and Shoe2004; Lenton et al. Reference Lenton, Boyle, Poulton, Shields-Zhou and Butterfield2014) and, finally, the last with the radiation of vertebrates and the rise of mammals (Table 1). While it is conceivable that these transitions just happen to coincide with the formation and dissipation of supercontinents, there is at least the suggestion that these geological and biological transitions are linked. Certainly, in terms of information content, supercontinent assembly and disassembly will provide a suitably challenging repertoire of environments with a high and varying information density. Moreover, as subduction processes extract more continental rock from the mantle, each supercontinent will be bigger than its predecessor. Therefore, the information content of the Earth's habitable surface has been growing over time as the possible configuration of land and environment has proliferated (Peters & Gaines Reference Peters and Gaines2012).
In this regard, the Ediacaran–Cambrian boundary is marked by a widespread geological unconformity (Peters & Gaines Reference Peters and Gaines2012). This coincides with the fragmentation of the supercontinent Pannotia and widespread marine transgression. Such a significant event produced an abundance of high-information, oxygenated and shallow, marine environments within which complex multicellular life could evolve. While the presence of oxygen allowed predation and greater mobility (Chen et al. Reference Chen2015; Fox Reference Fox2016), an additional critical factor was the capacity to expand both cellular numbers and the frequency and complexity of signalling within and between cells. Such networks require ATP and GTP to operate and these molecules can be generated more efficiently by aerobic respiration (West et al. Reference West, Bianconi, Severini and Teschendorff2012).
Oxygen also permits the expansion of the genome by accelerating the rate at which it can be replicated (Koonin Reference Koonin2010). Oxygen also eliminates hydrogen sulphide (Hong & Xue-Feng Reference Hong and Xue-Feng2008). This should also accelerate the rate of respiration in aerobic organisms, in turn permitting the other changes. Finally, oxygenation of the atmosphere produces an ozone shield. While the focus might be on the general impact on the habitability of land surfaces, in our model the principle impact is on information density. By permitting life to advance onto land, organisms now have complete access to the wealth of environments that are produced by geological processes. Consequently, the terrestrial ozone shield causes a rapid expansion in the number of available niches and vastly increases the information content available to life.
Extrapolating to other worlds, we assume that the only planets that will host complex, multicellular life will be those with complex, information-dense habitable surfaces. The information content of the habitable surface dictates the complexity of the information available to organisms and, therefore, the evolutionary pace of life on the planetary surface. A complex habitable surface produces sufficient information that can provide a selective advantage to those organisms that can detect and process it. Therefore, Mars, if it does host life, will have limited complexity because the variety of landscapes and hence niches is limited. Similarly, any biologically active ocean under the icy crust of Europa or Enceladus will also host only simple life, because of limited oxygen and limited environmental complexity.
Application to extraterrestrial life
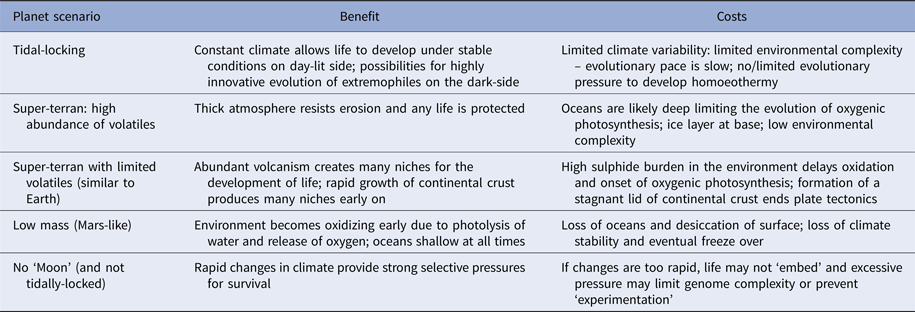
Planets orbiting red dwarf stars are of particular interest to astrobiologists as these likely constitute the vast majority of potentially habitable worlds in the Universe (Heath et al. Reference Heath, Doyle, Joshi and Haberle1999; Anglada-Escudé et al. Reference Anglada-Escudé2016). These planets are tidally-locked to their host stars, meaning one hemisphere permanently faces its star and is lit for billions or trillions of years, while the other face languishes in perpetual night. Such planets may be very rich in volatile elements and have surfaces devoid of dry land (Alibert & Benz Reference Alibert and Benz2017). In this situation, environmental information density may be comparable to the early Earth, and will be low. However, one hemisphere is perpetually dark and cold and likely experiences a static climate. Ocean floors will be warmer but still dark. On the opposing hemisphere, there is light and warmth, the intensity of which varies with distance from the sub-stellar point (Heath et al. Reference Heath, Doyle, Joshi and Haberle1999; Alibert & Benz Reference Alibert and Benz2017) (Table 2).
Table 2. Various scenarios for ‘habitable planets’ and the complications each may cause
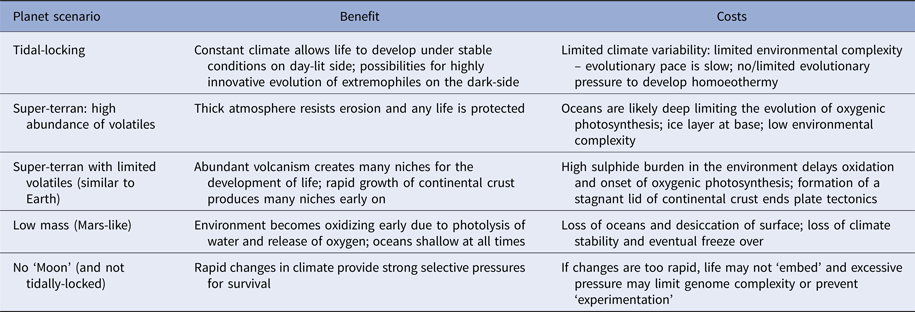
On a tidally-locked planet conditions are very stable which helps life develop, but may slow evolutionary pace by limiting environmental complexity. The converse scenario – a planet that wobbles excessively may have a climate so unstable life cannot embed and remains locked at a very early stage. Others are discussed in the text.
On ocean worlds (aquaplanets) there will be a persistently low information density: this is irrespective of whether they are tidally-locked or not. The environment will be largely dark over the planet's surface, and a deep ocean will have a frozen layer at its base (Vance et al. Reference Vance, Bouffard, Choukroun and Sotin2014; Alibert & Benz Reference Alibert and Benz2017). These worlds will have a low information density and any life that does emerge will only evolve at a snail's pace. Aquaplanets will also lack an appreciable carbonate–silicate cycle and as such will be subject to partial atmospheric collapse as carbon dioxide dissolves in an increasingly acidic ocean (Alibert & Benz Reference Alibert and Benz2017). Without carbon dioxide, any plants that evolve near the ocean's surface will exhaust the available supply, die and rain their organic material to a dark, anoxic floor. Low information density will prevent the development of any complex species and without active photosynthesis – caused by cold and low levels of atmospheric carbon dioxide, there will be no prospect of there being an increase in information density in the planet's lifetime (Fig. 3).
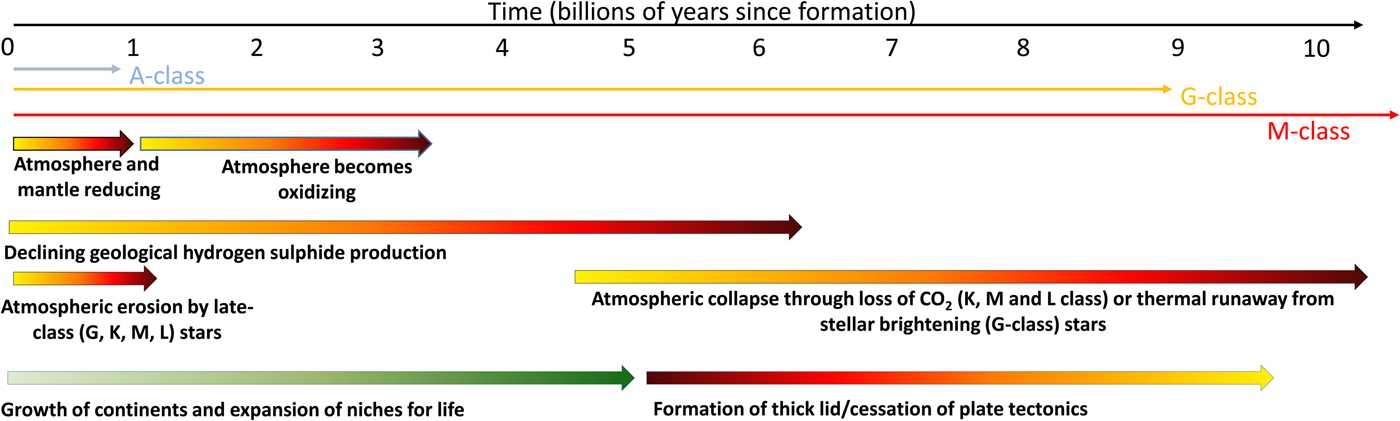
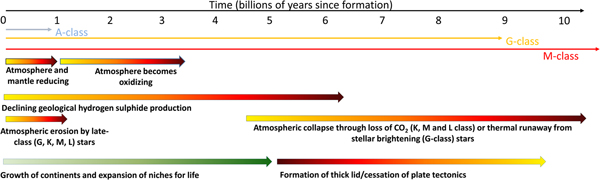
Fig. 3. The factors affecting the rise and eventual fall of life on planets. The origin of life almost certainly requires a reducing environment to support organic molecules. However, this environment will restrict the development of complex life as this almost certainly requires free oxygen. The growth of continents supports the expansion of life and the development of an oxidizing environment. Atmospheric erosion, far from being a problem, may also drive complexity by removing reducing conditions and favouring the formation of oxygenic photosynthesis. At late times life will be restricted and eventually eliminated by the overheating of the environment on planets around G-class stars, or the collapse of the atmosphere around lower class stars when carbon dioxide levels decline. The formation of a thick lid or the loss of plate tectonics will likely accelerate this loss. More massive A-class stars do not live long enough for their planets to develop a high information density.
On tidally-locked planets without a globally-pervasive ocean, tectonic processes should also generate continental crust. However, the environmental information density will be lower than found on Earth. Diurnal cycles effectively double or quadruple the information density of the environment, because most complex organisms are only active during one part of the cycle; that is they are nocturnal, crepuscular or active in the day. This allows more organisms to occupy the same environment at different times. Consequently, we conclude that evolutionary processes will operate more slowly on tidally-locked worlds because the density of available information will be less than half those found on the Earth.
At the other extreme, it is likely that all small terrestrial worlds, such as Mars, will lose most of their atmosphere early in their history. Low mass stars, of stellar classes L, M, K or G, generate strong ultraviolet and X-ray fluxes that readily strip gases from the atmospheres of terrestrial worlds (Som et al. Reference Som, Catling, Harnmeijer, Polivka and Buick2012, Reference Som, Buick, Hagadorn, Blake, Perreault, Harnmeijer and Catling2016; Mahaffy et al. Reference Mahaffy2013; Webster et al. Reference Webster2013; de Wit et al. Reference de Wit2016; Wheatley et al. Reference Wheatley, Louden, Bourrier, Ehrenreich and Gillon2017). This also maintains largely sterile conditions at the surface of such worlds. With few available niches and limited information complexity, life will remain restricted in terms of its complexity – assuming that it evolves at all. These are illustrated in Fig. 2.
Conclusions
While we assume that biological processes, such as gene duplication and mutation occur at broadly consistent rates, their impact will not. In terms of evolutionary pace, the driving factor is the growth in information complexity of the environment in which organisms exist. On planets without plate tectonics, or where there is no dry continental surface, evolutionary processes will be restricted. Organisms evolve into the space they are given and this is a measure of the information density of their environment. Low information landscapes (including aquaplanets) can never evolve complex or intelligent life because the information available to organisms is limited. Conversely, planets that provide a complex information landscape will be those in which the density and complexity of information drives the expansion in the cellular networks and consequently the diversification of multicellular life.