Published online by Cambridge University Press: 19 January 2004
For the diagnosis of imported malaria, optical or immunochromatographic methods are known to be less sensitive and less specific than PCR-based methods, which are conversely more complicated and time-consuming. An original strategy, based upon the sequential use of a multiplex competitive real-time PCR detecting Plasmodium falciparum or Plasmodium spp. infection, followed by, if necessary, a single real-time PCR for species identification, was therefore performed and then tested versus conventional PCR in routine conditions. Conventional PCR has been used since October 1999 in the Department of Parasitology, University Hospitals in Toulouse, as a 2nd line diagnostic method. Out of 183 patients tested, 48 were found to be harbouring a falciparum infection by conventional microscopy, 60 by conventional PCR and 60 by multiplex competitive real-time PCR. Nine further patients had a non-falciparum infection, and concordant species identifications were obtained by both conventional PCR and single real-time PCR. The major value of PCR-based methods, when compared to microscopical techniques, was to ascertain the negativity of a suspect sample. Moreover, real-time PCR allows simplification of the operating procedure, with a diagnosis being made within 2 h.
Imported malaria exerts a growing pressure on the health system of developed countries. In France, as reported by the National Reference Centre for Imported Diseases (NRCID), 2927 cases were notified in 1978, 4071 in 1999 and 4240 in 2000, with an estimated total which would be 2-fold higher (Danis et al. 2002). Eighty-three percent of the infections were due to Plasmodium falciparum. Among these patients, 42% followed incorrect chemoprophylaxis (regimen or drug), what can cause an uncommon clinical picture (Klement et al. 2001), along with very low falciparum parasitaemias.
For the laboratory diagnosis of imported malaria, the PCR-based molecular methods have been found to be more sensitive and specific than the conventional optical (thin and thick blood smear examination, Quantitative Buffy Coat™ [QBC™]) or immunochromatographic techniques (Barker et al. 1989, 1992; Jaureguiberry et al. 1990; Brown et al. 1992; Snounou et al. 1993 a,b; Craig & Sharp, 1997; Humar et al. 1997; Makler, Palmer & Ager 1998; Ciceron et al. 1999; Krudsood et al. 1999; Rubio et al. 1999 a,b, 2001; Tham et al. 1999; Zhong & Kain, 1999).
In 1999, the Department of Parasitology of the University Hospitals, in Toulouse, France, consequently introduced a conventional PCR method (c-PCR). Since then, this technique has been routinely applied along with both thin blood smear examination and QBC™. A comparative assessment of c-PCR and optical methods in a 529-patient set confirmed and strengthened previously published data from more limited samples (Morassin et al. 2002). Particularly, it was observed that conventional methods for the diagnosis of imported malaria missed 32 out of 136 cases found positive by c-PCR. Low parasitaemias, often encountered among imported malaria cases (immune immigrants, or travellers with partially efficient prophylaxis), explained this high rate of false negative results as determined by optical diagnostic techniques.
However, albeit the gold standard for both sensitivity and specificity, c-PCR was found to be inconvenient for the emergency diagnosis of malaria, given the minimal time required (6 h). The so-called real-time PCR, carried out with a LightCycler™ apparatus (Roche Diagnostics, Mannheim, Germany) appeared likely to reduce this technical drawback of c-PCR. In real-time PCR, the amplification step is shortened since capillary tubes, which are better for heat spreading, are used, and a final visualization by electrophoresis or hybridization is not required. Moreover, operating in sealed vials dramatically limits the risk of contamination.
An original diagnostic strategy for imported malaria, based upon the sequential use of a multiplex competitive real-time PCR (mcrt-PCR), followed by a single real-time PCR (srt-PCR) was therefore set up, and its results were compared to those from both microscopical examination and c-PCR.
From mid-June 2001 to the end of October 2001, 183 patients attending the emergency and tropical medicine units of the University Hospitals in Toulouse were tested. Any patient undergoing a malaria diagnostic test was included. All diagnostic tests, including microscopy (thin blood smear examination and QBC™) and molecular methods, were performed daily.
For each patient, 5 ml of whole blood were collected into an EDTA vial. The study used the routine sample provided by the clinical units for microscopy, so patients' informed consent was not required, according to the general position of the Board for Clinical Research of the University Hospital.
Thin smears were made using blood from the EDTA vial, then stained with May-Grünwald-Giemsa (MGG). MGG-coloured slides were examined for at least 20 min by a pool of 4 senior clinical biologists from the malaria unit in the Department of Parasitology. QBC™ (Becton-Dickinson, Le Pont de Claix, France) was performed on the EDTA tubes according to the manufacturer's instructions. QBC™ was read for at least 10 min by the members of the above-cited pool of senior clinical biologists.
Regarding sensitivity, the combined use of thin blood smear and QBC™ is considered as equivalent to thick blood smear examination (Lowe et al. 1996).
DNA extraction was carried out within 24 h from 200 μl of whole blood from the EDTA vial, stored at 4 °C. A commercial DNA extraction kit (High pure PCR™ template preparation kit, Roche Diagnostics, Neuilly-sur-Seine, France) was used according to the manufacturer's specifications. The volume of the DNA template was 200 μl, and the DNA concentration varied between 400 and 600 μg of DNA/ml, depending roughly on the value of the white blood cell count. The c-PCR and real-time PCR were then performed on the same DNA extract.
The detailed process has been previously described (Morassin et al. 2002). Briefly, this PCR procedure was based upon an amplification in microtubes performed in a DNA thermal cycler type 480 (Perkin-Elmer, Courtaboeuf, France), followed by electrophoresis and ethidium bromide staining. Routinely, 2 distinct PCR assays were performed simultaneously, one that detected parasites belonging to the genus Plasmodium (Pspp-PCR), and one that specifically detected P. falciparum (Pf-PCR). Both tests were coupled with an internal amplification control of the human β-globin gene, to check for the absence of inhibitors. For every sample displaying a positive Pspp-PCR and/or Pf-PCR, a second set of 3 PCR assays was subsequently performed 1 day later. These additional PCR assays were specific for P. malariae, P. ovale and P. vivax. Primers used for c-PCR are displayed in Table 1.

Originally, the working hypothesis had been to perform a multiplex real-time PCR for the simultaneous detection of any of the 4 Plasmodium species. Such multiplex real-time PCR assays have been previously proposed for diagnosis, e.g. bacterial (Corless et al. 2001; Sloan et al. 2002) and viral (Mackay, Arden & Nitsche, 2002; Tucker et al. 2001) diseases, and have been found to be efficient and fast. However, an initial experimental investigation showed that the technical constraints for amplification, which were identical for the primer pairs used for the detection of Plasmodium genus and Plasmodium falciparum species, substantially differed when primer pairs for the species diagnosis were employed. Consequently, it was decided to use 2 designs of real-time PCR sequentially, both of which included fluorescence visualization using SYBR Green I (Roche Diagnostics, Mannheim, Germany). The first step of the diagnosis relied upon a multiplex competitive real-time PCR (mcrt-PCR) detecting first a P. falciparum infection, if present, and then an infection by Plasmodium genus. Competitive real-time PCR has been mostly applied as a quantitative method (Widada et al. 2001). In the present study, the competition effect was used in order to bias the PCR reaction towards the exclusive detection of P. falciparum or of Plasmodium genus, according to the type of infectious species.
If the result of mcrt-PCR was positive, a second diagnostic step was carried out, that comprised 3 different single real-time PCR (srt-PCR) for the detection of P. malariae (Pm) or P. ovale (Po) or P. vivax (Pv), and of a mixed infection, if any.
Table 1 displays the primers which were employed. Five μl of extracted DNA were dropped in a capillary tube for LightCycler™, along with 15 μl of LC FastStart DNA Master SYBR Green I, 4 μM MgCl2, 0·08 μM BG07 and BG08 primer, 0·3 μM SSU1/SSU2 primer, and 0·7 μM Pf1/Pf2 primer. Samples were put in the LightCycler™ apparatus along with negative and positive controls in order to undergo, first 8 min of denaturation at 95 °C, then 45 amplification cycles for 15 sec at 95 °C, followed by 10 sec of hybridization at 62 °C and finally 24 sec of elongation at 72 °C. Once the amplification step had ended, the total amount of double-stranded DNA was quantified by the LightCycler™ software that measured the fluorescence from SYBR Green I. This reagent is fluorescent only when fixed onto double-stranded DNA. For every sample, a curve was plotted by the software, that had the number of amplification cycles on the X-axis, and the intensity of fluorescence, expressed as an index, on the Y-axis (Fig. 1).
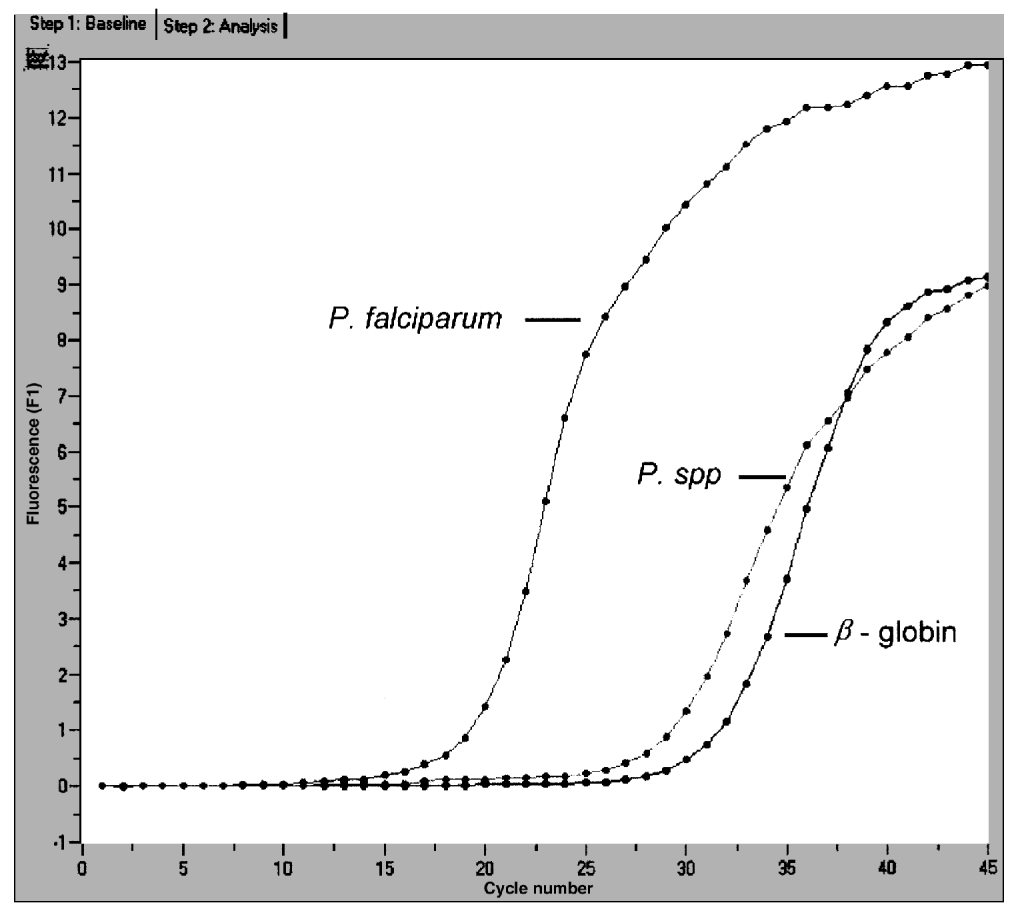
Fig. 1. Fluorescence curves in mcrt-PCR of a falciparum infection, or of a Plasmodium species infection (P. ovale) or from a negative subject (amplification of the human β-globin gene).
With the LightCycler™ method, the specificity of the reaction when a given primer was used was assessed by a specific melting temperature (Tm), which was correlated with the dissociation of double-stranded DNA. Following the amplification step, the temperature in the reaction tubes quickly dropped off from 95 °C to 65 °C, then progressively increased, thus eliciting DNA dissociation. Tm corresponded on the fluorescence curve to 50% of the initial fluorescence index. The LightCycler™ software analysed this dissociation reaction and plotted a curve where fluorescence was on the Y-axis and temperature on the X-axis. The software calculated the derived function of the initial sigmoid curve, so the Tm was in fact a breakpoint, and corresponded with the apex of a peak (Fig. 2). This peak, which indicated the Tm, was different for every Plasmodium species and for the β-globin gene. During the developmental stage of the tests, the specificity of each Tm was checked by the measurement of the size of real-time PCR products by agar gel electrophoresis (Fig. 3).

Fig. 2. Melting curves in mcrt-PCR of a falciparum infection (left), or of a Plasmodium species infection (P. ovale, centre) or from a negative subject (amplification of the β-globin gene, right).

Fig. 3. Electrophoresis gel of real-time PCR products. Lanes 1 and 7, base pair markers; lane 2, sample positive for a non-falciparum infection; lane 3, control sample positive for a non-falciparum infection; lanes 4 and 5, samples positive for falciparum infection; lane 6, control sample positive for falciparum infection.
The primers which were used for identification of P. vivax, P. malariae, and P. ovale are shown in Table 1. As for c-PCR, an additional set of 3 different real-time PCR was used to detect any non-falciparum infection.
Infected red blood cells from continuous cultures of 2 P. falciparum strains (FCM 29, Cameroon, and F32, Tanzania) were diluted in total human blood. The initial parasitaemia was 35000 trophozoïtes/μl, and the final concentration, after serial dilution using an 1[ratio ]10 ratio, was 0·00035 parasites/μl. The mcrt-PCR was performed after DNA extraction of 200 μl of each diluted sample.
The L1 L2 primers used for c-PCR for Plasmodium spp. were targeted to regions of the mitochondrial cox1 gene, according to Tan et al. (1997) and Tham et al. (1999), but these authors did not give any Accession number to GenBank. The GenBank references of the nucleotide sequences of the other genes used for the diagnosis were M76611, M54897, L48986 and X13926 (real-time PCR for Plasmodium spp./P. falciparum, P. malariae, P. ovale, and P. vivax, respectively). The targeted loci were the mitochondrion complete genome of P. falciparum for M76611, the small subunit (SSU) ribosomal RNA gene of P. malariae for M54897, the complete sequence of the clone 26 SSU ribosomal RNA of P. ovale for L48986, and the SSU ribosomal RNA gene of P. vivax for X13926.
Using LightCycler™, the amplification of Plasmodium DNA elicited an increase in fluorescence, the position of which on the X-axis depended on the level of parasitaemia (Fig. 1). The negative controls indicated the background signal. Patient's status was yielded by the analysis of the corresponding Tm, and by the comparison with those from positive and negative control samples. That is, a negative sample only peaked at 87 °C, which was specific for the amplification of β-globin gene, and indicated a lack of PCR inhibitors. A patient with a P. falciparum infection peaked at 78 °C. Since the molar ratio between the oligonucleotide pairs was calculated to prioritize in this competitive amplification, first the falciparum primers, then the Plasmodium genus primers, and finally the β-globin primers, a patient having a falciparum peak did not display any positivity for the β-globin gene, and only a moderate peak for the Plasmodium genus. When an infection by a Plasmodium species other than falciparum was encountered, only a peak at 80·5 °C was observed.
Any sample exhibiting a positive result for P. falciparum or for Plasmodium genus was then tested by 3 specific srt-PCR. Typical Tm was 83·80 °C for a malariae infection, 84·20 °C for an ovale infection, and 77·30 °C for a vivax infection.
Whatever the P. falciparum strain used, FCM 29 or F32, the mcrt-PCR was found to have an experimental sensitivity of 0·035 infected red blood cells/μl, i.e. 35 P. falciparum trophozoites/ml. Out of 183 patients, 48 were found to be harbouring a falciparum infection by conventional microscopy, 60 by c-PCR and 60 by mcrt-PCR, respectively (Table 2). Mc Nemar's χ2 result with Yates's correction was 12·0 (P<0·001), thus indicating a significantly improved sensitivity of both molecular diagnostic methods. Moreover, c-PCR and mcrt-PCR detected the same positive individuals. Regarding non-falciparum species, 9 patients were found positive by QBC™ and thin blood smear examination (1 P. malariae, 4 P. ovale and 4 P. vivax infections). Concordant species identifications were yielded by both c-PCR and srt-PCR.
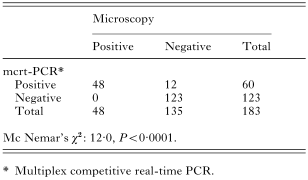
Results from mcrt-PCR and srt-PCR were available within 2 h every working day. The cost of a diagnosis including reagents and technicians' labour was estimated at approximately 4.8€ for a negative sample, and 12.7€ when positive. The depreciation of the equipment accounted for a 17.0€ extra charge, on the basis of approximately 700 tests a year.
The most important technical failure was encountered with mcrt-PCR, given the competition between the 3 pairs of primers. It looked like an increase in the height of the β-globin peak, coupled with a simultaneous decrease in that of both P. falciparum and Plasmodium spp. peaks. Such a problem occurred when a newly synthesized batch of primers was used for the first time, and was fixed by a strict checking of mcrt-PCR's efficacy, followed by an adjustment of molar proportions, if needed.
Since 1903, the year when Ronald Ross introduced the thick smear method, the laboratory diagnosis of malaria has not basically changed. During roughly the last decade, several major improvements have arisen, namely the report of a PCR-based method in 1989 (Barker et al. 1989), the introduction of the QBC™ also in 1989 (Levine, Warldaw & Patton, 1989), and finally the so-called dip-stick technique in 1993 (Shiff, Premji & Minjas, 1993). The value of PCR for malaria diagnosis was recently reviewed (Hänscheid & Grobusch, 2002). The authors stressed that the benefits from PCR would be (a) to confirm the diagnosis of malaria in patients with symptoms, but having repeatedly negative microscopy examinations, (b) to confirm species identification, (c) to detect mixed infections, and (d) to monitor the response to treatment. However, the availability of a PCR result in a clinically useful time-frame was stressed, a requirement not met by c-PCR. Currently, there is a single report on real-time PCR, concerning simply the quantification of the parasitaemia during falciparum infection (Hermsen et al. 2001). Hänscheid & Grobusch (2002) concluded therefore that real-time PCR remained only a potentially interesting method.
The present study, which reports the first comparative assessment of real-time PCR for the routine diagnosis of imported malaria, addresses the above concerns. Since October 1999, c-PCR has been used in the Department of Parasitology of the Toulouse University Hospitals, and has become the reference method (Morassin et al. 2002). Tested versus c-PCR in parallel on any patient for whom a malaria diagnostic was required, namely in the same routine condition of laboratory testing, real-time PCR displayed comparable sensitivity (mcrt-PCR) and specificity (mcrt and srt-PCR). When the operating procedures were compared, real-time PCR exhibited a decisive advantage: from the beginning of DNA extraction to the result on the LightCycler apparatus, the elapsed time was 2 h vs 6 h for c-PCR. Moreover, real-time PCR appeared to be less complicated to use routinely than c-PCR, since the whole diagnostic process was done in a single run and in a single tube. The electrophoresis step was suppressed, and the raw results were stocked as computer files, instead of Polaroid™ pictures. Discarding electrophoresis improved safety, since contamination hazards were avoided.
More sensitive than optical methods, certainly more specific than dip-stick assays, faster and easier to use than c-PCR and straightforwardly quantifiable, mcrt-PCR and/or srt-PCR currently appears to be a useful 2-line test in specialized laboratories, being particularly useful to quickly rule out any malaria infection in a patient found negative by classical investigation methods.
The authors gratefully acknowledge Joëlle Beaudou, Sandra Bernadets, Françoise Bertin, Annie Monier, Catherine Proheze and Jean-Louis Duclos for their technical assistance.

Table 1. Oligonucleotides used for conventional PCR or real-time PCR

Fig. 1. Fluorescence curves in mcrt-PCR of a falciparum infection, or of a Plasmodium species infection (P. ovale) or from a negative subject (amplification of the human β-globin gene).

Fig. 2. Melting curves in mcrt-PCR of a falciparum infection (left), or of a Plasmodium species infection (P. ovale, centre) or from a negative subject (amplification of the β-globin gene, right).
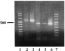
Fig. 3. Electrophoresis gel of real-time PCR products. Lanes 1 and 7, base pair markers; lane 2, sample positive for a non-falciparum infection; lane 3, control sample positive for a non-falciparum infection; lanes 4 and 5, samples positive for falciparum infection; lane 6, control sample positive for falciparum infection.
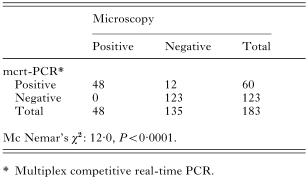
Table 2. Comparative results from microscopy (QBC™ and thin smear) and from multiplex competitive real-time PCR for the diagnosis of falciparum infection