INTRODUCTION
Endosymbiont microbes that disrupt host reproductive biology are common parasites of arthropods (Bandi et al. 2001). These reproductive parasites obligately inhabit host tissues and achieve transmission only within the egg when female hosts reproduce. To such vertically transmitted bacteria, males are therefore an evolutionary dead end (Hurst, 1991). Transmission to offspring is typically not perfect and many infections impose fitness costs on female hosts. Nevertheless, reproductive parasites can proliferate through populations by biasing host offspring sex ratios in favour of the transmitting sex (females). Sex ratio distortion may be achieved by 3 different mechanisms: male-killing, feminization, and parthenogenesis induction. An alternative reproductive manipulation strategy to sex ratio distortion is cytoplasmic incompatibility (Bandi et al. 2001).
Male-killing bacteria cause male offspring death during embryonic development, resulting in low egg hatch rates in clutches laid by infected females. This is probably the most common mechanism by which reproductive parasites achieve sex ratio distortion and is known only from insects (Hurst and Jiggins, 2000). The phenotype is selected for when the death of male embryos elevates the immediate fitness of sibling females which also harbour the bacterium (Hurst, 1991). This occurs if male death reduces the opportunities for deleterious sibling inbreeding, reduces antagonistic sibling interactions (e.g. egg cannibalism, competition for resources), or if neonate female survival is enhanced by the consumption of dead male embryos within the brood (Hurst and Majerus, 1993). The spread of male-killers in host populations is therefore crucially dependent on host ecology, in addition to genetic interactions between insect and parasite.
The bacterial genus Wolbachia has been considered exceptional amongst reproductive parasite taxa. Infections are very common; most estimates suggest that 17–30% of arthropod species are hosts (Werren, Windsor and Guo, 1995; Werren and Windsor, 2000). Initial reports indicated that Wolbachia was unique in its ability to perform all 4 types of parasitic reproductive manipulation. However, more recently this has been challenged by the findings that members of the Cytophaga-Flavobacterium-Bacteriodes phylum can also induce all 4 phenotypes (Hurst et al. 1997; Weeks, Marec and Breeuwer, 2001; Zchori-Fein et al. 2001; Hunter, Perlman and Kelly, 2003), although the proportion of species infected appears lower than for Wolbachia (Zchori-Fein and Perlman, 2004). In contrast to the limited taxonomic range of bacteria known to have adopted parthenogenesis induction, feminization and cytoplasmic incompatibility, a phylogenetically diverse array of microbes is responsible for male-killing. Male-killers have been reported from 5 different bacterial taxa: Spiroplasma, γ-proteobacteria, Rickettsia, Flavobacteria and Wolbachia (Hurst and Jiggins, 2000).
Majerus and Hurst (1997) suggested that the aphidophagous Coccinellidae are particularly prone to invasion by male-killers due to their ecology: indeed, over 50% of species are estimated to be infected (Hurst, Jiggins and Majerus, 2003). Thus coccinellids are ideal organisms for investigating the diversity of symbionts responsible for male-killing. The majority of known host species are generalists, with broad ecological requirements. In contrast, Anisosticta novemdecimpunctata is a habitat specialist found only on emergent aquatic plants or terrestrial vegetation immediately adjacent to water bodies (Majerus, 1991). Majerus and Hurst (1997) outlined four criteria which may predispose a coccinellid species to male-killing infection: it should be aphidophagous, lay eggs in clutches, indulge in larval sibling egg cannibalism, and starvation should be a major cause of neonate larval mortality. Anisosticta novemdecimpunctata fulfils the first three of these, and whilst the latter has not been investigated, it seems probable it applies. Hence, we predicted that A. novemdecimpunctata would be an ecologically suitable host for male-killer infection. Our discovery of females that produced only daughters provided an opportunity to investigate the nature and cause of this sex ratio trait.
MATERIALS AND METHODS
The sex ratio trait
Anisosticta novemdecimpunctata were collected from Phragmites and Typha dominated aquatic habitats. Two English locations were sampled, one in the East (Cambridge: grid reference, TL 434587) and one in the Southwest (Chew Lake, Somerset: grid reference ST 575592) in October 2001 and March 2002 respectively. Adult beetles were maintained in the laboratory in 9 cm diameter Petri dishes at 21 °C under a 24 h L photo-period and fed pea aphids (Acyrthosiphon pisum) and artificial diet (Majerus et al. 1989). Petri dishes were changed daily; those with egg clutches were retained, eggs counted and hatch rates of clutches subsequently determined. Larvae were reared and sexed when adult under carbon dioxide anaesthesia. Male and female A. novemdecimpunctata are distinguished by a prominent anteriorly-directed groove in the posterior-most ventral abdominal segment of females and a clear ingression of the same region in males (Randall, Majerus and Forge, 1992). Female-biased broods were identified and inheritance investigated in the F1 generation. To determine whether sex ratio distortion was bacterial in origin, F1 females were treated with the antibiotic tetracycline hydrochloride in golden syrup (10% w/w) for 4 h daily over 10 days (Hurst, Majerus and Walker, 1992). Eggs were collected before, during and after this treatment and resulting larvae reared to adulthood. Golden syrup alone does not influence offspring sex ratios in other coccinellids (Hurst et al. 1992). To confirm this, 1 F1 female was fed only golden syrup as a control. Offspring samples produced during treatment were preserved for molecular analysis.
Infectivity of the sex ratio trait
Donor A. novemdecimpunctata from all-female lineages were fed excess aphids for 1 week to promote ovary development and ensure adequate tissue volume. Adalia bipunctata from equitable sex ratio lines and uninfected by male-killers were reared to pupal stage to act as recipients. Adalia bipunctata was employed because its larger size facilitated injection and laboratory rearing is more successful than for A. novemdecimpunctata. Following the method of Hurst et al. (1999b), donor A. novemdecimpunctata abdomens were removed and homogenized in sterile 0·7% NaCl solution (10 μl per abdomen) on ice. Injection needles were pulled from glass capillaries (1·1 mm internal diameter) and attached to a 10 μl Hamilton syringe. Recipient A. bipunctata pupae were injected with 1 μl of homogenate adjacent to the dorsal midline, between abdominal segments 2 and 3. Following pupal eclosion, males were discarded and females maintained for 1 month to permit bacterial replication, then mated and bred.
Identification of the bacterium responsible
Following the method of Jiggins et al. (2000), a modified version of the Chelex 100 protocol was employed to extract genomic DNA. Beetle abdomens were homogenized in buffer containing 200 μl of sterile distilled water, 0·01 g Chelex-100 ion-exchange resin (Bio-Rad), 7 μl of 1 M dithiothreitol and 1 μl of proteinase K (20 mg ml−1), then digested for 70 min at 56 °C. Extractions were subsequently incubated at 96 °C for 10 min and centrifuged at 16000 g for 5 min. Bacterial presence was investigated by diagnostic PCR using primers specific for male-killing bacterial taxa: Rickettsia RSSUF–RSSUR (Schulenburg et al. 2001), Wolbachia wsp81f–wsp691r (Zhou, Rousset and O'Neill, 1998), Flavobacteria FL1–FL2 (Hurst et al. 1997) and Mollicutes MGSO–Ha-In-1 (van Kuppeveld et al. 1992; Hurst et al. 1999b). Mitochondrial DNA primers J2630–N3014 (Schulenburg et al. 2002) were used to verify the presence of amplifiable template and samples not yielding product were discarded. PCR reactions were run using a premix containing 12·6 μl of sterile distilled water, 2 μl of 10× PCR reaction buffer (Sigma), 2 μl of MgCl2 (25 mM: Sigma), 2 μl of dNTPs (2 mM per nucleotide: Bioline), 0·2 μl of each primer (50 pM) and 0·6 μl of REDTaq™ DNA polymerase (1 U μl−1: Sigma). DNA extraction supernatant (0·5 μl) was added and reactions run in either a Techne (Progene and Genius models) or Hybaid (OmniGene) thermal cycler including appropriate positive and negative controls. For primers wsp81f–wsp691r, FL1–FL2 and J2630–N3014, cycle conditions were: 2 min at 95 °C, then 35 cycles of 20 sec at 95 °C, 1 min at 60 °C and 1 min at 72 °C, followed by 10 min at 72 °C. For primers RSSUF–RSSUR and MGSO–Ha-In-1, cycle conditions were: 2 min at 95 °C, then 35 cycles of 20 sec at 95 °C, 30 sec at 57 °C and 30 sec at 72 °C, followed by 10 min at 72 °C. PCR products were electrophoresed on 1% agarose gels containing 0·5 ng ml−1 ethidium bromide in 1×TAE buffer, then visualized and photographed under UV light.
The bacterial 16S rDNA gene and adjoining internally transcribed spacer (ITS) region were amplified from 2 all-female lineages in 2 overlapping fragments using primer pairs 27f–MGSO and Ha-In-1–SPITS-N2, specific for the Mollicutes and Spiroplasma genus respectively (van Kuppeveld et al. 1992; Hurst et al. 1999b). Both strands were sequenced using the PCR primers and one internal primer (AnSpInt1r: 5′-GTT TGG GCC GTG TCT CAT C-3′). Products were electrophoresed, bands excised and DNA purified using GenElute™ gel extraction columns (Sigma) following manufacturer's instructions. Sequencing employed the ABI PRISM Dye Terminator cycle sequencing ready reaction kit (Perkin Elmer) and an ABI 384 automated sequencer (Perkin Elmer). Sequence homology was first investigated by BLAST search. The A. novemdecimpunctata sequence (EMBL Accession number AM087471) was then manually aligned with published Spiroplasma ribosomal sequences: 3 male-killing and 3 non-male-killing spiroplasmas from the Spiroplasma ixodetis clade and 2 sequences from the Spiroplasma citri group. The variable ITS region was unalignable for more distantly related sequences, therefore 2 data sets were separately investigated using PAUP* version 4.0b8 (Swofford, 1998). Initially, only the 16S rDNA region of all sequences was studied, followed by the full-length sequence for members of the S. ixodetis clade to which this novel Spiroplasma belongs. Tree topology was evaluated by maximum likelihood using a heuristic search, branch swapping by nearest neighbour interchange and employing the HKY85+G substitution model (Hasegawa, Kishino and Yano, 1985). Model parameter values were calculated from an initial tree constructed using maximum parsimony and a tree bisection and reconnection branch swapping protocol. Statistical support for trees was sought by performing 1000 nonparametric bootstrap replicates. MEGA version 3.1 (Kumar, Tamura and Nei, 2004) was employed for sequence comparisons, calculating number of nucleotide changes and Kimura 2-parameter distances (using pair-wise gap deletion).
RESULTS
Sex ratio trait
Offspring were reared from 26 females, 13 from each site; mean offspring number per female was 53·6 (range 3–118). Progeny sex ratios of 7 females differed significantly from equity (Fisher's exact tests, Bonferroni corrected for multiple comparisons) (Table 1). Sex ratio biases were always accompanied by low egg hatch rates, indicative of male-killing (Table 1). A further female (Wa/13) exhibiting a low egg hatch rate produced only 3 offspring before dying, all were female. These 8 females had 293 female and 3 male offspring in total. Their mean egg hatch rate was 0·24 (n=8), exactly half that of the remaining females (0·48, n=18): hatch rates of these groups differed significantly (Mann Whitney U Test, U(n=8,18)=136; P=0·0004).
Table 1. Breeding data for two UK samples of Anisostica novemdecimpunctata from Cambridge (Wa/) and Chew (Chew/) (Lines displaying male-killing marked in bold. Egg hatch rates (proportion hatched) and offspring sex ratios (proportion male) are given (sample sizes in parentheses). Asterisks denote significant female-biased deviations from equitable sex ratios (Fisher's exact test, Bonferroni corrected for 26 multiple comparisons). Results of diagnostic PCR tests using Mollicute specific primers are indicated.)

DNA was extracted from 1 F1 female from 2 biased (Wa/6 and Wa/2) and 2 equitable ratio lines (Wa/3 and Wa/4) and PCR tests performed. None produced amplification products using Rickettsia, Wolbachia or Flavobacteria specific primers. Both sex ratio lines did produce a product of approximately 430 bp with Mollicute-specific primers MGSO–Ha-In-1, but the 2 unbiased lines did not. The association between this bacterium and the sex ratio trait was assessed across all lines. PCR tests on either the parental female or 6 F1 females revealed a perfect association between this Mollicute bacterium and sex ratio distortion (Table 1). The single male offspring from sex ratio line Chew/13 that was tested carried the infection. Both population samples had a bacterial prevalence of 31% (n=13; 95% CI=6·6–52·0%).
Trait inheritance and antibiotic sensitivity
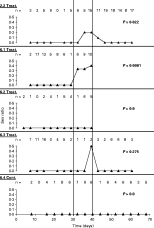
The inheritance of the sex ratio trait was investigated in 2 infected lines (Wa/2 and Wa/6). Eggs were collected from 5 F1 females for approximately 25 days, before 4 were treated with antibiotic. Prior to treatment, all individuals produced only female offspring (mean=30·4, range 16–50), accompanied by low hatch-rates (mean=0·26, range 0·18–0·34). During and immediately following treatment, 3 of 4 females produced male offspring (range 1–9) (Fig. 1). Also, notably elevated hatch-rates occurred in 3 cases (Fig. 2). Sex ratio and hatch rate increases during antibiotic treatment were significant in 2 of 4 and 3 of 4 cases respectively (Figs 1 and 2). Alleviation of male-killing symptoms was temporary: complete male mortality returned when antibiotic treatment ceased. The control line that received only golden syrup displayed low hatch-rates and produced solely female offspring throughout. All offspring (F2) produced prior to antibiotic treatment that were tested were infected (n=15) as were 22 F2 females and 11 F2 males derived afterwards. All control line offspring tested (6 before and 6 after treatment) were also infected.

Fig. 1. Offspring sex-ratios produced by Spiroplasma-infected F1 females of Anisostica novemdecimpunctata. Vertical lines delimit an antibiotic treatment period during which females 2·2, 6·1, 6·2 and 6·3 received tetracycline in golden syrup (treat.), female 6·4 was a control and received only golden syrup (cont.). Each data-point represents mean offspring production over a 4-day period, numbers (n) above each figure provide sample sizes. Females 6·1 and 6·2 died before the experiment finished. Data for each female before and after treatment were pooled and sex ratios compared to those produced during treatment. P-values represent significance of sex ratio increases during treatment (Fisher's exact test, Bonferroni corrected for 5 comparisons).

Fig. 2. Hatch rates of eggs produced by Spiroplasma-infected F1 females of Anisostica novemdecimpunctata. Vertical lines delimit an antibiotic treatment period during which females 2·2, 6·1, 6·2 and 6·3 received tetracycline in golden syrup (treat.), female 6·4 was a control and received only golden syrup (cont.). Each data-point represents mean egg production over a 4-day period, numbers (n) above each figure provide sample sizes. Females 6·1 and 6·2 died before the experiment finished. Data for each female before and after treatment were pooled and hatch rates compared to those produced during treatment. P-values represent significance of hatch rate increases during treatment (Fisher's exact test, Bonferroni corrected for 5 comparisons).
Trait infectivity
Abdomen homogenate of infected A. novemdecimpunctata females was injected into A. bipunctata pupae derived from 2 females producing equitable offspring sex ratios (0·46, n=69; 0·45, n=83) and carrying no known sex ratio distorting bacteria. Six females survived and laid eggs: all produced significantly female-biased offspring sex ratios (Fisher's exact tests, Bonferroni corrected for multiple comparisons) (Table 2). However, only 2 had exclusively female progeny and 1 produced numerous males (34 of 117 offspring). The mean egg hatch rate from these 6 injected A. bipunctata females was 0·51 (range 0·23–0·68) (Table 2). All male and female offspring tested were infected by the Mollicute bacterium (n=34 males and 9 females, representing all 6 lines).
Table 2. Breeding data for six Adalia bipunctata females injected with abdominal homogenate from female-biased Anisostica novemdecimpunctata lineages (sample sizes in parentheses) (All A. bipunctata offspring sex ratios deviate significantly from equity (Fisher's exact test, Bonferroni corrected for 6 comparisons). Male-killing occurred in the novel species but was not complete.)

Phylogenetic affiliation
The 16S and ITS rDNA region was sequenced for the A. novemdecimpunctata male-killer from 2 females providing 1672 bases for analysis and achieving approximately 80% double-stranded coverage. One site producing double peaks in the sequence of both forward and reverse strands for both individuals was ignored. The A. novemdecimpunctata Mollicute belonged to the Spiroplasma genus. Tree construction using maximum likelihood placed the male-killer in the Spiroplasma ixodetis clade, which is strongly evolutionarily divergent from the Spiroplasma citri clade containing the Drosophila male-killing spiroplasmas (SROs). Nonparametric bootstrapping provided no support for any branches except the S. ixodetis – S. citri clade divergence. All S. ixodetis clade sequences had very close homology (less than 1·5% difference), but were considerably divergent (more than 15%) from those in the S. citri clade (Table 3).
Table 3. Homology and divergence of rDNA sequences (Number of nucleotide differences and Kimura 2-parameter distances are given above and below the diagonal respectively. Bacteria are designated by insect host species name except for Spiroplasma ixodetis, S. citri and S. poulsonii. The Anisostica novemdecimpunctata, Harmonia axyridis, Danaus chrysippus and Adalia bipunctata infections as well as S. poulsonii cause male-killing. The others are not associated with sex ratio distortion. Sequence names from the S. citri clade are displayed in bold, those of the S. ixodetis clade in normal font. It was not possible to align the ribosomal ITS region for inter-clade comparisons, therefore homology statistics for the S. poulsonii and S. citri sequences are calculated only for the 16S gene, whereas other comparisons employ the full length 16S and ITS sequences.)

DISCUSSION
Male-killing bacteria are common parasites of insects where they distort population sex ratios in favour of females (Hurst et al. 2003). Here we report a new male-killing symbiosis; between a coccinellid beetle and a Spiroplasma bacterium. A number of other coccinellids are hosts to male-killers (Hurst and Jiggins, 2000), thus our discovery lends support to the suggestion of Majerus and Hurst (1997) that coccinellid ecology predisposes this group to invasion by male-killing parasites. The identification of a Spiroplasma as the bacterium responsible brings the number of coccinellid male-killers known of this bacterial genus to 3 (Hurst et al. 1999b; Majerus et al. 1999).
The male-killing trait was maternally inherited in all cases tested and transmission demonstrated across 3 host generations. In other coccinellids male-killer transmission occurs transovarially (Hurst et al. 1999a). The possibility of paternal transmission was not investigated here due to mortality of the 3 male offspring surviving from sex ratio lines, but seems unlikely. One F1 male that was tested carried the infection, therefore its survival was not due to complete transmission failure. It seems probable that surviving males received low bacterial densities, permitting embryonic survival. Spiroplasma transmission was perfect, or near-perfect, under laboratory conditions, as observed for other coccinellid male-killers of this genus (Majerus et al. 1998; Hurst et al. 1999b). The apparent lack of inefficient transmission questions the mechanism for maintenance of this infection at moderate (31%) prevalence in the populations studied. In the majority of male-killing symbioses uninfected females are constantly generated by bacterial transmission failure, preventing parasite fixation and host extinction (Hurst and Jiggins, 2000). It is possible that field environmental factors may reduce transmission rates in natural populations.
The partial alleviation of male-killing symptoms during antibiotic treatment provides compelling evidence for the bacterial origin of this trait. Complete concordance of the sex ratio condition with the presence of the Spiroplasma supports this. The absence of a complete cure during antibiotic treatment was probably due to insufficient treatment length or dose. However, tetracycline exposure caused ladybird deterioration and 2 died, administering increased doses might have caused further mortality. Another Spiroplasma male-killer similarly proved difficult to cure by antibiotics (Majerus et al. 1998). Survival of infected male offspring following treatment suggests tetracycline either reduced bacterial density below the threshold required for embryo death, or disrupted male-killing directly. Dyer, Minhas and Jaenike (2005) found maternal bacterial density was inversely correlated with male offspring survival in Drosophila. Previous authors have proposed that the transmission or phenotype of reproductive parasites may be impaired by exposure of the host to environmental factors such as naturally occurring antibiotics or high temperatures (Stevens, 1989; Hoffmann, Turelli and Harshman, 1990). Rapid recovery of full male-killing in A. novemdecimpunctata following treatment suggests that unless these influences are persistent, such effects on male-killer performance might be short-lived.
Experimental transfer of the Spiroplasma into Adalia bipunctata permitted investigation of its infectivity and ability to function in a novel host, a species in which some females also carry a native male-killing Spiroplasma (Hurst et al. 1999b). The bacterium established in all cases and caused female-biased offspring sex ratios, demonstrating an ability to transmit and kill males in this new species. Not all male offspring were killed; those surviving carried the Spiroplasma. Male-killing requires an interaction between the bacterium and host sex determination pathways (Veneti et al. 2005), as well as appropriate bacterial density in maternal tissues (Dyer et al. 2005); either factor may have been altered following transfer. It is notable that A. bipunctata and the native host are not closely related. The long-term success of vertically transmitted parasites must depend on occasional horizontal transmission to new host species; indeed whilst transfer routes are currently unclear, phylogenetic studies reveal that these events characterize reproductive parasite evolutionary history (Bandi et al. 2001). Our study did not set out to investigate the fitness of this Spiroplasma in the novel host, nevertheless it emphasizes the potential for such natural interspecific transmission.
Phylogenetic analysis demonstrated that the A. novemdecimpunctata bacterium belongs to the S. ixodetis clade of the Spiroplasma genus. Three other coccinellid and lepidopteran male-killers also lie in this clade (Hurst et al. 1999b; Majerus et al. 1999; Jiggins et al. 2000). These spiroplasmas diverged 200–400 million years ago from those in the S. citri clade some of which cause male-killing in Drosophila, indicating a minimum of 2 independent evolutions of male-killing within this genus (Hurst et al. 1999b). The S. ixodetis clade is basal to the rest of the Spiroplasma genus, differing substantially in both morphology and genome size (Gasparich et al. 2004). The 16S and ITS rDNA phylogeny gave no resolution within the S. ixodetis clade, providing no information to predict whether male-killing amongst these spiroplasmas is mono- or polyphyletic. Gasparich et al. (2004) suggested that previous studies demonstrating close 16S homology of male-killing spiroplasmas with S. ixodetis were flawed and had been confounded by long branch attraction and the availability of few closely related sequences. The current analysis clearly demonstrates that these male-killing parasites do lie within this clade. Very close 16S and ITS homology existed between S. ixodetis and this new Spiroplasma; furthermore the increasing numbers of bacteria allied to this clade provide a considerable number of comparators.
Anisosticta novemdecimpunctata differs from most previously described coccinellid male-killer hosts by being highly habitat specific (Majerus, 1991). Many aphidophagous coccinellid larvae starve shortly after hatching because they fail to find aphid prey (Hurst and Majerus, 1993). Killing males benefits the bacterium partly because infected female larvae consume dead embryos in their clutch, extending aphid search opportunities (Hurst and Majerus, 1993). In generalist coccinellid species adult females migrate between plant taxa during the reproductive season, ovipositing near productive aphid colonies where larval survival is relatively high (Sloggett and Majerus, 2000). Thus bacterial fitness returns of larval egg consumption may be small. However, habitat specialists are restricted to a narrow range of plants, more frequently laying eggs where aphids are scarce and bacterial fitness high. Therefore we predict male-killer prevalence may be greater in specialist than generalist hosts. Nonetheless, prevalence in both A. novemdecimpunctata populations was 31% and within the range typical for coccinellid male-killers (Hurst and Jiggins, 2000). Comparisons across more populations and species will be necessary to further test this hypothesis.
This study has revealed the existence of a new male-killing Spiroplasma parasite, closely phylogenetically allied to the other non-dipteran male-killers of this genus. Hurst et al. (2003) reviewed male-killer diversity across all insect species and concluded that the currently diminishing rate of discovery of new male-killing bacterial clades perhaps suggests that the majority have now been identified. That this male-killer also belongs to the Spiroplasma genus supports their argument. The present findings provide further demonstration of the widespread occurrence of male-killing as a strategy employed by a variety of parasitic agents in insect hosts.
We thank Frank Jiggins for much helpful advice and assistance and are grateful to Ian Wright and Dennis Farrington for technical support. M.C.T. was funded by a BBSRC studentship.