1. Introduction
The Luoping Biota (Anisian, Middle Triassic, Yunnan Province, southwest China) is an exceptional fossil Lagerstätte that contains abundant and diverse marine reptiles, actinopterygians, echinoderms, crustaceans, molluscs, brachiopods and plants. Many new taxa have been described since it was discovered in 2007 by the Chengdu Center of the China Geological Survey (CGS) (Zhang & Zhou, Reference Zhang and Zhou2008). These exceptionally preserved fossils were found in the second member of the Guanling Formation, which is of Anisian age, Middle Triassic (Zhang et al. Reference Zhang, Zhou, Lü, Xie, Lou, Liu, Sun and Jiang2008, Reference Zhang, Zhou, Lü, Xie, Lou, Liu, Sun, Huang and Zhao2009; Hu et al. Reference Hu, Zhang, Chen, Zhou, Lü, Xie, Wen, Huang and Benton2011) (Fig. 1). There are three major quarries, made during large excavations in 2009 and 2015, and these have become scenic spots for the Luoping Biota National Geopark. Currently, thousands of fossil specimens have been obtained and are available at the Land and Resources Bureau of Luoping County for further taxonomic study.

Figure 1. Location map and stratigraphic column.
Fossil fishes are the most abundant and diverse taxa among the marine vertebrates of the Luoping Biota. They are mostly well preserved and include Chondrichthyes, Chondrostei, Neopterygii and Sarcopterygii. Of these, the crown-group Neopterygii includes most taxa in the Luoping fish assemblage, making up nearly 55% of the total, based on counts of the collected specimens. Common elements include the fusiform Sangiorgioichthys and Robustichthys, naked Marcopoloichthys and Gymnoichthys, and deep-bodied Luoxiongichthys and Kyphosichthys (Tintori et al. Reference Tintori, Sun, Lombardo, Jiang, Sun and Hao2007, Reference Tintori, Sun, Lombardo, Jiang, Sun and Hao2010; López-Arbarello et al. Reference López-Arbarello, Sun, Sferco, Tintori, Xu, Sun, Wu and Jiang2011; Wen et al. Reference Wen, Zhang, Hu, Zhou, Xie, Huang, Chen and Benton2012; Xu & Wu, Reference Xu and Wu2012; Xu, Zhao & Coates, Reference Xu, Zhao and Coates2014c). This assemblage of taxa provides an excellent example of the radiation of neopterygian fishes, a key part of the biotic recovery in the sea after the Permian–Triassic mass extinction (Tintori et al. Reference Tintori, Sun, Lombardo, Jiang, Sun and Hao2007; Lombardo et al. Reference Lombardo, Sun, Tintori, Jiang and Hao2011; Chen & Benton, Reference Chen and Benton2012; Benton et al. Reference Benton, Zhang, Hu, Chen, Wen, Liu, Huang, Zhou, Xie, Tong and Choo2013; Tintori et al. Reference Tintori, Hitij, Jiang, Lombardo and Sun2014; Romano et al. Reference Romano, Koot, Kogan, Branyard, Minikh, Brinkmann, Bucher and Kriwet2016). Basal neopterygians are also quite abundant and comprise mainly Perleidiformes and Peltopleuriformes, namely Peltopleurus, Habroichthys, Placopleurus, Altisolepis, Peltoperleidus, Luopingichthys, Perleidus, Luopingperleidus, Fuyuanperleidus and Diandongperleidus (Sun et al. Reference Sun, Tintori, Jiang, Lombardo, Rusconi, Hao and Sun2009; Lin et al. Reference Lin, Sun, Tintori, Lombardo, Jiang and Hao2011; Lombardo et al. Reference Lombardo, Sun, Tintori, Jiang and Hao2011; Geng et al. Reference Geng, Jin, Wu and Wang2012).
Saurichthyid fishes are diverse and abundant (Wu et al. Reference Wu, Sun, Hao, Hand, Xu, Sun and Tintori2009, Reference Wu, Sun, Xu, Hao, Jiang and Sun2010; Zhang et al. Reference Zhang, Zhou, Lü and Bai2010). Other groups of fishes are relatively less diverse than Neopterygii, like the stem-actinopterygian Pteronisculus (Xu, Shen & Zhao, Reference Xu, Shen and Zhao2014b) and coelacanths (Wen et al. Reference Wen, Zhang, Hu, Benton, Zhou, Xie, Huang and Chen2013). Hybodus is the only representative of Chondrichthyes up to now.
Platysiagum sclerocephalum was the first described species of Platysiagum (Egerton, Reference Egerton1872), and both species, Platysiagum minus and Platysiagum sclerocephalum, were included in Platysiagidae by Brough (Reference Brough1939). Helmolepis gracilis was considered to be the plesiomorphic sister group of Platysiagum minus and Platysiagum sclerocephalum by Bürgin (Reference Bürgin1992). Neuman & Mutter (Reference Neuman and Mutter2005) added Helmolepis cyphognathus to Platysiagidae. Coelathichthys was first erected as a member of Paleonisciformes by Lombardo (Reference Lombardo2002). However, it was ascribed to Platysiagidae by Neuman & Mutter (Reference Neuman and Mutter2005), and it was considered to be most parsimoniously closely related to Platysiagidae by Mutter (Reference Mutter2005). Consequently, three genera, Helmolepis, Platysiagum and Caelatichthys, were assigned to the Platysiagidae.
These three genera include seven species, which were widespread from the Early Triassic to Early Jurassic over eastern Greenland (Griesbachian), northwest Madagascar (Dienerian to early Smithian), western Canada (Early Triassic), Italy/Switzerland (Anisian–Ladinian boundary, upper Ladinian) and Great Britain (Liassic) (Stensiö, Reference Stensiö1932; Brough, Reference Brough1939; Nybelin, Reference Nybelin1977; Bürgin, Reference Bürgin1992; Mutter, Reference Mutter2005; Neuman & Mutter, Reference Neuman and Mutter2005; Kogan & Romano, Reference Kogan and Romano2016).
Four specimens among these exceptionally preserved fossil fish materials from the upper fossiliferous layers of the Luoping Biota are assignable to Platysiagidae (Fig. 1), all of which occur, as noted, in the western Tethys region. The new specimens therefore represent the first record of platysiagid fishes from the eastern Tethyan region. In addition, the clade Platysiagidae remains problematic in terms of classification. The new, well-preserved specimens provide more detailed anatomical information than ever, and thus could help better understand the nature of this family and its position among Neopterygii.
2. Materials and methods
2.a. Materials
The materials under study are housed at the Chengdu Center of the CGS. They include four specimens preserved in micrite, all of which have similar standard lengths of c. 43 mm. The first is the best-preserved specimen (LPV-11797, holotype; Fig. 2). The second is an almost complete specimen (LPV-11014, paratype; Fig. 3). The third specimen, LPV-10302, lacks its anal and caudal fins distally (Fig. 4). The cheek, gular and fin regions are broken in LPV-33426 (Fig. 5). The specimens were collected from the same strata and assigned to the same species because of the structure of the dermal bones and fin elements.
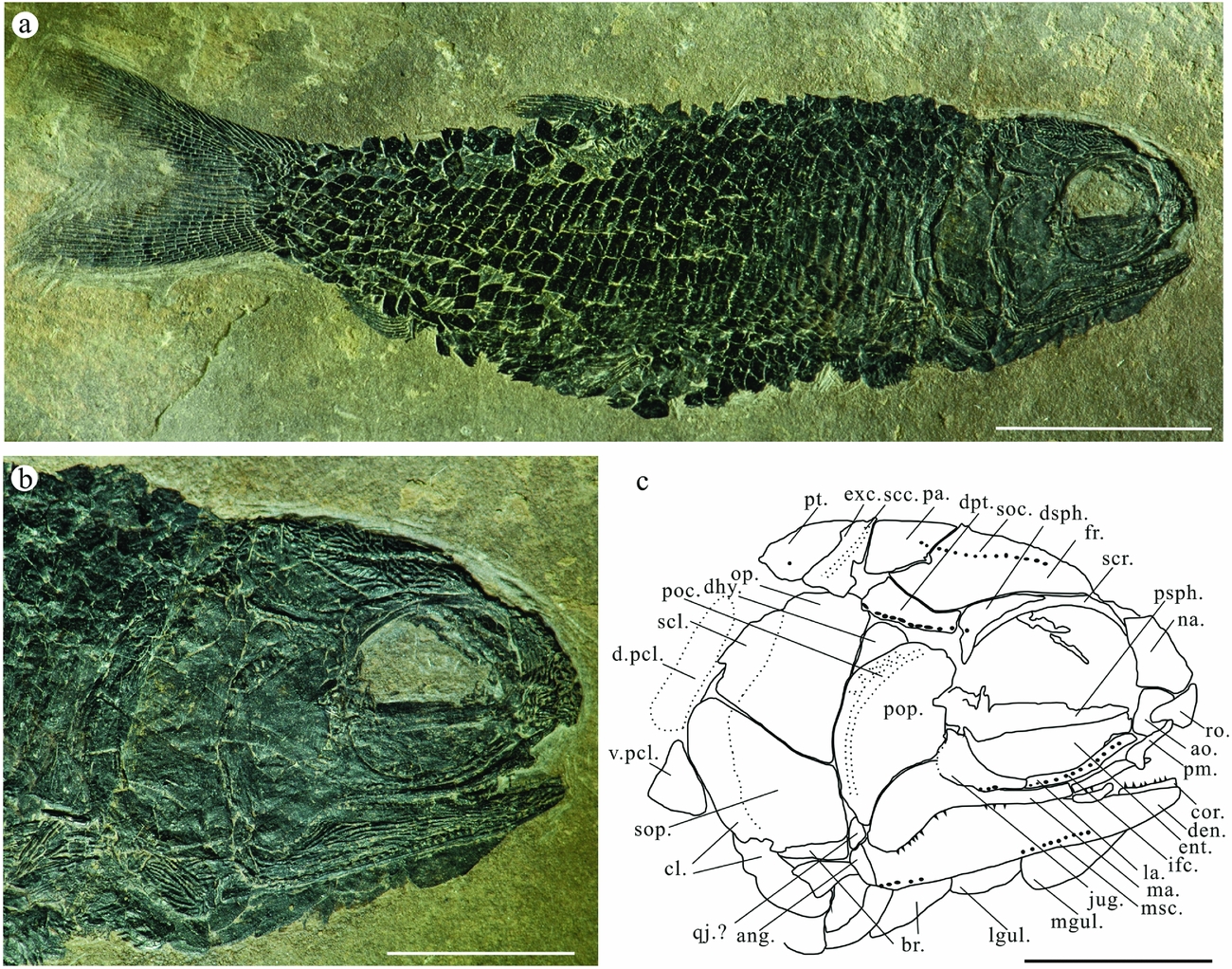
Figure 2. Platysiagum sinensis sp. nov. (a) Photograph of the holotype (LPV-11797). Scale bar = 10 mm. (b) Photograph of the skull. Scale bar = 5 mm. (c) Line drawing of the skull. Scale bar = 5 mm. Abbreviations: ang, angular; ao, antorbital; br, branchiostegal rays; cl, cleithrum; cor, coronoid; den, dentalosplenial; dhy, dermohyal; d.pcl, dorsal postcleithrum; dpt, dermopterotic; dsph, dermosphenotic; ent, entopterygoid; exc, extrascapula; fr, frontal; ifc, infraorbital sensory canal; jug, jugal; la, lachrymal; lgul, lateral gular; ma, maxilla; mgul, median gular; msc, mandibular sensory canal; na, nasal; op, operculum; pa, parietal; pas, parashphenoid; pcl, postcleithrum; poc, preoperculum canal; pop, preoperculum; psph, parasphenoid; pt, posttemporal; qj, quadratojugal; ro, rostral; scc, supratemporal commissural canal; scl, supracleithrum; scr, sclerotic ring; soc, supraorbital sensory canal; sop, suboperculum; v.pcl, ventral postcleithrum.

Figure 3. Platysiagum sinensis sp. nov. (a) Photograph of the paratype. Scale bar = 10 mm. (b) Photograph of the skull. Scale bar = 5 mm. (c) Line drawing of the skull (LPV-11014). Scale bar = 5 mm. Abbreviations: ao, antorbital; br, branchiostegal rays; cl, cleithrum; dhy, dermohyal; dpt, dermopterotic; dsph, dermosphenotic; exc, extrascapula; fr, frontal; ifc, infraorbital sensory canal; io, infraorbital; jug, jugal; la, lachrymal; lden, left dentalosplenial; lgul, lateral gular; ma, maxilla; mgul, median gular; msc, mandibular sensory canal; na, nasal; op, operculum; pa, parietal; pas, parashphenoid; pcl, postcleithrum, poc, preoperculum canal; pop, preoperculum; psph, parasphenoid; pt, posttemporal; rden, right dentalosplenial; ro, rostral; scc, supratemporal commissural canal; scl, supracleithrum; soc, supraorbital sensory canal; sop, suboperculum.
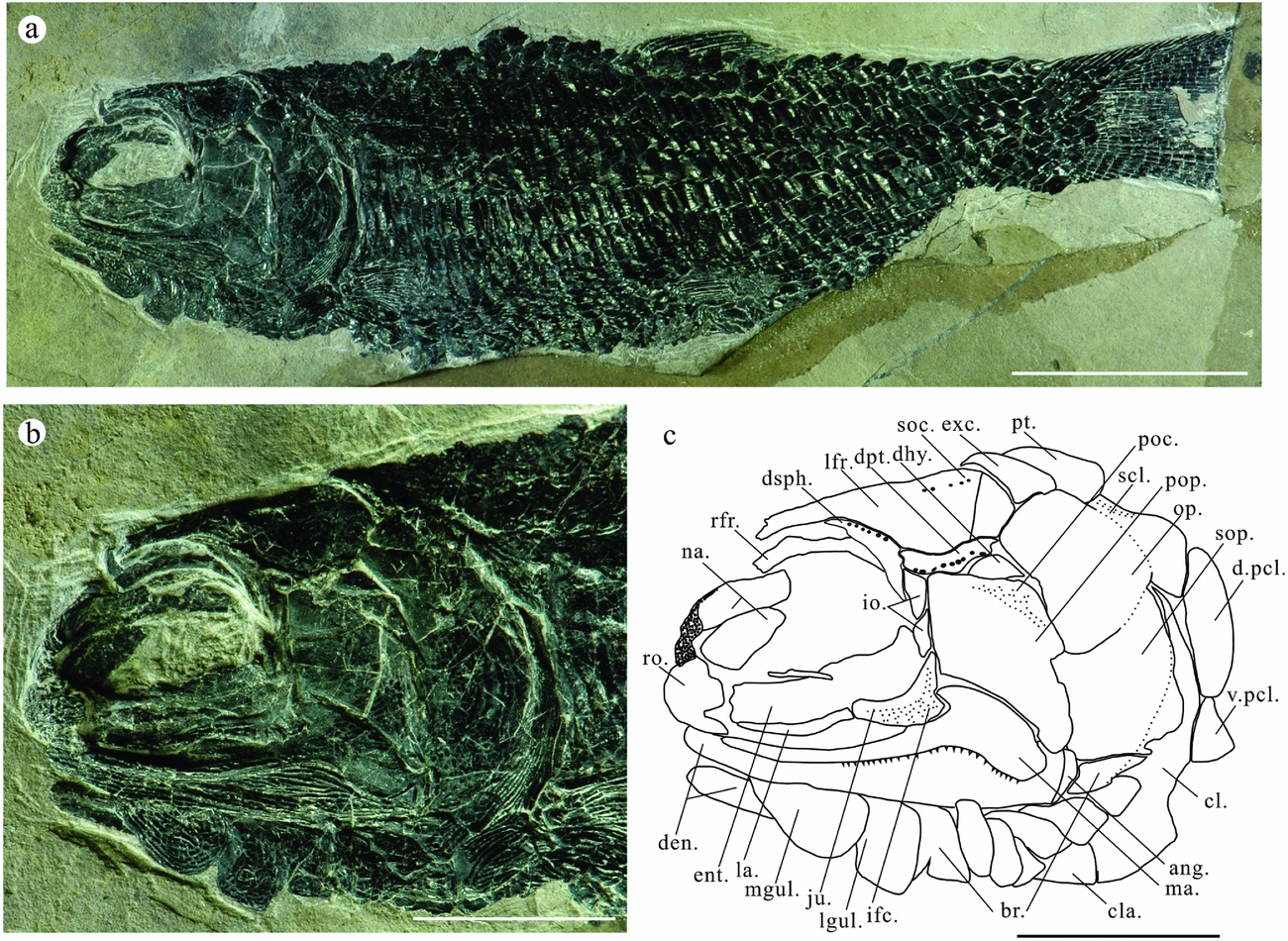
Figure 4. Platysiagum sinensis sp. nov. (a) Photograph of specimen LPV-10302. Scale bar = 10 mm. (b) Photograph of the skull. Scale bar = 5 mm. (c) Line drawing of the skull. Scale bar = 5 mm. Abbreviations: ang, angular; ao, antorbital; br, branchiostegal rays; cl, cleithrum; cla, clavicle; den, dentalosplenial; dhy, dermohyal; d.pcl, dorsal postcleithrum; dpt, dermopterotic; dsph, dermosphenotic; ent, entopterygoid; exc, extrascapula; ifc, infraorbital sensory canal; io, infraorbital; jug, jugal; la, lachrymal; l.fr, left frontal; lgul, lateral gular; ma, maxilla; mgul, median gular; na, nasal; op, operculum; pa, parietal; pcl, postcleithrum; poc, preoperculum canal; pop, preoperculum; pt, posttemporal; rfr, right frontal; ro, rostral; scl, supracleithrum; soc, supraorbital sensory canal; sop, suboperculum; v.pcl, ventral postcleithrum.

Figure 5. Platysiagum sinensis sp. nov. (a) Photograph of the skull for specimen LPV-33426. Scale bar = 5 mm. (b) Close-up of the nasals of specimen LPV-33426. Scale bar = 2 mm. (c) Line drawing of the nasals meeting along the midline. Scale bar = 2 mm. Abbreviations: na, nasal; soc, supraorbital sensory canal.
2.b. Methods
All specimens were prepared with needles under a microscope (Leica M80) at the Chengdu Center of the CGS. Photos were taken using a Nikon D800 camera. Illustrations were drawn manually using Coreldraw X4. A cladistic parsimony analysis was conducted with TNT 1.5 (Goloboff, Farris & Nixon, Reference Goloboff, Farris and Nixon2008), using the traditional search settings and TBR (tree bisection and reconnection) branch swapping, and the strict and majority-rule consensus trees were calculated, with bootstrap values (1000 replicates) and Bremer supports for each node. All characters were treated as unordered and equally weighted. Characters were coded mainly based on Xu, Gao & Coates, (Reference Xu, Gao and Coates2015) and published data, together with codings of Platysiagum minus based on Brough (Reference Brough1939), Bürgin (Reference Bürgin1992, Reference Bürgin, Arratia and Viohl1996) and specimens in the Natural History Museum, London (holotype NHMUK P.19408 and paratype NHMUK P.19420 of Platysiagum minus), Helmolepis based on Mutter (Reference Mutter2005), Neuman & Mutter (Reference Neuman and Mutter2005), and Altisolepis based on Mutter & Herzog (Reference Mutter and Herzog2004), Sun et al. (Reference Sun, Lombardo, Tintori and Jiang2015) and our new specimens.
2.c. Anatomical nomenclature
The terminology used for dermal skull bones here follows the traditional approach rather than implying strict homologies using ‘frontal’ instead of ‘parietal’ and ‘parietal’ instead of ‘postparietal’ (Wiley, Reference Wiley, Arratia, Schultze and Wilson2008) to make comparisons to previously described taxa, although we advocate employing strict homology criteria in the future. Schultze & Arsenault (Reference Schultze and Arsenault1985), Schultze (Reference Schultze, Arratia, Schultze and Wilson2008) and Wiley (Reference Wiley, Arratia, Schultze and Wilson2008) provide reviews of the homology of dermal skull roofing bones and extensive discussions of the weaknesses and strengths of using traditional nomenclatural schemes or those based on homology criteria. The scale counts are expressed in a scale formula following Westoll (Reference Westoll1944).
2.d. The usage of ‘Neopterygii’
The monophyly of Neopterygii is well supported by morphological characters (e.g. Patterson, Reference Patterson1982; Olsen, Reference Olsen1984; Gardiner, Reference Gardiner1985; Gardiner & Schaeffer, Reference Gardiner and Schaeffer1989; Olsen & McCune, Reference Olsen and McCune1991; Coates, Reference Coates1998, Reference Coates1999; Arratia, Reference Arratia2001; Cloutier & Arratia, Reference Cloutier, Arratia, Arratia, Wilson and Cloutier2004; Gardiner, Schaeffer & Masserie, Reference Gardiner, Schaeffer and Masserie2005; Hurley et al. Reference Hurley, Mueller, Dunn, Schmidt, Friedman, Ho, Prince, Yang, Thomas and Coates2007). The only exceptions are studies by Jessen (Reference Jessen, Greenwood, Miles and Patterson1973) based on quantitative analyses indicating closer relationships between chondrosteans and teleosts, and Hurley et al. (Reference Hurley, Mueller, Dunn, Schmidt, Friedman, Ho, Prince, Yang, Thomas and Coates2007) based on mitochondrial data supporting an ‘ancient fish clade’ rather than a monophyletic Neopterygii. However, most other molecular analyses also support monophyletic Neopterygii (e.g. Betancur-R et al. Reference Betancur-R, Broughton, Wiley, Carpenter, Lopez, Li, Holcroft, Arcila, Sanciango, Cureton, Zhang, Buser, Campbell, Ballesteros, Roa-Varon, Willia, Borden, Rowley, Reneau, Hough, Lu, Grande, Arratia and Orti2013). We consequently use the term ‘Neopterygii’ here in a strictly phylogenetic context based on morphological characters including extant and extinct taxa (e.g. Xu, Gao & Finarelli, Reference Xu, Gao and Finarelli2014a), which excludes Chondrostei from this clade, but unites both in a larger monophyletic clade as sister groups.
3. Systematic palaeontology
Class Osteichthyes Huxley, Reference Huxley1880
Infraclass Actinopterygii Cope, Reference Cope1887
Superdivision Neopterygii Regan, Reference Regan1923
Family Platysiagidae Brough, Reference Brough1939
Genus Platysiagum Egerton, Reference Egerton1872
Emended diagnosis (Bürgin, Reference Bürgin1992, Reference Bürgin, Arratia and Viohl1996; Neuman & Mutter, Reference Neuman and Mutter2005). – Small to large-sized (52–600 mm in total length) actinopterygians. Elongate fusiform body with a deeply forked, equilobate and hemi-heterocercal caudal fin. Dorsal and anal fins segmented entirely. Head characterized by a large and broad preoperculum and a maxilla with a long and narrow posterior plate. Dermohyal present. The terminal axial scale lobe reaches over half of the upper caudal fin lobe length. No epaxial rays. Fin rays branch distally. Fringing fulcra on the surfaces of marginal fin rays. Scales with smooth surfaces and serrated posterior border.
Type species. Platysiagum sclerocephalum (Egerton, Reference Egerton1872).
Type locality and type horizon. Early Jurassic of England (Lyme Regis, Dorset).
Stratigraphic and geographic distribution. Anisian–Ladinian of the Besano Formation (Lombardy, North Italy; Canton Ticino, Switzerland). Early Jurassic of England (Lyme Regis, Dorset).
Platysiagum sinensis sp. nov.
Holotype. LPV-11797, a complete specimen, laterally compressed, showing the best-preserved skull and paired and unpaired fins.
Paratype. LPV-11014, an almost complete specimen, lacking the distal part of the caudal fin.
Referred specimens. LPV-10302, lacking its anal fin and caudal fin distally. LPV-33426 with broken cheek region, gular region and fin system.
Type locality. Daaozi Village, Luoxiong Town, Luoping County, Qujing City, Yunnan Province, China.
Stratigraphic distribution. Member II, Guanling Formation, Middle Triassic (Nicoraella kockeli Zone, late Pelsonian, middle–late Anisian).
Etymology. The species name is the Latin adjective ‘sinensis’, meaning ‘from China’.
Diagnosis. Small-sized platysiagid, average standard length 43 mm (average total length 52 mm). Nasals large, meeting in the midline. Post-rostral absent. Preoperculum broad dorsally. Dermosphenotic keystone in shape. Suboperculum larger than operculum or of equal size. Dermohyal present and triangular in shape. No supraorbital. Two small infraorbitals between dermosphenoic and jugal. Tiny sharp teeth distributed on both maxilla and dentalosplenial. Maxilla slightly shorter than dentalosplenial, with posterior region curved downward. Premaxilla slender. Last branchiostegal modified. Medial gular ovoid in shape and larger than lateral gular. One pair of extrascapulars. Two postcleithra. Fin rays entirely segmented with fringing fulcra. No epaxial rays. Scales extend to the upper lobe of caudal fin. Squamation formula is D18/P12, A21, C31/T35. Anterior flank scale rows deepened. Posterior margins of scales serrated.
4. Description
4.a. Skull roof
The dermal bones belonging to the skull roof are complete in the holotype. The long frontals occupy the main part of the skull roof. It extends from the middle part of the otic region to the ventral margin of the parietal, equal to the position of the first two-thirds of the length of the dermopterotic. Its greatest depth appears at the postero-dorsal corner of the orbit. The parietals are triangular in shape and suture to the frontals. The boundary between frontal and parietal is not obvious in specimen LPV-10302. There is only one pair of extrascapulars, which are slender and wedge-like in shape. The triangular posttemporals have round posterior borders. The supratemporal sensory canal runs straight across the extrascapular and extends to the posttemporal (Fig. 2b). The dermopterotic is long and narrow, connecting with the parietal and frontal ventrally. The supraorbital sensory canal runs through the frontal and extends caudally to the parietal, following the basic pattern seen in many basal actinopterygians (Neuman & Mutter, Reference Neuman and Mutter2005). All dermal bones of the skull roof are ornamented with flattened, irregular ridges and tubercles.
4.b. Snout
The rectangular nasal bones are large, being half the length of the frontals. The nasal is longer than wide, forming the rostral border of the orbit. In specimens LPV-10302, LPV-11014 and LPV-33426, the nasals of both sides are exposed. They meet in the midline. The boundary between the left and right nasals is not very obvious in LPV-33426 due to the strong ornamentation (Fig. 5b). The left nasal in specimen LPV-10302 twists inwards. No post-rostral was detected in any specimen, which is different from most other perleidid fishes and ‘Palaeonisciformes’. The shape of the rostral is preserved in specimen LPV-11014. A distinct notch for the anterior nostril is present at the middle level of the lateral margin of this bone. The rostral widens medially, reaches its maximum width (dorso-ventral extension) just anterior to the nostril notch, and then narrows anteriorly, with the ethmoid sensory canal running transversely through the widest portion of this bone. No teeth were detected. In the holotype, only a triangular part of the rostral is preserved, overlapping the rectangular antorbital.
4.c. Opercular apparatus
The operculum is large, conspicuously deeper than wide and has a round dorsal margin. The antero-dorsal margin of the operculum abuts against a triangular dermohyal. The suboperculum is equal in size to the operculum. Both anterior borders of operculum and suboperculum are concave to connect with the preoperculum. The preoperculum is a large, wedge-shaped bone with a broad dorsal portion and a somewhat pointed ventral limb. The preopercular sensory canal is located along the posterior margin and branches into at least six extremities in the dorsal portion in the holotype. The ventral margin of the preoperculum is also concave and contacts the maxilla. Below the preoperculum, there is a separate bone in the holotype. It is, however, impossible to identify it either as a quadratojugal or as a fragment of the peroperculum (Fig. 2). The tubercles and ridges in the opercular apparatus bones are less pronounced than on other dermal bones of the skull.
4.d. Gular region
The oval median gular is well preserved as well as the lateral gular. There are seven to eight branchiostegal rays present. They are triangular in shape with an acute rostral corner and a convex ventral margin. The uppermost branchiostegal ray is modified. It directly connects to the ventral margin of the suboperculum in specimens LPV-10302 and LPV-11797 (Figs 2, 4). The gular region is ornamented in the same way as the elements of the skull roof.
4.e. Circumorbital series
The dermosphenotic appears to be present in all of our specimens, displaying some conspicuous pores for the connection of the supratemporal commissural canal (Figs 3, 4). It is a keystone-shaped bone and resembles that of Perleidus canadensis (A. G. Neuman, unpub. thesis, 1986; note that Neuman & Mutter (Reference Neuman and Mutter2005) considered it a nomen nudum) and Caelatichthys (Lombardo, Reference Lombardo2002). No supraorbital exists, based on all of our specimens. One elongated bone is found in the holotype, but there is no ornament on its surface, and it is covered by the dermosphenotic at the dorsal corner, so it is better to interpret it as part of the sclerotic ring.
The infraorbitals consist of distinct jugal, lachrymal and antorbital bones. The jugal is sickle-shaped, overlapping the postorbital part of the maxilla. A long and slender lachrymal is attached to the jugal. The most anterior bone in the circumorbital series is the antorbital. It seems that there are two small fragments of infraorbitals that connect the dermosphenotic and jugal bones in both specimens LPV-11014 and LPV-10302. Sensory canals are conspicuous on the skull of LPV-11014. The infraorbital sensory canal has five branches at the posterior corner of the jugal, which is similar to that of the preoperculum. The antorbital bears the commissure of the supraorbital and infraorbital sensory canals (Fig. 3).
4.f. Upper and lower jaws
The anterior part of the maxilla, which is located below the orbit, is narrow and bent upward. Its postorbital part is not expanded dorsally, but it is strongly inclined downward, overlapping the dentalosplenial bone. The inclined part is about one-third of its total length. Its dorsal corner is concave, which is always overlapped by the jugal (LPV-11014). The maxilla is slightly shorter than the dentalosplenial. The premaxilla is preserved in our holotype. It is slender and seemingly edentulous, overlapping the maxilla (Fig. 2a, b). There is an expanded bone ventrally to the antorbital in LPV-11014. It is located to the right of the premaxilla, and according to its shape, ornamentation and location it may represent the anterior and slightly dislocated part of the left dentalosplenial in medial view (Fig. 3a, b). The dentatoplenial is a long bone with a slender angular bone posteriorly. The coronoid process is not very conspicuous; only a swelling part can be observed in both holotype and paratype. Longitudinal ridges cover the surface of the dentalosplenial except the smooth swelling part, where the adductor mandibulae muscles inserted. The mandibular sensory canal runs along the ventral margin of the dentalosplenial. Tiny and pointed teeth are distributed along almost the entire length of the maxilla. The teeth on the dentalosplenial are similar in size to those on the maxilla. The jaw articulation is not exposed in any of our specimens. The parasphenoid is exposed in the holotype and paratype. Several blunt teeth can be observed distributed on the ventral surface of the entopterygoid in LPV-11014 (Fig. 3).
4.g. Pectoral girdle and fins
The pectoral girdle is best exposed in LPV-10302 (Fig. 4a, b). The cleithrum is very strong. Its dorsal limb is narrowed to a tip. The ventral limb of the cleithrum is broad, with a posterior notch for the pectoral fin. The oval supracleithrum bears the sensory canal passing through the posttemporal to the flank scales. Its anterior margin is overlapped by the operculum. Two postcleithra can be observed. The upper one is rectangular and the lower one is triangular. A clavicle is present rostral to the cleithrum. The cleithrum and supracleithrum are overlapped by the operculum and suboperculum in both the holotype and LPV-11014. The ventral line of the cleithrum in LPV-33426 is broken. The surfaces of the cleithrum and supracleithrum are ornamented by inclined ridges.
The pectoral fins are small and consist of at least 13 completely segmented rays. The uppermost spinous ray is un-jointed. The fin rays branch distally. Fringing fulcra are not visible in any of our specimens.
4.h. Pelvic girdle and pelvic fins
The pelvic girdle is not preserved in any of our specimens. The pelvic fin is small, inserting at about the 12th scale row. It is closer to the anal than to the pectoral fin. Nearly ten fin rays can be counted. They are entirely segmented and distally branched. Fringing fulcra are preserved on the surface of the marginal fin rays in LPV-10302 (Fig. 4a).
4.i. Unpaired fins
The dorsal and anal fins are well preserved in the holotype and LPV-11014. The dorsal fin is situated at about the 18th scale row, containing at least 16 segmented rays. It is closer to the pelvic fin than to the anal fin. The anal fin originates at about the 20th scale row with c. 12 segmented rays. The radial bones of the dorsal and anal fins are exposed in the holotype. Each radial supports several rays, which is different from perleidid fishes (Fig. 6). Both dorsal and anal fins are preceded by a series of basal fulcra and fringing fulcra posteriorly. Fringing fulcra lie on the surface of marginal leading rays. Fin rays branch at least once distally.

Figure 6. Platysiagum sinensis sp. nov. (a) Relationship between radial and fin rays of dorsal fin on holotype with arrow. Scale bar = 5 mm. (b) Line drawing of the relationship between radial and fin rays of dorsal fin on holotype. (c) Relationship between radial and fin rays of anal fin on holotype with arrow. Scale bar = 5 mm. (d) Line drawing of the relationship between radial and fin rays of dorsal fin on holotype. Peg structures of scales are highlighted by white arrows in line drawings.
4.j. Caudal fin
The holotype has the most complete caudal fin. It is deeply forked and of hemiheterocercal type with 33 segmented fin rays. They are branched at least twice distally. The upper lobe of the caudal fin is hemmed by c. 10–11 basal fulcra and smaller fringing fulcra. The lower lobe of the caudal fin bears only one basal fulcrum and smaller fringing fulcra (Fig. 7). There are no epaxial rays.

Figure 7. Platysiagum sinensis sp. nov. (a) Photograph of the caudal fin for the paratype LPV-11014. Scale bar = 5 mm. (b) Line drawing of caudal fin on holotype LPV-11797. Scale bar = 5 mm. (c) Photography of the caudal fin for the paratype LPV-11014. Scale bar = 5 mm. (d) Line drawing of caudal fin on holotype LPV-11014. Scale bar = 5 mm. Scale line in red colour are the terminal axial scales. Abbreviations: bf, basal fulcra; ff, fringing fulcra.

Figure 8. Reconstruction of Platysiagum sinensis based on LPV-11797, LPV-11014, LPV-10302 and LPV-33426. Scale bar = 10 mm.
4.k. Squamation
There are 34–35 vertical and 15–16 longitudinal scale rows that can be counted at the level of the dorsal fin. The squamation formula is D18/P12, A20, C30/T34. The lateral line runs slightly above the mid-lateral level of the body. The first ten rows of vertical scales are deepened. The depth of the exposed surface is two-thirds longer than its width. The ratio reaches its highest value at the longitudinal scale row beneath the scale row bearing the lateral line. It decreases posteriorly and ventrally, so that the posterior scales are rhombic in outline. In the scales around the pelvic fin, the width is greater than the depth (holotype). There is a long terminal axial scale lobe, which runs along the base of the dorsally situated basal fulcra (Fig. 7). The surface of all scales is smooth and most scales have a serrated posterior margin. The serrated margin is weaker in the peduncle region. Some of them have an unserrated posterior margin. Those scales in the dorsal and ventral regions are rhombic with a ridge protruding from the postero-ventral corner. Scutes appear in front of the pelvic fin, dorsal fin and both upper and lower lobes of the caudal fin. Peg-and-socket articulations are observed on the scales near the anal fin of the holotype (Fig. 6b, d, black arrows).
5. Discussion
5.a. Assignment to Platysiagidae, and their relationships
The new specimens from the Luoping Biota undoubtedly belong to the clade Platysiagidae since the nasals meet in the midline, the post-rostral is absent, the preoperculum is dorsally broad and the suboperculum is larger than the operculum or of equal size. Further, the shape of the maxilla, number of branchiostegal rays (7–8) and squamation identify it as belonging to the genus Platysiagum. The number of branchiostegal rays in Platysiagum sinensis sp. nov. also resembles Platysiagum minus (7–8), is more than in Helmolepis gracilis (6) and less than in Helmolepis cyphognathus (usually 9, even 11) and Caelatichthys nitens (11). The gular region is not preserved in Helmolepis manis (Mutter, Reference Mutter2005).
Platysiagidae is a clade of small to medium-sized actinopterygian fishes with enlarged uppermost branchiostegal rays, a dorsally broad preoperculum, absent post-rostral bone, nasals meeting in the midline, scales extending to the upper lobe of the caudal fin, and a hemi-heterocercal caudal fin. They were previously classified as ‘subholosteans’ (Brough, Reference Brough1939), and then thought probably to be members of the Peltopleurus group (Gardiner & Schaeffer, Reference Gardiner and Schaeffer1989). The characters used to diagnose the Peltopleurus group, however, cannot be found in Platysiagum (Neuman & Mutter, Reference Neuman and Mutter2005). Subsequently, platysiagids were considered to be perleidid fishes, bearing both plesiomorphic and derived features (Bürgin, Reference Bürgin1992). Platysiagum displays similarities with cf. Perleidus and Perleidus canadensis according to the description of Lower Triassic materials from western Canada (Schaeffer & Mangus, Reference Schaeffer and Mangus1976; A. G. Neuman, unpub. thesis, 1986). Bürgin (Reference Bürgin1992) suggested that Platysiagidae should include cf. Perleidus and Perleidus canadensis, but this opinion was later rejected (Neuman & Mutter (Reference Neuman and Mutter2005) considered they are nomina nuda). Platysiagum conversely was assigned to Perleidiformes because it resembles members of this group in many aspects, for example in having the suboperculum slightly larger than the operculum or of equal size, the dorsally broad preoperculum, the dermohyal present, and the maxilla, which is still attached to the preoperculum (Bürgin, Reference Bürgin1992). Although the enlarged last branchiostegal ray was thought to be an incipient interoperculum in Platysiagum minus by Bürgin (Reference Bürgin1992), it certainly is not a real one. Further, the absence of a post-rostral, nasals meeting in the midline, each radial supporting several rays, and the absence of epaxial rays make Platysiagidae distinct from perleidid fishes. Some vestigial epaxial rays were mentioned in Helmolepis cyphognathus, but they are not obvious from the figure (Neuman & Mutter, Reference Neuman and Mutter2005, fig. 6).
The fixed maxilla, numerous branchiostegal rays, entirely segmented fin rays, and the relationship between radials and fin rays all resemble features of ‘palaeoniscid’ fishes (Brough, Reference Brough1939). The type species P. sclerocephalum is incomplete, and its caudal region is almost completely absent. Its head is typically palaeoniscid based on the jaw and opercular region. However, the tail of Platysiagum sinensis sp. nov. is distinct from the full heterocercal condition. The absence of the post-rostral, the dermosphenotic, which is not in contact with the nasal, the maxilla with inclined postorbital part, and the presence of the premaxilla also differentiate it from ‘Palaeonisciformes’ (like Pteronisculus, Palaeoniscum and Ptycholepis).
Helmolepis is undoubtedly the sister taxon of Platysiagum given the absence of the post-rostral, nasals meeting in the midline, the shape of the maxilla and preoperculum, and the medial gular and hemi-heterocercal caudal fin. Caelatichthys is different from Platysiagum and Helmolepis in the shape of the rostral, two postorbitals and a more inclined preoperculum (Lombardo, Reference Lombardo2002; Neuman & Mutter, Reference Neuman and Mutter2005). Although these differences were interpreted as of amblypterid type (Mutter, Reference Mutter2005), our phylogenetic analysis (Fig. 9) suggests that Caelatichthys cannot be included in Platysiagidae any longer, which is consistent with Lombardo (Reference Lombardo2002).

Figure 9. Strict consensus of two trees (TL (tree length) = 144, CI (consistency index) = 0.568 and RI (retention index) = 0.747), illustrating the phylogenetic position of Platysiagidae. Character states supporting the clades include A, 16(1), 17(1), 18(1), 23(0), 37(1)*, 41(1)*, 43(2)*, 72(1); B, 7(1)*, 11(1); C, 35(1)*; D, 23(2), 59(2), 62(2)*; E, 17(1); F, 16(2); G, 8(2);H, 64(1), 69(1)*; I, 33(1)*, 34(1)*; J, 62(0)*, 72(1); K, 43(1)*; L, 5(1), 6(1)*, 8(1), 71(1); M, 31(1), 32(1)*, 36(1); N, 37(1)*, 67(1); O, 19(1), 21(1)*, 22(1), 50(1)*; P, 15(1)*, 20 (1)*, 30(1)*, 65(1); Q, 1(0), 14(1), 40(1)*, 42 (1), 44(1), 45 (1)*, 46(1)*, 56(1); R, 26(2)*, 39(1), 57(1); S, 12(1), 27(1)*, 54(1); T, 28(1)*, 48(1), 49(1)*, 61(1)*, 68(2)*. Character states with an asterisk have a CI of 1.0.
The shape of the dermosphenotic is uncertain in previous specimens of Helmolepis and Platysiagum (Brough, Reference Brough1939; Bürgin, Reference Bürgin1992; Mutter, Reference Mutter2005; Neuman & Mutter, Reference Neuman and Mutter2005). However, the dermosphenotic is well preserved in all of our specimens. Besides, the premaxilla was not well described in either Platysiagum or Helmolepis due to poor preservation. In Helmolepis cyphognathus, the premaxilla was thought to have existed and was perhaps fused with the rostral (Neuman & Mutter, Reference Neuman and Mutter2005), but its exact shape is unknown. A slender premaxilla is preserved in the holotype (LPV-11797). Two infraorbitals are present between the dermosphenotic and jugal, the maxilla is slightly shorter than the dentalosplenial, premaxilla and clavicle present, and two postcleithra, as a combination of characters, confirm that this is a new species of Platysiagum. One radial support for two fin rays and entirely segmented fin rays suggest that it is more plesiomorphic than previously assumed (Mutter, Reference Mutter2005). Nasals meeting in the midline can be seen in some other basal actinopterygians, such as Manlietta, Procheirichthys and Mendocinichthys (Neuman & Mutter, Reference Neuman and Mutter2005). This is also seen in Paraperleidus changxingensis from South China, dated as Griesbachian (Zhao & Lu, Reference Zhao and Lu2007). Maybe this consequently cannot be considered as a synapomorphic character in Platysiagidae. The shape of the maxilla resembles that in some ‘perleidid’ and peltopleurid fishes, like ‘Perleidus canadensis’, Meridensia and Altisolepis (Neuman, unpub. thesis, 1986; Bürgin, Reference Bürgin1992; Sun et al. Reference Sun, Lombardo, Tintori and Jiang2015). The position of the supraorbital described in Platysiagum minus is the same as that in Platysiagum sinensis sp. nov. Additionally, there is no sculpture on its surface. As a result, it is more appropriate to interpret the supraorbital with a question mark in Platysiagum minus as a sclerotic ring (Bürgin, Reference Bürgin1992). The four supraorbitals identified in Helmolepis gracilis by Mutter (Reference Mutter2005) are not so clear. There is no supraorbital described in Caelatichthys. Maybe the absence of a supraorbital is a synapomorphic character in the platysiagid group. No distinct coronoid process exists, which is similar to the condition seen in Platysiagum minus (Bürgin, Reference Bürgin1992). Besides, the new species of Platysiagum is the smallest species within Platysiagidae, with a standard length of 43 mm and total length of 60 mm. The type species P. sclerocephalum is a very large platysiagid, with total length 600 mm. The size of the new species is most like Helmolepis manis, with a standard length of 53 mm, and Helmolepis cyphognathus, with a common total length of 60 mm (Fig. 8).
5.b. Broader significance of the find
The new finds from the Luoping Biota confirm its importance as a major new source of information on marine fossil vertebrates of the Middle Triassic (Hu et al. Reference Hu, Zhang, Chen, Zhou, Lü, Xie, Wen, Huang and Benton2011). Further, the fact that Platysiagum is a neopterygian, albeit a basal one, confirms the significance of the dominance of neopterygians among the Luoping fishes. Recent work has corroborated details of the rather slow recovery of life from the catastrophic Permo-Triassic mass extinction (Chen & Benton Reference Chen and Benton2012), with several fitful bursts of evolution among some fast-evolving groups such as ammonoids and foraminifera through the Early Triassic, but with repeated crises caused by sharp global warming crises. Vertebrate remains are rather sporadic in the Early Triassic of China, with well-preserved faunas first appearing in the latest Olenekian at Chaohu and other sites (Benton et al. Reference Benton, Zhang, Hu, Chen, Wen, Liu, Huang, Zhou, Xie, Tong and Choo2013). New marine reptile clades such as ichthyosaurs and sauropterygians then expanded rapidly in diversity, and size and ecological range in the Anisian. The Luoping biota and others of the same age represent the beginning of this explosion of new taxa. Importantly, the rise of neopterygian fishes, once seen as being largely a feature of the Late Triassic and Jurassic (Tintori, Reference Tintori1998), and a key component of the Mesozoic marine revolution (Vermeij, Reference Vermeij1977), has now been firmly shifted down to the explosive recovery of life in the first half of the Triassic, following the mass extinction.
6. Phylogenetic analysis
The phylogenetic position of the new species was cladistically tested based mainly on an analysis of the data matrix created by Xu, Gao & Coates (Reference Xu, Gao and Coates2015). We made six changes. (1) The repeated character 52 has been deleted. (2) Character 56 (Suborbital/maxilla contact absent) has been deleted because too many taxa scored with character 56(1): Suborbital/maxilla contact absent. (3) Characters 10 and 59 have been merged as ‘Supratemporal–intertemporal/ dermopterotic area’ according to Mutter (Reference Mutter, Upchurch, McGowan and Slater2011), because most taxa in the matrix do not have suborbitals. (4) ‘Lateral gulars’ is evaluated in the data matrix as character 60. (5) Perleidus specimens from the Early Triassic (except those from Southern China) have recently been assigned to Teffichthys (Maramà et al. Reference Maramà, Lombardo, Tintori and Carnevale2017), so the genus name is also revised in the data matrix. (6) Three additional genera (Helmolepis, Caelatichthys, Altisolepis) and the new species were added to identify their positions within the platysiagid group and relationships to other stem-group neopterygians. Additional characters employed here come from specimens housed in the Natural History Museum (London) and from previous studies (Lehman, Reference Lehman1952; Lombardo, Reference Lombardo2002; Mutter & Herzog, Reference Mutter and Herzog2004; Mutter, Reference Mutter2005; Neuman & Mutter, Reference Neuman and Mutter2005; Sun et al. Reference Sun, Lombardo, Tintori and Jiang2015).
In the phylogenetic analysis, two most parsimonious trees (MPTs) were found. The strict consensus of the two MPTs (Fig. 9) has a tree length of 144, a consistency index of 0.568 and a retention index of 0.747. The tree confirms the monophyly of several clades like Platysiagidae, Cleithrolepididae and Thoracopteridae. The relationships of some taxa, however, such as the peltopleurids Altisolepis and Peltopleurus, the perleidid Perleidus, Plesiofuro, the pseudobeaconiid Pseudobeaconia, and Peltoperleidus, which are positioned near the base of the crown clade, remain unresolved. The Cleithrolepididae, Platysiagidae and Pholidopleuridae are positioned basal to these.
The analysis identifies Platysiagum as a basal neopterygian, forming with Helmolepis the Platysiagidae. The basal position of Platysiagidae (as well as that of Perleidiformes) within Neopterygii found here is in good agreement with the results of Xu, Gao & Coates (Reference Xu, Gao and Coates2015), Xu & Ma (Reference Xu and Ma2016) and Xu & Zhao (Reference Xu and Zhao2016).
Platysiagidae was previously assumed to be closely related to Perleididae (Bürgin, Reference Bürgin1992; Mutter, Reference Mutter2005; Neuman & Mutter, Reference Neuman and Mutter2005). The shape of the maxilla, entirely segmented fin rays, the relationship between radials and fin rays, and the absence of epaxial rays are characters identifying this group as more plesiomorphic than Perleidiformes (Perleididae, Polzbergidae, Cleithrolepidae, Gabanellidae, Luganoidae, Pseudobeaconiidae and Colobodontidae). Platysiagidae did not originate from the Perleidiformes, but their ancestor is among more basal groups, confirming the previous hypothesis of Mutter (Reference Mutter2005). Mutter (Reference Mutter, Upchurch, McGowan and Slater2011) tested the relationships between Ptycholepidae and other Acrolepiformes referred to Platysiagidae. His phylogenetic analysis revealed that Acrolepiformes forms a sister-group relationship together with Ptycholepidae plus Platysiagidae. The characters linking platysiagids with ptycholepids are: fewer than ten branchiostegal rays; conspicuous enlargement of the first branchiostegal ray; two pairs of extrascapulars; and equal-sized teeth. Although Platysiagidae is more plesiomorphic than previously assumed, it nevertheless is more derived than Ptycholepidae based on the absence of postrostral and intertemporal. Caelatichthys was placed in Palaeonisciformes by Lombardo (Reference Lombardo2002), but it was later assigned to the Platysiagidae (Mutter Reference Mutter2005; Neuman & Mutter, Reference Neuman and Mutter2005). Our result indicates that Caelatichthys is more plesiomorphic than Platysiagidae, and thus better excluded from the latter group. Altisolepis is also better assigned to the Peltopleuriformes than the Perleidiformes, as suggested by Sun et al. (Reference Sun, Lombardo, Tintori and Jiang2015).
7. Conclusion
The newly found fish materials from the Luoping Biota, southwest China, provide additional anatomical information for the basal neopterygian Platysiagum, particularly in the shape of the dermosphenotic, rostral, infraorbitals and premaxilla, and the relationships between endoskeleton radials and the median fins. The characters confirm that these specimens represent a new species of Platysiagidae. The small teeth and wide gaps between them indicate a diet of small planktonic or nektonic organisms (Bürgin, Reference Bürgin, Arratia and Viohl1996). The phylogenetic analysis confirms that Platysiagidae is more basal within Neopterygii than Perleidiformes. Although the origin of platysiagids remains unknown, it is, however, an isolated phylogenetic lineage that was diverse in the Triassic. Platysiagum sinensis sp. nov. is also the first record of Platysiagidae from eastern Tethys, indicating closer biogeographic relationship between both sides of the Tethys than previously thought.
Acknowledgements
We thank Guang Hui Xu for helpful comments on an early version of the manuscript and illustration. We thank Dr Lorna Steel for access to fossil material in the Natural History Museum (London) and reviewers for constructive suggestions for improving the manuscript. This work is supported by four research grants from the China Geological Survey (DD20160020, 12120114068001, 1212011140051, 12120114030601 and 1212010610211) and National Natural Science Foundation of China (No. 41772022 and 41661134047).
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0016756818000079











