The cascade of reactions leading to plasminogen activation is controlled by a complex network of molecular interactions between plasminogen activators (urokinase-type, u-PA and tissue-type, t-PA) and at least two types of plasminogen activator inhibitors (PAI-1 and PAI-2). The cellular receptor for u-PA (u-PAR) is a cell membrane protein that binds u-PA with high affinity. The primary function of u-PAR is to localize plasminogen activation at the cell surface because u-PA bound to its receptor retains its proteolytic activity. The u-PA/u-PAR system plays a crucial role in the control of fibrinolysis, cell migration, matrix degradation and cell signalling (Alfano et al. Reference Alfano, Franco, Vocca, Gambi, Pisa, Mancini, Caputi, Carriero, Iaccarino and Stoppelli2005).
The notion that a strong correlation exists between cell proliferation and expression of u-PA has been known from the early 1990s. The most powerful growth regulators of cultured cells are among the main factors regulating u-PA expression in vitro (Politis, Reference Politis1996). More recent data suggesting that an intricate relationship exists between expression of the u-PA/u-PAR system and cell growth have reopened the issue. In particular, Alfano et al. (Reference Alfano, Franco, Vocca, Gambi, Pisa, Mancini, Caputi, Carriero, Iaccarino and Stoppelli2005) reviewed several studies and concluded that the u-PA may support cell proliferation and the extensive matrix remodelling associated with tissue regeneration by plasmin-mediated activation of other proteinase classes and growth factors as well as through u-PAR-dependent signalling. Gandhari et al. (Reference Gandhari, Arens, Majety, Dorn-Beineke and Hildenbrand2006) reported that binding of the high molecular weight form of u-PA to u-PAR induced proliferation of MDA-MB-231 breast cancer cells. Silencing of the u-PA/u-PAR genes resulted in inhibition of MDA-MB-231 cell proliferation (Jo et al. Reference Jo, Thomas, Takimoto, Gaultier, Hsieh, Lester and Gonias2007; Ahmad et al. Reference Ahmad, Kong, Wang, Sarkar, Banerjee and Sarkar2009).
There is limited evidence regarding the role of genes implicated in the plasmin-plasminogen system in the bovine mammary gland. Rabot et al. (Reference Rabot, Sinowatz, Berisha, Meyer and Schams2007) reported that u-PA, u-PAR and PAI-1 expression increased during late involution (days 14–28 of the dry period) suggesting that high levels of expression of these enzymes might be a typical characteristic of the non-lactating gland. However, in the same study and in contrast with what is known in other species, high levels of expression of all three genes were also observed in mammary tissue obtained from lactating dairy cows. Thus, the dairy cow might represent a unique case in the family of lactating animals. With respect to cell growth, the evidence regarding the role of u-PA/u-PAR/PAIs is limited and conflicting as well. Politis et al. (Reference Politis, Zavizion, White, Goldberg, Baldi and Akers1995) reported that insulin and insulin growth factor 1 (IGF-1) increased u-PA gene expression and activity and enhanced proliferation of the MAC-T bovine mammary epithelial cells. In contrast, Cheli et al. (Reference Cheli, Politis, Rossi, Fusi and Baldi2003) showed that retinoids inhibited BME-UV cell proliferation but this inhibition did not correlate with any changes in u-PA gene expression or activity. Furthermore, phorbol myristate acetate increased both u-PA and u-PAR expression but caused a small effect on inhibition of the BME-UV mammary epithelial cells (Politis Reference Politis1996). To the best of our knowledge, there are no studies on whether growth factors such as IGF-1 and EGF and lactogenic hormones (oestrogen, hydrocortisone, prolactin, insulin) known to affect mammary function (Akers Reference Akers1985; Baumrucker & Stemberger, Reference Baumrucker and Stemberger1989; Purup et al. Reference Purup, Vestergaard and Sejrsen2000; Zhou et al. Reference Zhou, Capuco and Jiang2008) also affect u-PAR expression in bovine mammary epithelial cells. Because activation of the plasmin-plasminogen system might be the result of down-regulation of PAIs, it is interesting to investigate whether enhanced bovine mammary cell proliferation is correlated with down-regulation of PAI-1 and PAI-2.
Further knowledge of the regulation of PA-related genes by growth factors and lactogenic hormones may provide novel insight regarding the significance of the plasminogen activation cascade in the bovine mammary gland during the lactation cycle. The objective of the present study was to determine the effect of growth factors (IGF-1 and EGF) and three hormones (insulin, dexamethasone, prolactin) on expression of plasminogen activator (PA)-related genes (u-PA, u-PAR, PAI-1, PAI-2) and BME-UV cell proliferation. The three hormones were selected based on published data suggesting that insulin enhances proliferation, hydrocortisone acts mainly as an inhibitor of proliferation and prolactin causes no effect on cell proliferation in various mammary cell culture models (Politis et al. Reference Politis, Zavizion, White, Goldberg, Baldi and Akers1995; Lipka et al. Reference Lipka, Mankertz, Fromm, Lubbert, Buhler, Kuhn, Ragosch and Hundertmark2004; Rubis et al. Reference Rubis, Grodecka-Gazdecka, Lecybyl, Ociepa, Krozowski and Trzeciak2004).
Materials and Methods
Cell culture
The BME-UV1 cell line was established from primary bovine mammary epithelial cells by stable transfection with a plasmid, carrying the sequence of the simian virus 40 early region mutant tsA58, encoding the thermolabile large T antigen (Zavizion et al. Reference Zavizion, van Duffelen, Schaeffer and Politis1996) and is maintained in the Laboratory of Cell Culture, Department of Veterinary Science and Technologies for Food Safety of University of Milan. Cells are routinely cultivated into 75-cm2 tissue culture flasks (Corning Life Sciences, Corning NY, USA), in normal growth medium which consisted of 50% DMEM-F12, 30% RPMI-1640 and 20% NCTC-135 (Sigma-Aldrich, St. Louis MO, USA), supplemented with 10% fetal bovine serum (FBS) (Lonza, Basel, Switzerland), 0·1% lactose, 0·1% lactalbumin hydrolysate, 1·2 mm-glutathione, 1 μg/ml insulin, 5 μg/ml transferrin, 1 μg/ml hydrocortisone, 0·5 μg/ml progesterone, 10 μg/ml l-ascorbic acid and antibiotics (penicillin 100 IU/ml; streptomycin 100 μg/ml). All medium supplements are from Sigma-Aldrich. Cells are maintained at 37°C in a humidified 5%-CO2 incubator until confluence.
Cell proliferation
The first experiment examined the effect of the various hormonal and growth factor treatments on cell proliferation using two methodologies. The first method included the use of the MTT assay, which measures the production of the chromophore formazan from 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide (MTT) in cultured cells (Sigma-Aldrich), as previously described (Baldi et al. Reference Baldi, Losio, Cheli, Rebucci, Sangalli, Fusi, Bertasi, Pavoni, Carli and Politis2004). Formazan is produced in viable cells by the mitochondrial enzyme succinate dehydrogenase. The second methodology used was direct cell enumeration. All details of this method have been described by Politis et al. (Reference Politis, Zavizion, White, Goldberg, Baldi and Akers1995). Results parallelled those of the MTT assay and therefore are not presented in detail in the Results section.
Cells were seeded (2·5×104 cells) in each well of 96-well tissue culture plastic plates (Corning Life Sciences) in normal growth medium. After 24 h media were removed, cells were washed twice with Hank's balanced salt solution (HBSS) and test medium was added. Test medium consisted of serum-free DMEM alone, used as control, and serum-free DMEM containing insulin (bovine; 0·1 and 1 μg/ml), EGF (human; 50 ng/ml), IGF-1 (mouse; 50 ng/ml), prolactin (ovine; 0·01 and 1 μg/ml), dexamethasone (100 nm), dexamethasone (100 nm) plus insulin (1 μg/ml) and dexamethasone (100 nm) plus prolactin (1 μg/ml). All hormones and growth factors were purchased from Sigma-Aldrich. Preliminary time-course experiments showed that both IGF-1 and EGF affected cell proliferation at 24, 36 and 48 h but not at 12 h of incubation. Therefore, incubation for 24 h was selected in all subsequent experiments. Furthermore, other preliminary experiments with various doses of the growth factors were performed to detect the optimum concentrations of IGF-1 and EGF. With respect to IGF-1, three concentrations were tested (1, 10 or 50 ng/ml) and the optimal concentration was found to be the 50 ng/ml which is consistent with the concentration used by Zavizion et al. (Reference Zavizion, van Duffelen, Schaeffer and Politis1996) in the BME-UV cells. With respect to EGF, two concentrations were tested (10 or 50 ng/ml) and they were equally effective but the concentration of 50 ng/ml gave more consistent results. This concentration is higher than the 10 ng/ml utilized by Zavizion et al. (Reference Zavizion, van Duffelen, Schaeffer and Politis1996) and Accornero et al. (Reference Accornero, Martignani, Miretti, Cucuzza and Baratta2009) in the BME-UV cells. The concentrations of all three hormones (insulin, dexamethasone and prolactin) were similar to those utilized by Politis et al. (Reference Politis, Zavizion, White, Goldberg, Baldi and Akers1995) in the MAC-T cell line which was derived in a similar manner with BME-UV cell line.
After 24 h of incubation, treatment test medium was removed, then 150 μl MTT stock solution (5 mg/ml) in PBS was added to each well and finally the plates were incubated for 3 h at 37°C in a humidified chamber. The reaction was terminated by removing the incubation solution and adding 150 μl of dimethyl sulphoxide to dissolve the formazan. The optical density of the dimethyl sulphoxide solution at 540 nm was determined on a Biorad 680 microplate reader (Biorad, USA). The percentage of cell proliferation was calculated as follows: Percentage cell proliferation=(mean optical density in presence of treatment/mean optical density of control)×100. For each treatment three biological replicates were used and the experiment was performed twice.
Gene expression of PA-related genes
The second experiment examined the effects of insulin, EGF, IGF-1, prolactin and dexamethasone (Sigma-Aldrich) on expression of four PA-related genes. Cells were seeded in 6-cm culture dishes (106 cells) in normal growth medium. After 24 h media were removed, cells were washed twice with Hank's balanced salt solution (HBSS) and test medium was added for another 24 h. Test medium used was the same as described in the first experiment. After 24 h of incubation, cells were removed by complete trypsinization and RNA was extracted using TRI reagent (Sigma-Aldrich).
Relative levels of mRNA were quantified with real-time, quantitative RT-PCR. A pair of primers for each of the target genes (u-PA, u-PAR, PAI-1 and PAI-2) and the housekeeping gene (β-actin) was constructed using PERLprimer software (Marshall, Reference Marshall2004). Primers for u-PA, PAI-1 and PAI-2 were designed based on Bos taurus sequences, while b-actin and u-PAR primers were designed in highly homologous regions, between different species, of their respective cDNAs. PCR products from all primer pairs used were verified by sequencing. All primer pairs are presented in Table 1. The amount of sample RNA was normalized by using b-actin as a housekeeping gene. Equal amounts of total RNA were reverse transcribed with the iScript™cDNA Synthesis Kit (Biorad, Hercules CA, USA) according to the manufacturer's instructions using a mix of random hexamers and oligo-dT primers. Real time PCR was performed in the MyiQ2 cycler (BioRad) using the SsoFast™ EvaGreen® Supermix (BioRad) according to the manufacturer's protocol. Each reaction (total volume 20 μl) for the quantification of the housekeeping gene and the target genes contained 50 ng RNA equivalents as well as 450 nm of forward and reverse primers for b-actin and PAI-1 and 350 nm of forward and reverse primers for u-PA, u-PAR and PAI-2. The reactions were incubated at 95°C for 30 s followed by 40 cycles of 5 s at 95°C and 10 s at 60°C. This was followed by a melt curve analysis to determine the reaction specificity. For each treatment two biological replicates were used, each sample was measured in duplicate and the experiment was performed twice. The comparative Ct method was used for relative quantification. The amount of target, normalized to b-actin and relative to a calibrator, is given by 2−ΔΔCT.
Table 1. Nomenclature, nucleotide sequences, length of the primers used and the size of the resulting amplicons for all Real time PCR reactions

Determination of PA activity
The third experiment examined the effect of the insulin, EGF, IGF-1, prolactin and dexamethasone on cell-associated, membrane-bound and secreted u-PA activity by BME-UV cells. Cells were seeded (1·5×105 cells) in each well of 24-well tissue culture plastic plates (Corning Life Sciences) in normal growth medium. After 24 h media were removed, cells were washed twice with Hank's balanced salt solution (HBSS) and test medium was added for another 24 h. Test medium used was the same as described in the first experiment. After hormonal treatment the medium was recovered and stored for the determination of secreted PA activity. The protocol used for recovery of cell-associated and membrane-bound PA has been described previously (Stoppelli et al. Reference Stoppelli, Tacchetti, Cubellis, Corti, Hearing, Cassani, Appella and Blasi1986). Briefly, membrane-bound u-PA, was recovered by a 3-min treatment at room temperature with 50 mm-glycine–HCl buffer, pH 3·0, containing 0·1 m-NaCl, then quickly neutralized with 0·5 m-Hepes buffer, pH 7·5 containing 0·1 m-NaCl. Cell-associated u-PA was obtained from cells after lysis with 1% Triton X-100, 20 mm-Hepes buffer, pH 7·5, containing 10% glycerol. PA activity in all three fractions was quantified using the method described by Politis et al. (Reference Politis, Zavizion, White, Goldberg, Baldi and Akers1995) and Politis (Reference Politis1996). This method is based on the conversion of exogenously provided plasminogen to plasmin from the PA present in the fraction. Plasmin so produced, is subsequently allowed to attack the chromogenic substrate Valine-Leucine-Lysine-p-nitroaniline adjacent to Lysine and liberate the free chromophore p-nitroaniline (V 7221). In this system, changes in colour are directly related to plasmin levels and therefore indirectly to PA activity. All forms of PA activity were normalized by the number of cells. Cell numbers were quantified using a haemocytometer after trypsinization. For each treatment three biological replicates were used, each sample was measured in triplicate and the experiment was performed twice.
Statistical analysis
All data are presented as means and sem. The effect of the various treatments was assessed by ANOVA. Fischer's LSD test was used post hoc, with a 95% confidence interval. All analyses were performed using the PASW Statistics 18 release 18.01 (SPSS Inc., USA) program.
Results
The effect of growth factors and lactogenic hormones on cell proliferation following a 24-h incubation was determined and the results are presented in Fig. 1. Both IGF-1 and EGF caused significant increases in cell proliferation. However, EGF was a more effective (P<0·01) mitogen than IGF-1. No effect (P>0·05) was observed when cells were cultured in the presence of insulin, prolactin, dexamethasone. The combinations of dexamethasone with insulin or prolactin showed a trend for increased cell proliferation, but it was not significant.
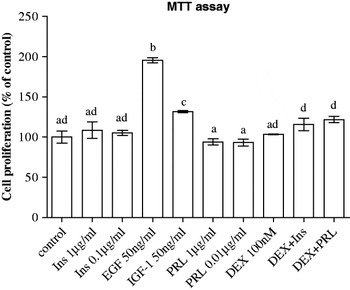
Fig. 1. Effect of lactogenic hormones [insulin (Ins), prolactin (PRL) and dexamethasone (DEX)] and growth factors (EGF and IGF-1) on cell proliferation of BME-UV1 mammary epithelial cells after 24-h treatment. Treatment combinations were made using the highest concentration for each compound. All data are presented as means±sem (n=6). Means without a common letter differ (P<0·05).
The effect of growth factors and lactogenic hormones on expression of u-PA and u-PAR, by bovine mammary epithelial cells was examined and the results are presented in Fig. 2. IGF-1 caused 2-fold increases (P<0·05) in expression of u-PA (Fig. 2A) and u-PAR (Fig. 2B). EGF caused a 3·8-fold increase (P<0·01) in expression of u-PAR (Fig. 2B). Treatment of cells with prolactin at the high concentration (1 μg/ml) caused a 2-fold increase (P<0·05) in expression of u-PA. Dexamethasone alone or in combination with insulin or prolactin showed a tendency to decrease expression of u-PA but this decrease did not reach the designated level of significance (P>0·05).
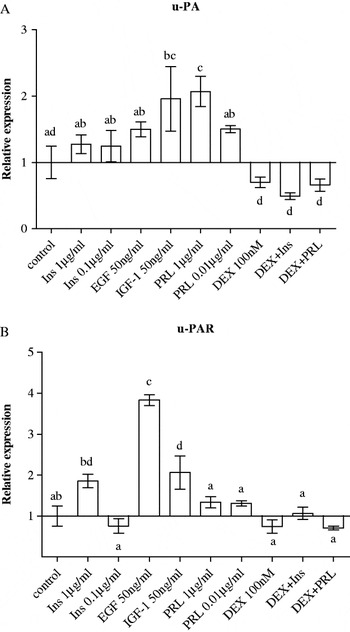
Fig. 2. Effect of lactogenic hormones [insulin (Ins), prolactin (PRL) and dexamethasone (DEX)] and growth factors (EGF and IGF-1) on gene expression of (A) urokinase plasminogen activator (u-PA) and (B) u-PA receptor (u-PAR) in BME-UV1 mammary epithelial cells after 24-h treatment. Treatment combinations were made using the highest concentration for each compound. All data are presented as means±sem (n=4). Means without a common letter differ (P<0·05).
The effect of growth factors and lactogenic hormones on mRNA expression of PAI-1 and PAI-2 by bovine mammary epithelial cells was examined and the results are presented in Fig. 3. Dexamethasone alone and when combined with insulin or prolactin increased by 2–3·5-fold expression of PAI-1 (Fig. 3A) and by 3–4-fold that of PAI-2 (Fig. 3B). Treatment of cells with insulin had no effect (P>0·05) on expression of all four PA-related genes (u-PA, u-PAR, PAI-1 and PAI-2). However, treatment of cells with the high concentration of insulin (1 μg/ml) showed a trend for increasing expression of PAI-2, but was not significant (Fig. 3B). IGF-1 and EGF had no effect on PAI-1 and PAI-2 gene expression.
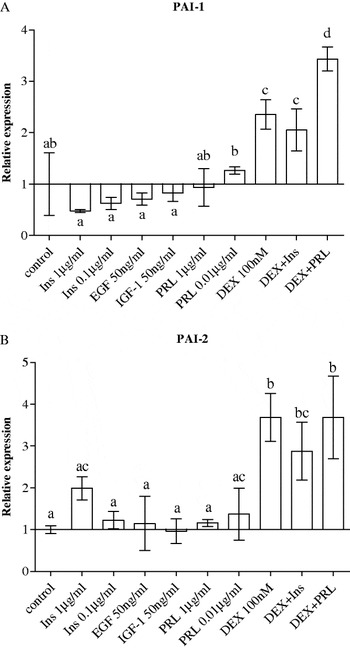
Fig. 3. Effect of lactogenic hormones [insulin (Ins), prolactin (PRL) and dexamethasone (DEX)] and growth factors (EGF and IGF-1) on gene expression of (A) plasminogen activator inhibitor type 1 (PAI-1) and (B) plasminogen activator inhibitor type 2 (PAI-2) in BME-UV1 mammary epithelial cells after 24-h treatment. Treatment combinations were made using the highest concentration for each compound. All data are presented as means±sem (n=4). Means without a common letter differ (P<0·05).
The effect of growth factors and lactogenic hormones on total cell-associated, membrane-bound and secreted u-PA activity was examined and the results are presented in Fig. 4. IGF-1 and EGF increased (P<0·001) total, membrane-bound and secreted u-PA activity on a per cell basis (Fig. 4); EGF increased total cell-associated and membrane-bound activity more (P<0·01) than did IGF-1. Treatment of cells with prolactin at both concentrations (0·01 or 1 μg/ml) had no effect on all forms of u-PA activity. Insulin did not cause any significant (P>0·05) change in any form of u-PA activity when compared with control values (Fig. 4). Consistent with the main effect concerning up-regulation of PAI-1 gene expression, dexamethasone when combined with insulin or prolactin decreased (P<0·05) all three forms of u-PA activity (Fig. 4) but surprisingly treatment with dexamethasone alone had no effect on u-PA activity.
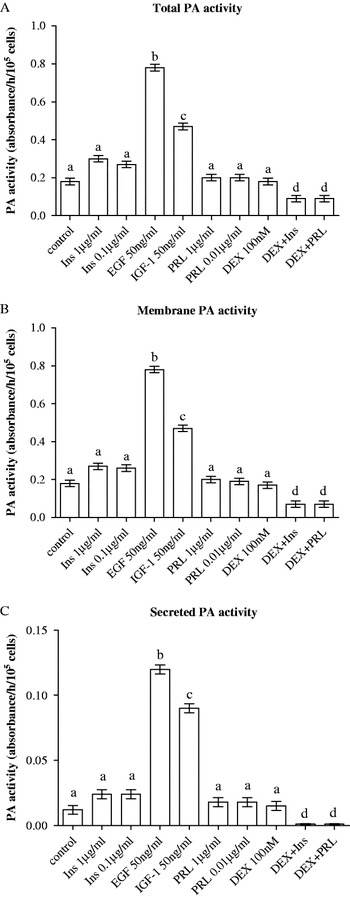
Fig. 4. Effect of lactogenic hormones [insulin (Ins), prolactin (PRL) and dexamethasone (DEX)] and growth factors (EGF and IGF-1) on (A) total, (B) membrane-bound and (C) secreted plasminogen activator (PA) activity in BME-UV1 mammary epithelial cells after 24-h treatment. Treatment combinations were made using the highest concentration for each compound. All data are presented as means±sem (n=6). Means without a common letter differ (P<0·05).
Discussion
We investigated whether IGF-1 and EGF regulated the expression of genes implicated in activating (u-PA, u-PAR) or blocking (PAIs) the plasminogen activating cascade in the BME-UV non-invasive bovine mammary epithelial cell line. Our working hypothesis was that both growth factors enhance cell growth and up-regulate genes implicated in activating the system (u-PA, u-PAR) and/or down-regulate genes implicated in blocking the activation of the system. We showed that both EGF and IGF-1 increased cell proliferation. With respect to gene expression, we found some subtle differences in the mode of action of these two growth factors. For example, EGF increased expression of u-PAR while IGF-1 up-regulated expression of both u-PA and u-PAR. Both growth factors affected neither the expression of PAI-1 nor the expression of PAI-2 by BME-UV1 cells. In a manner consistent with the expression data, both EGF and IGF-1 increased all forms of measured u-PA activity (total cell-associated, membrane-bound and secreted). These data taken together suggest that a relationship exists between cell proliferation and expression of the two genes implicated in the activation of the plasmin-plasminogen system (u-PA, u-PAR).
Our data suggest that EGF was a more effective mitogen than IGF-1 (Fig. 1). Furthermore, EGF increased the amount of u-PA present in cell membranes more than that caused by IGF-1 presumably because EGF induced more u-PAR expression than IGF-1 (Figs 2 and 4). These data are consistent with those of others (Alfano et al. Reference Alfano, Franco, Vocca, Gambi, Pisa, Mancini, Caputi, Carriero, Iaccarino and Stoppelli2005) who indicated that what truly matters is the physical binding of u-PA to u-PAR, in addition to increased expression of u-PAR. In fact, Jo et al. (Reference Jo, Thomas, Takimoto, Gaultier, Hsieh, Lester and Gonias2007) proposed that both conditions are necessary for increased cell proliferation.
Previous studies have provided conflicting results concerning whether activation of u-PA gene or activity is implicated in the mechanism through which IGF-1 affects cell proliferation. The present data suggesting that IGF-1 up-regulated u-PA and u-PAR without affecting any of the PAIs agree with those of Politis et al. (Reference Politis, Zavizion, White, Goldberg, Baldi and Akers1995) who similarly reported up-regulation of u-PA together with elevated u-PA activity on a per cell basis in response to IGF-1. Similar results suggesting that IGF-1 is acting through induction of the u-PA/u-PAR system were provided by Dunn et al. (Reference Dunn, Torres, Nihei and Barrett2000; Reference Dunn, Torres, Oh, Cykert and Barrett2001) using the highly invasive MDA-MB-231 breast cancer cell line. However, against this notion, Cheli et al. (Reference Cheli, Politis, Rossi, Fusi and Baldi2003) showed that retinoids inhibit insulin/IGF-1 mediated BME-UV cell proliferation but have no effect on u-PA mRNA levels and activity. Future u-PA/u-PAR gene silencing experiments will solve the discrepancy between these studies.
With respect to the lactogenic hormones, we observed that dexamethasone alone and when combined with insulin or prolactin up-regulated expression of PAI-1 and PAI-2 by BME-UV1 cells. The combination of dexamethasone and prolactin was more effective than dexamethasone alone or the combination of dexamethasone and insulin indicating a synergistic effect of dexamethasone and prolactin in up-regulating PAI-1 but not PAI-2 synthesis. On the other hand, dexamethasone alone had no effect on u-PA expression but when used in combination with prolactin down-regulated the prolactin-induced increase in u-PA synthetic ability of the BME-UV1 cells. With respect to u-PA activity, dexamethasone alone had no effect but when combined with insulin or prolactin reduced all forms of measured u-PA activity (total cell-associated, membrane-bound and secreted). Thus, the prominent effect of dexamethasone in the BME-UV cells is its ability to down-regulate the plasminogen activating cascade.
None of the lactogenic hormones when used alone or combined had any effect on cell proliferation. This finding taken together with the expression data leads to two conclusions. First, the induction of PAI-1 and PAI-2 synthesis caused by dexamethasone and the overall down-regulation of the plasminogen activating cascade at the molecular and the protein levels are not related to cell growth. Second, the ability of prolactin to induce u-PA expression without an effect on u-PA activity suggests that changes in the u-PA system at the protein level together with physical binding of u-PA to u-PAR might be necessary for induction of cell growth.
Our findings suggest that all modulators of mammary function studied regulate both plasminogen activator inhibitors (PAI-1 and PAI-2) in a similar manner, thus creating an apparently functionally redundant mechanism for inhibition of the system in bovine mammary epithelial cells. Both PAIs share a similar manner of action in blocking the PAs. Therefore, one of them could easily block the system, thus, making the second one redundant. The functional role of PAIs may no longer be simply to inhibit over-expressed PAs but they may serve some additional role in regulating mammary cell functions.
Results from various mammary cell systems suggest that glucocorticoids exercise mainly an anti-proliferative effect on breast cancer cells (Lipka et al. Reference Lipka, Mankertz, Fromm, Lubbert, Buhler, Kuhn, Ragosch and Hundertmark2004; Rubis et al. Reference Rubis, Grodecka-Gazdecka, Lecybyl, Ociepa, Krozowski and Trzeciak2004). Furthermore, glucocorticoids suppress u-PA gene expression and activity (Henderson & Kefford, Reference Henderson and Kefford1993; Politis et al. Reference Politis, Zavizion, White, Goldberg, Baldi and Akers1995; Sasaki et al. Reference Sasaki, Lin and Passaniti1999). Busso et al. (Reference Busso, Belin, Failly-Crepin and Vassalli1987) reported that the glucocorticoid-induced inhibition of the plasminogen activating cascade was related to both a decrease in the synthesis of u-PA and a concomitant increase in the production of PAI-1 in the highly invasive MDA-MB-231 mammary carcinoma cell line. We showed that dexamethasone in BME-UV cells down-regulated the plasminogen activating cascade at the molecular level by causing an increase in expression of both PAIs and by inhibiting the prolactin-induced increase in expression of u-PA.
Our data concerning the effect of prolactin and the lack of an insulin effect on expression of u-PA related genes and cell proliferation deserve some comments. With respect to prolactin, Politis et al. (Reference Politis, Zavizion, White, Goldberg, Baldi and Akers1995) showed that prolactin had no effect on proliferation of MAC-T bovine mammary epithelial cells. In contrast, others have shown that prolactin enhanced proliferation of bovine mammary epithelial cells (Olazabal et al. Reference Olazabal, Munoz, Ogueta, Obregon and Garcia-Ruiz2000) and that of the invasive MCF7 cells (Doll et al. Reference Doll, Pfeilschifter and Huwiler2007). Our data indicate that prolactin up-regulated expression of u-PA but had no effect on all forms of u-PA activity by BME-UV1 cells. Furthermore, as expected, prolactin had no effect on proliferation of BME-UV1 cells. These data taken together indicate that the induction of the u-PA gene alone does not support mammary epithelial cell proliferation but binding of u-PA to u-PAR might be necessary to prime mammary epithelial cells for enhanced proliferation. With respect to the lack of the insulin effect on u-PA expression, it was very surprising to us that insulin did not act in a similar manner to IGF-1. This is because the concentration of insulin used should have activated the IGF-1 receptor. The similarity of the effectiveness between insulin and IGF-1 in increasing both gene expression and activity of u-PA was demonstrated in the MAC-T cell line by Politis et al. (Reference Politis, Zavizion, White, Goldberg, Baldi and Akers1995), which was derived in a similar manner to the BME-UV1 cell line. This discrepancy between the earlier and the present study cannot be explained, but we believe that the lack of the effect is not an artifact or a problem related to biological activity of the insulin used because the combination of insulin and dexamethasone was more effective in reducing all forms of PA activity than dexamethasone alone.
In conclusion, IGF-1 and EGF increased cell proliferation and in parallel caused induction of the u-PA/u-PAR system using the non-invasive bovine mammary epithelial BME-UV cells as a model system. On the other hand, the prominent effect of dexamethasone was to induce PAI-1 and PAI-2 expression and overall to down-regulate the plasminogen activating cascade at the molecular and protein levels. Alterations in the expression of PA-related genes and those observed at the protein level following treatment of BME-UV1 cells with dexamethasone or prolactin (induction of u-PA) are not related to bovine mammary epithelial cell growth. Further studies using various techniques of gene silencing will provide the ultimate answer as to whether u-PA and u-PAR play a role in bovine mammary epithelial cell proliferation.
This study was partly supported by a short-term scientific mission (STSM) grant awarded to Georgios Theodorou (COST-STSM-FA0802-5739) and has been funded by Feed for Health, COST Action FA 0802 (www.feedforhealth.org).







