Introduction
Understanding species-habitat relationships and fine-scale distributions including microhabitat preferences are critical in biodiversity conservation (Michael et al. Reference Michael, Cunningham and Lindenmayer2010). In regional conservation planning, an ecosystem approach is more effective in preserving species and associated habitats rather than focusing on only rare species (Hierl et al. Reference Hierl, Franklin, Deutschman, Regan and Johnson2008; Harvey et al. Reference Harvey, Gounand, Ward and Altermatt2017; Marini et al. Reference Marini, Bartomeus, Rader and Lami2019). A guild-based approach can reveal habitat associations, requirements, and behavioural adaptations of different species of a community, which ultimately helps in implementing effective habitat conservation across multiple species (Bishop & Myers Reference Bishop and Myers2005). However, most of such functional analyses are restricted to bird communities (Holmes et al. Reference Holmes, Bonney and Pacala1979; Nally Reference Nally1994; Caprio et al. Reference Caprio, Ellena and Rolando2009; Korňan et al. Reference Korňan, Holmes, Recher, Adamík and Kropil2013) and have rarely been addressed in herpetofauna (Inger et al. Reference Inger, Shaffer, Koshy and Bakde1987; Michael et al. Reference Michael, Cunningham and Lindenmayer2010; Michael et al. Reference Michael, Kay, Crane, Florance, MacGregor, Okada, McBurney, Blair and Lindenmayer2015). A recent study on temperate Australian reptiles highlighted that guild-based analysis is useful for developing habitat management strategies for multiple species in human-modified landscapes (Michael et al. Reference Michael, Kay, Crane, Florance, MacGregor, Okada, McBurney, Blair and Lindenmayer2015).
Reptiles are more associated with habitat structure than vegetation composition (Webb & Shine Reference Webb and Shine2000; Garden et al. Reference Garden, Mcalpine, Possingham and Jones2007). Narrow distribution limits and niche requirements make reptiles highly susceptible to threats such as habitat loss and degradation (Gibbons et al. Reference Gibbons, Scott, Ryan, Buhlmann, Tuberville, Metts, Greene, Mills, Leiden, Poppy and Winne2000). Being ectotherms, reptiles are often adapted to specific microhabitats and associated microclimatic conditions, so availability and preference for microhabitats can be indirect evidence of resource limitation (Abrams Reference Abrams1980; Whitfield & Pierce Reference Whitfield and Pierce2005). Collecting microhabitat data is important for executing effective habitat management and conservation strategies for reptiles (Michael et al. Reference Michael, Kay, Crane, Florance, MacGregor, Okada, McBurney, Blair and Lindenmayer2015).
In this study, we examined microhabitat preferences of a tropical reptile community to understand community structure and inform targeted habitat management strategies within the Western Ghats biodiversity hotspot of India. The Western Ghats, also known as the Sahyadri, is one of the richest biodiversity hotspots in the world and is also a UNESCO World Heritage site (Myers et al. Reference Myers, Mittermeier, Mittermeier, Da Fonseca and Kent2000). This region covers an area of 140,000 km2 and stretches 1,600 km along the western coast of the Indian Peninsula (Rodgers et al. Reference Rodgers, Panwar and Mathur2002). These mountains are often ignored in conservation planning, have high species endemism, with 62% of reptile species endemic to the region (Das et al. Reference Das, Krishnaswamy, Bawa, Kiran, Srinivas, Kumar and Karanth2006). Also, studies show substantial evidence of local endemism in the partially isolated hill ranges of the Western Ghats (Inger et al. Reference Inger, Shaffer, Koshy and Bakde1987). However, microhabitat preferences of reptiles are not well-known, limited to very few species in this region (Inger et al. Reference Inger, Shaffer, Koshy and Bakde1987; Chandramouli Reference Chandramouli2011). Nevertheless, recent discoveries and new distribution records of reptiles (Jins et al. Reference Jins, Bhupathy and Panigrahi2014; Pal et al. Reference Pal, Vijayakumar, Shanker, Jayarajan and Deepak2018; Mallik et al. Reference Mallik, Achyuthan, Ganesh, Pal, Vijayakumar and Shanker2019; Deepak et al. Reference Deepak, Narayanan, Das, Rajkumar, Easa, Sreejith and Gower2020) establish the southern Western Ghats as a key region harbouring many unexplored base lineages of reptiles that need urgent and tailored conservation efforts. One such conservation priority zone within the southern Western Ghats biodiversity hotspot is the Agasthyamalai-Periyar landscape (Das et al. Reference Das, Krishnaswamy, Bawa, Kiran, Srinivas, Kumar and Karanth2006). This landscape is subject to significant pressure from different anthropogenic activities such as tourism and other human interventions resulting in habitat degradations (Jose et al. Reference Jose, Alex, Kumar, Varghese and Madhu2011; Panigrahi & Jins Reference Panigrahi and Jins2018).
In this context, we categorize reptiles into generalists and specialists with respect to their microhabitat use, evaluate the guild structure of the community based on microhabitat preferences, and derive conservation implications based on microhabitat associations.
Methods
Study site
We conducted this study in Agasthyamalai Hills (8.4°–8.8°N and 77.0°–77.4°E), part of the Agasthyamalai Biosphere Reserve (ABR), at the southern end of the Western Ghats. The western slope (windward side) of the Agasthyamalai Hills was the focal study area, comprising of two major protected areas—Neyyar and Peppara wildlife sanctuaries in the Kerala State (Figure 1). The focal study area covers an area of approximately 250 km2 with elevation ranging from 50–1,868 m a.s.l. The windward side of the Agasthyamalai Hills receives high rainfall (2000–5000 mm annually) with two to three dry months annually (Ramesh et al. Reference Ramesh, Menon and Bawa1997; Varghese & Balasubramanyan Reference Varghese and Balasubramanyan1999).
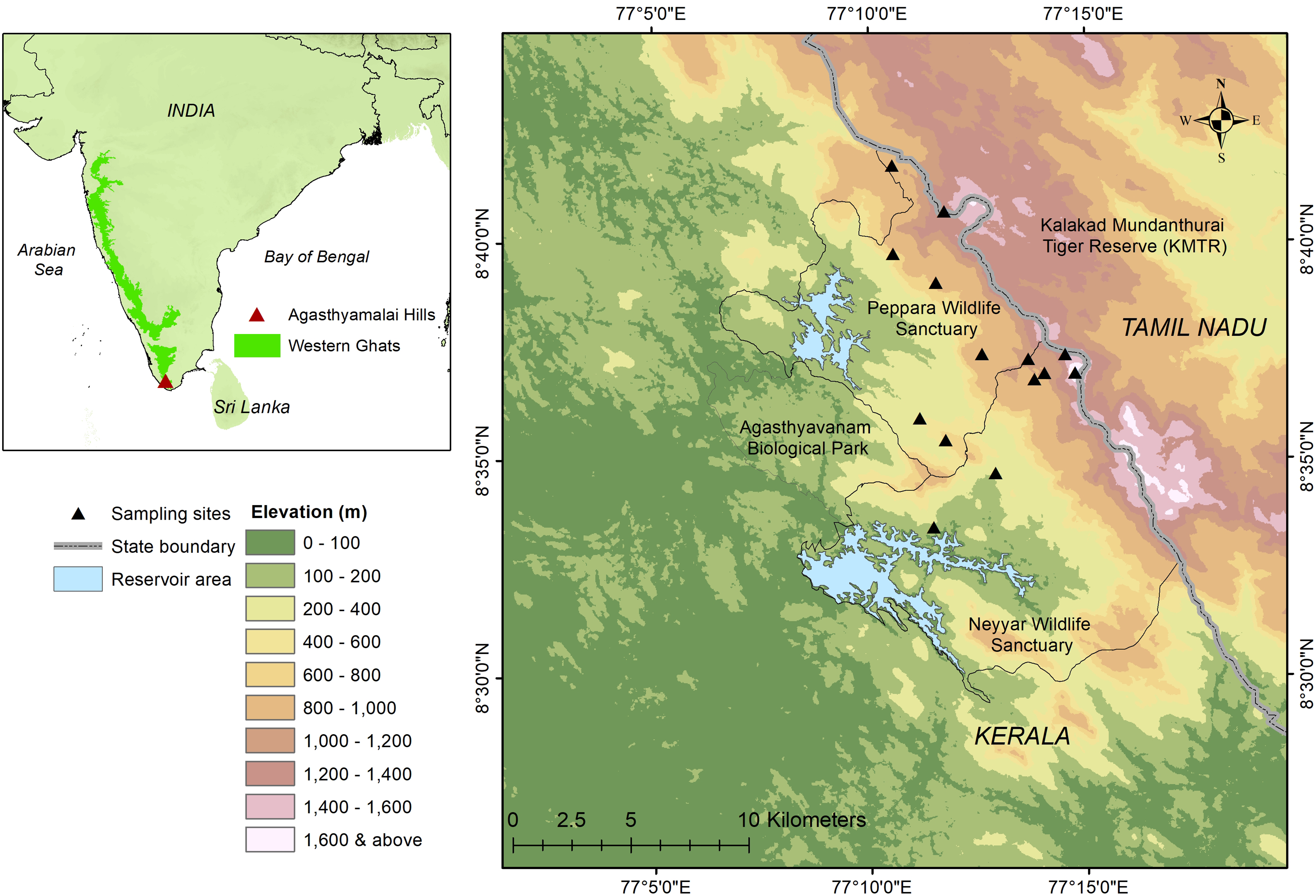
Figure 1. Sampling sites within Agasthyamalai Hills and study area location within the Indian Peninsula (inset).
The Agasthyamalai region is well-known for its high plant diversity and endemism (Nayar Reference Nayar1996; Manju et al. Reference Manju, Rajesh and Madhusoodanan2009). The vegetation of the area changes significantly with elevation and supports four major vegetation types: (1) southern moist mixed deciduous forest (<400 m a.s.l.); (2) west coast semi-evergreen (400–600 m a.s.l.); (3) west coast tropical evergreen (600–1200 m a.s.l.); and (4) southern hilltop tropical evergreen (>1200 m a.s.l.) (Champion & Seth Reference Champion and Seth1968). The deciduous and evergreen forests up to 1200 m a.s.l. are comprised of tall trees ranging from 10–35 m in canopy height. In contrast, the hilltop forest is dense, stunted evergreen vegetation with canopy height reaching a maximum of up to 10 m, mixed with open rocky and grassy areas (Varghese & Balasubramanyan Reference Varghese and Balasubramanyan1999).
Data collection
We sampled reptile communities in Agasthyamalai Hills from April 2012 to December 2014 using time-constrained visual encounter surveys (VES), covering all major habitats and elevation gradients (100–1,868 m a.s.l.). VES is a time-constrained technique (Campbell & Christman Reference Campbell and Christman1982; Crump & Scott Reference Crump, Scott, Heyer, Donnelly, McDiarmid, Hayek and Foster1994), which involves surveying an area or habitat for a prescribed time and systematically searching for animals in all possible microhabitats. The total search time was expressed in person-hours and each sample consisted of a 1-hour search by two people (i.e. two person-hours). Sampling was restricted to 08h00 and 18h00, and searches involved turning rocks and fallen logs, racking through leaf litter, scanning vegetation, branches, and bark of trees. When possible, we identified all individuals to species; however, we classified taxonomically uncertain species as unknown or comparable (cf.) species at the genus level for analyses. We recorded microhabitat use for each species based on the substratum used by a particular individual at the time of observation. We conducted a total of 1,304 VES person-hours, and details of sampling effort in each elevation zone are provided in the supplementary file (Table S1).
We assigned all species observations to 15 broad microhabitat categories representative of the study area (Inger et al. Reference Inger, Shaffer, Koshy and Bakde1987). The 15 microhabitats identified include (1) on tree buttress (BT); (2) in rock crevice (RC); (3) on open ground (OG); (4) on ground with grass or herb cover (GG); (5) in tree hole (TH); (6) inside water (IW); (7) in leaf litter (LL); (8) on log (OL); (9) on rock (OR); (10) on shrub stem (OS); (11) on tree trunk (TT); (12) under tree bark (UB); (13) under soil surface (UG); (14) under log (UL); and (15) under rock (UR).
Data analysis
All statistical analyses were carried out in the R statistical language (R Core Team 2019) with various packages (see below).
Niche breadth
To determine specialists vs. generalists based on microhabitat use, we conducted a niche breadth analysis. We calculated niche breadth using the CRAN package ‘spaa’ (SPecies Association Analysis) (Zhang et al. Reference Zhang, Ding and Huang2013) using frequency distributions of species under different microhabitat categories. We used Levin’s measure of niche breadth (Pianka Reference Pianka1973), derived from Simpson’s diversity index (Simpson Reference Simpson1949).
where B is the microhabitat niche value, Pi is the proportion of i th microhabitat category; B varies from 1 to n based on the Pi values. We classified species with B value <2 as microhabitat specialists and ≥2 as microhabitat generalists based on a natural break in the histogram of niche breadth values (Figure 2). Species with less than two observations were excluded from classifying specialists and generalists (Michael et al. Reference Michael, Kay, Crane, Florance, MacGregor, Okada, McBurney, Blair and Lindenmayer2015). In the present analysis, species with less than five observations were classified as ‘predicted specialists or generalists’ given the limited data.
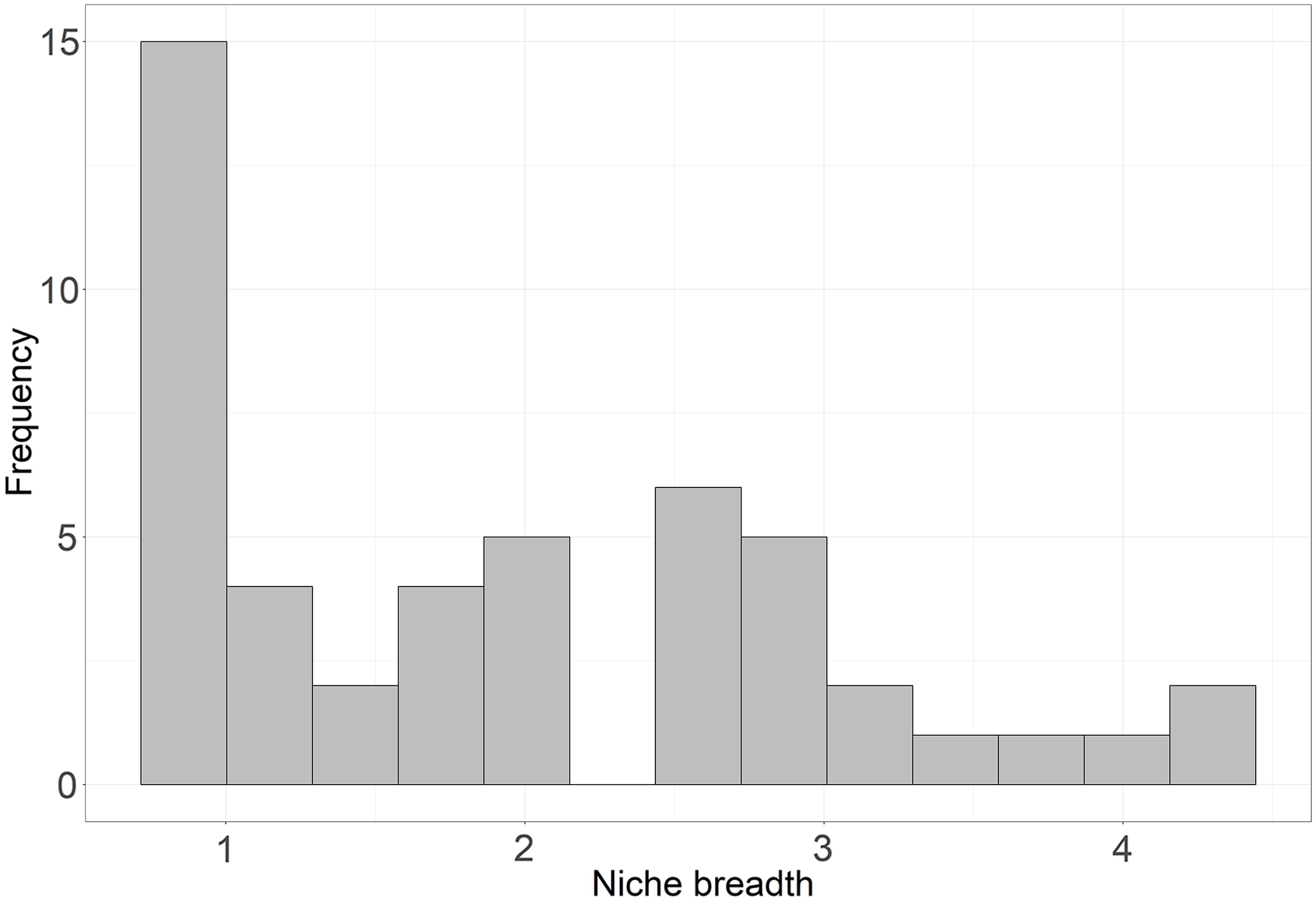
Figure 2. Frequencies of niche breadth values of all reptiles observed during the study.
Guild structure
For guild analysis, we used standardized frequencies (percentage) instead of actual counts to avoid biases due to large variations in the number of observations of different species in different microhabitat categories (Michael et al. Reference Michael, Kay, Crane, Florance, MacGregor, Okada, McBurney, Blair and Lindenmayer2015). We then created a Bray–Curtis dissimilarity matrix from these standardized frequency distributions. We conducted hierarchical cluster analysis on this distance matrix using the ‘single linkage’ method (nearest neighbour clustering) which generates clusters based on the minimum distance between the species (Pianka Reference Pianka1980). We carried out these analyses using the ‘vegan’ package in R code (Oksanen et al. Reference Oksanen, Blanchet, Kindt, Legendre, Minchin, O’hara, Simpson, Solymos, Stevens and Wagner2013) and categorized species groups into guilds based on general characteristics of the clusters generated (Michael et al. Reference Michael, Kay, Crane, Florance, MacGregor, Okada, McBurney, Blair and Lindenmayer2015).
Results
We recorded 47 reptile species from 1,554 observations comprising two major orders (Squamata and Testudines) and 11 families. These 47 species consisted of 24 lizards, 22 snakes and one tortoise (Indotestudo travancorica). Overall, nearly half (47.55%) of observations were attributed to two skink species: Eutropis macularia (n = 548, 35.26%) and Sphenomorphus dussumieri (n = 191, 12.29%). Five species were observed only once during the sampling period, four of which were snakes and the other was a lizard (Varanus bengalensis). We obtained microhabitat data for all 1,554 observations and calculated niche breadths. After excluding species with less than two observations, we were able to include 42 species (1,549 observations) in our analysis.
Niche breadth
Niche breadth values varied from 1.00 to 4.44 based on differences in microhabitat use among species (Table 1). We classified 19 (45%) species as microhabitat specialists and 23 (55%) species as generalists. Specialists included nine lizards, nine snakes, and one tortoise species. Family Pareidae (Xylophiinae) showed relatively high niche breadth (B = 2.67); however, this family was represented by a single species, Xylophis captaini, which is a burrowing and litter-dwelling snake. Family Gekkonidae exhibited the highest average niche breadth (n = 7, B = 2.53), followed by Scincidae (n = 8, B = 2.49) and Agamidae (n = 8, B = 2.28). Although we included 42 species (with at least two observations) for categorizing specialists or generalists, we classified 18 species with less than five observations as ‘predicted specialists or generalists’ (Figure 3).
Table 1. List of reptile species (from VES), with number of observations (#), niche breadth (B), and microhabitat categories.
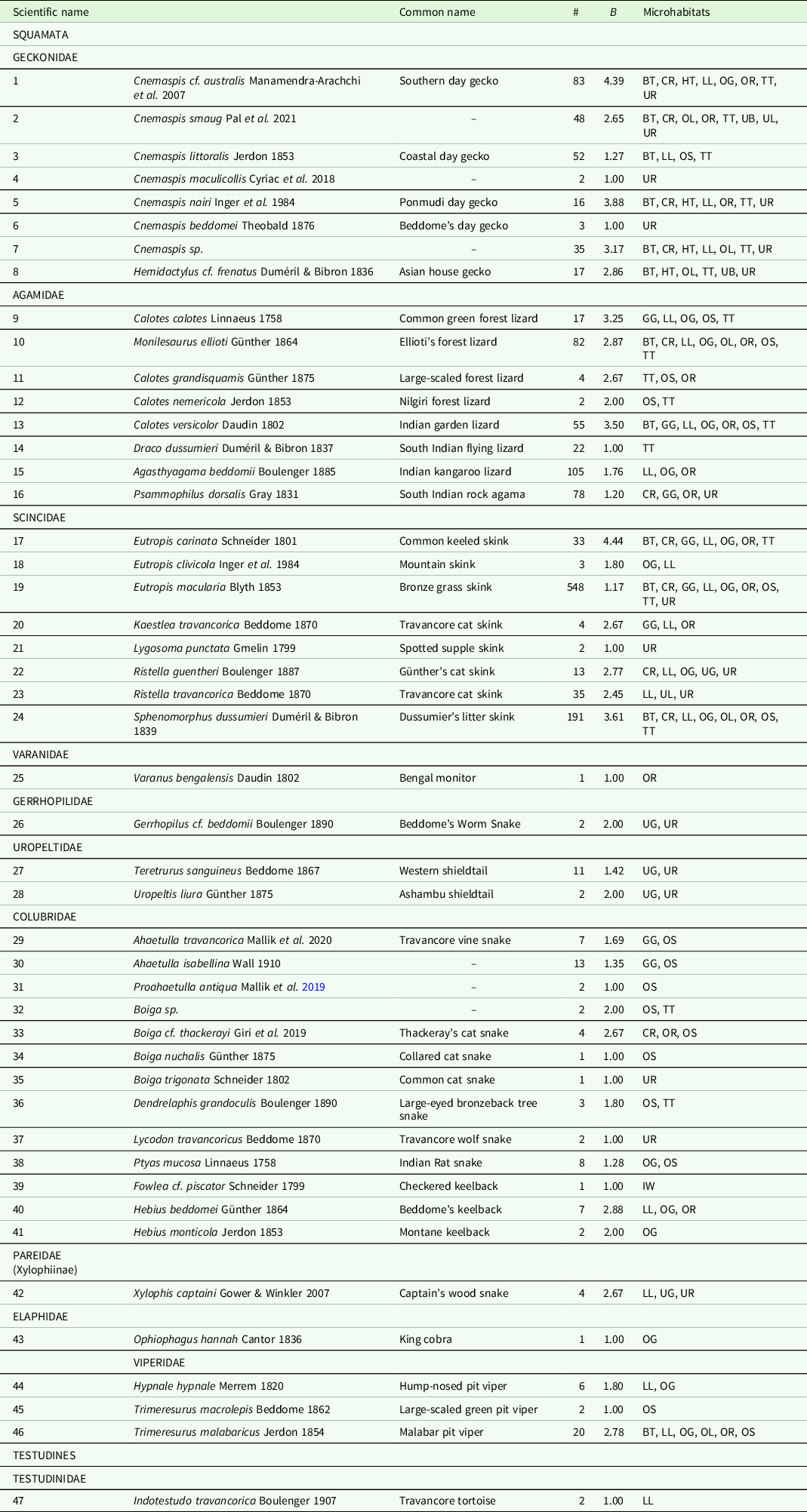
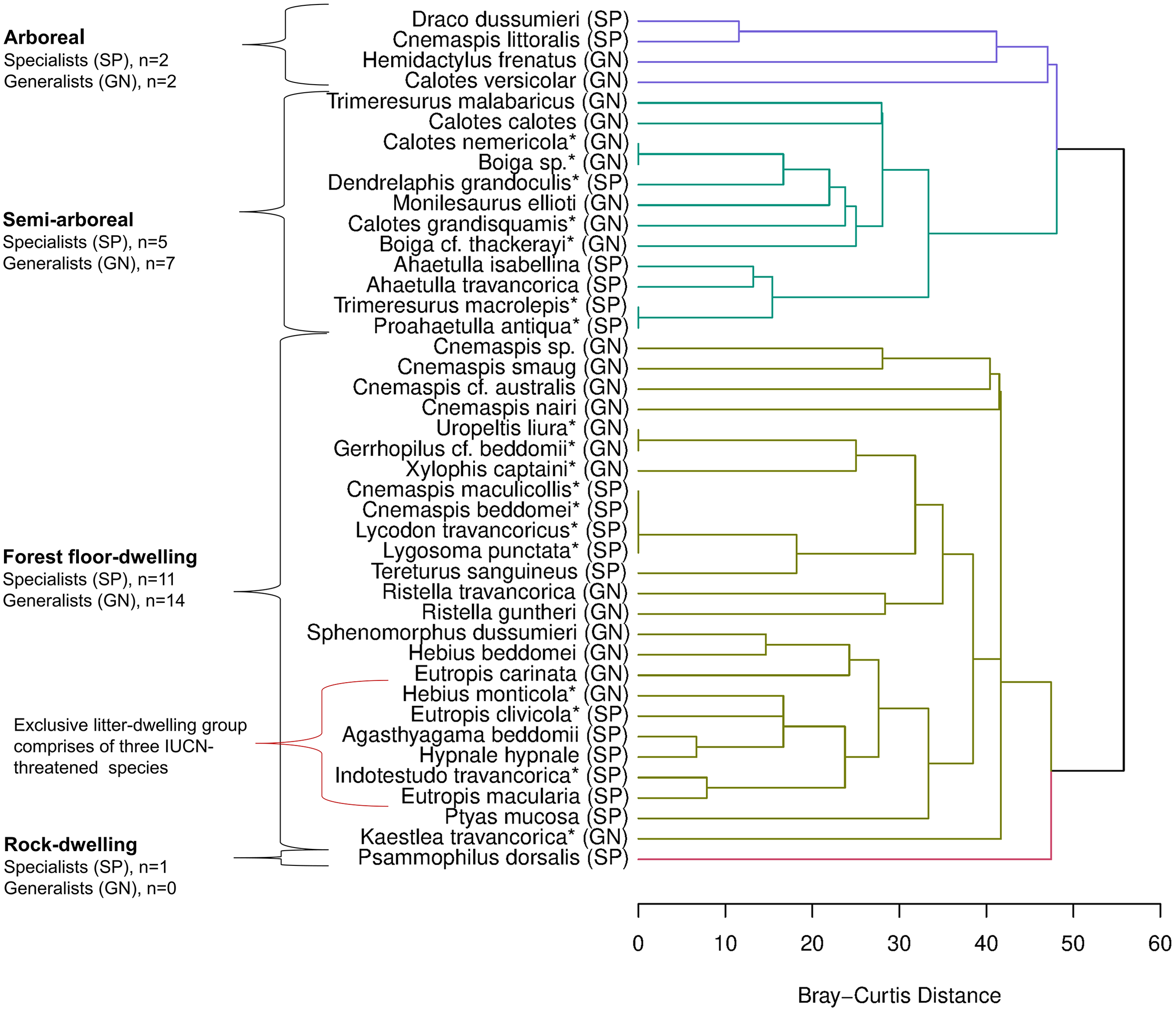
Figure 3. Cluster diagram depicting guild structure of reptiles based on microhabitat use. Assemblages of specialists (SP) and generalists (GN) within each guild are also shown (species with <2 observations were not included). *‘Predicted specialists/generalists’ (species with less than 5 observations).
Guild classification
Hierarchical clustering classified reptiles into four main guilds based on microhabitat utilization: (1) arboreal; (2) semi-arboreal; (3) forest floor-dwelling; and (4) rock-dwelling (Figure 3). Of these, the forest floor-dwelling guild formed the largest group with 25 species (9 snakes, 15 lizards, and a tortoise), including subgroups such as litter-dwelling, open ground-dwelling, and burrowing species. The arboreal guild was comprised of four lizard species. The semi-arboreal guild consisted of twelve species, including eight snakes and four agamid lizards. The rock-dwelling (cryptozoic) guild was comprised of a single species, Psammophilus dorsalis, which is found exclusively on rock or within rock crevices. Based on niche breadth values, the forest floor-dwelling group contained the maximum number of specialists (n = 11), followed by the semi-arboreal group (n = 5). Moreover, an exclusively litter-dwelling group (n = 6), within the forest floor-dwelling guild, contained five specialists, three of which are listed under IUCN-threatened species classifications: Agasthyagama beddomii (Endangered), Eutropis clivicola (Endangered), and Indotestudo travancorica (Vulnerable) (see Figures 3 and 4).

Figure 4. Exclusively litter-dwelling and threatened species in the study area: (a) Agasthyagama beddomii (Endangered); (b) Eutropis clivicola (Endangered); and (c) Indotestudo travancorica (Vulnerable).
Discussion
We found that many reptiles in the study area have relatively narrow niche breadths, making them potentially more vulnerable to habitat degradation than habitat generalists with larger niche breadths. The presence of a high number of specialists (n = 19) highlights the significance of the Agasthyamalai landscape for reptile conservation. As habitat fragmentation and disturbances are already reported from the region (Ramesh et al. Reference Ramesh, Menon and Bawa1997; Jose et al. Reference Jose, Alex, Kumar, Varghese and Madhu2011), further degradation of microhabitats may increase the susceptibility of reptiles to population decline and affect the survival of many habitat specialists.
Our cluster analysis revealed that forest floor-dwelling reptiles (both open ground and leaf litter species) dominate the reptile community in this study area. We also found that the forest floor guild contains more microhabitat specialists (n = 11) and is a group that is likely to be most vulnerable to habitat degradation like forest fires. Most of the species in this guild have strong associations with leaf litter. For example, some of the IUCN-threatened species observed in this study, Agasthyagama beddomii (Endangered), Eutropis clivicola (Endangered), and Indotestudo travancorica (Vulnerable), are exclusively found in leaf litter microhabitats (Jose et al. Reference Jose, Ramachandran and Nair2007; Deepak & Vasudevan Reference Deepak and Vasudevan2015) (Figure 4). Additionally, most of the skinks reported in our study also depend on leaf litter for breeding and foraging activity. Thus, the conventional practice of leaf litter removal from the forest edges during fire-lining may severely impact litter-dwelling species (Jose et al. Reference Jose, Ramachandran and Nair2007; Chandramouli Reference Chandramouli2011).
As spatial complexity is one of the major factors that determine reptile diversity (Pianka Reference Pianka1967; Bars-Closel et al. Reference Bars-Closel, Kohlsdorf, Moen and Wiens2017), microhabitat analysis gives insights on community patterns and can potentially help in identifying species that are vulnerable to habitat degradations. The guild-based approach has important application in understanding reptile community structure and habitat requirements, and developing effective habitat management strategies (Inger & Colwell Reference Inger and Colwell1977; Michael et al. Reference Michael, Kay, Crane, Florance, MacGregor, Okada, McBurney, Blair and Lindenmayer2015). This study aimed to evaluate microhabitat use in a tropical reptile community, rather than simply investigating species diversity. In doing so, our findings serve as a potential conservation tool for developing effective landscape management strategies for the region. For implementing practical conservation strategies, it is important to integrate species’ ecologies into spatial planning that involves modelling dynamic ecological processes across space and time (Opdam et al. Reference Opdam, Foppen and Vos2001; Harvey et al. Reference Harvey, Gounand, Ward and Altermatt2017). In this perspective, our approach of identifying species with similar microhabitat use patterns could be helpful in multi-species conservation planning at the landscape levels. For instance, in our study, forest floor species constituted 60% of the total species, highlighting the importance of leaf litter and open ground habitats for a diverse range of reptile species. A similar study on the herpetofaunal assemblage in the Ponmudi Range (a mountain range within the southern Western Ghats) reported a high proportion of reptiles and amphibians that used forest leaf litters and rocks as their major microhabitats (Inger et al. Reference Inger, Shaffer, Koshy and Bakde1987). There are a few studies that demonstrate a significant impact of habitat degradation on reptile diversity (Gillespie et al. Reference Gillespie, Howard, Stroud, Ul-Hassanah, Campling, Lardner, Scroggie and Kusrini2015; Theisinger & Ratianarivo Reference Theisinger and Ratianarivo2015). A study on Australian rainforest frogs also suggested that guild-based analyses are useful in identifying specific causal factors for population declines due to habitat degradation, as ecological guilds with more specialists and limited microhabitat resources are susceptible to changes in habitats (Williams & Hero Reference Williams and Hero1998).
The Western Ghats of India is a biodiversity hotspot extremely vulnerable to anthropogenic disturbances including forest fragmentation and annual forest fires because of high human population density (Kodandapani et al. Reference Kodandapani, Cochrane and Sukumar2008). During our surveys, we observed isolated forest fire events and the dumping of plastic and other waste within the core areas of ABR. These observations corresponded with the presence of tourists in the region (see Figure 5) suggesting a certain impact of tourism-related activities in the core areas. However, the same was not quantified in the present study and needs further detailed investigation. There are some landscape-level assessments from the southern Western Ghats showing a considerable amount of forest fragmentation and degradation due to human disturbances (Parthasarathy Reference Parthasarathy1999; Giriraj et al. Reference Giriraj, Murthy and Beierkuhnlein2010; Jose et al. Reference Jose, Alex, Kumar, Varghese and Madhu2011). A study from the northern part of Agasthyamalai range showed significant changes in forest types between the years 1971 to 2009 where a large area of evergreen patches transformed to semi-evergreen and moist deciduous forests due to disturbances (Jose et al. Reference Jose, Alex, Kumar, Varghese and Madhu2011). It is understood that such changes in forest types could largely affect habitat structure by changing spatial complexities including microhabitat availabilities. Additionally, removal of leaf litter and forest degradation have also been reported as a threat to herpetofauna from different parts of the Western Ghats, including the Agasthyamalai landscape (Jose et al. Reference Jose, Ramachandran and Nair2007; Chandramouli Reference Chandramouli2011). For example, the endangered and endemic (to the southern Western Ghats) Indian Kangaroo Lizard, Agasthyagama beddomii is found only in the evergreen or semi-evergreen habitats where the leaf litter composition is one of the major limiting factors for its presence (Jose et al. Reference Jose, Ramachandran and Nair2007; Chandramouli Reference Chandramouli2011). Such habitat and microhabitat specialists will be severely impacted by habitat degradations over the years. Considering the potential impact of existing tourism activities and human interventions in the region (Panigrahi & Jins Reference Panigrahi and Jins2018), we recommend management plans to protect these habitats from further degradation. Protecting the structural attributes of these habitats would ultimately conserve many such habitat specialists and endemic reptiles in the southern Western Ghats.

Figure 5. (a) Burnt grassland and adjacent evergreen forest patch (at 800m a.s.l.) in the core area of Agasthyamalai landscape with the Agasthyamalai peak in the backdrop; (b) Dumping of plastic and other wastes by tourists.
Conclusion
Our study demonstrates that microhabitat is a crucial element of ecosystem complexity and can serve as a proxy to predict the community structure of associated herpetofaunal taxa. In our study, 45% of reptile species in this area of the Western Ghats were microhabitat specialists with relatively narrow niche breadth. The 42 reptile species utilized 15 distinct microhabitat types, clustered into four guilds. In addition to these findings, we also found that 60% (25) of the species were forest floor dwellers with a significant number of specialists, including globally threatened species. Our results demonstrate potential effective implementations of microhabitat data for improved and comprehensive forest management practices, as opposed to conventional current management practices in eco-sensitive regions of the Western Ghats. The data generated in the present study along with existing information about microhabitat specialists might help us understand how they could be severely impacted by the degradation of different forest types in the region. Given the sampling constraints and limited duration of the present study, we were unable to collect a sufficient number of observations for many species. We believe that further investigations in the area would help in determining the microhabitat specializations and conservation importance of many reptiles found in the region. Although our study area is within the protected area network of Agasthyamalai Biosphere Reserve (ABR), we observed the area to be under sustained pressures from high anthropogenic activities especially tourism, thus making the landscape more prone to habitat degradation and forest fires. Hence, we recommend a dynamic approach to monitor habitat quality and regulate human interventions in this landscape to sustain the health of the habitat for the conservation of all the native and endemic species including the rich diversity of reptiles.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0266467422000190
Acknowledgements
We thank Kerala Forest Department for granting research permissions and logistic support for field studies [Letter No.WL 10/1540/2012]. We are grateful to the Director and staff at Sálim Ali Centre for Ornithology and Natural History (SACON), Coimbatore for their logistics. Constant encouragement and support from Bhoj K Acharya, Sikkim University to the first author are duly acknowledged. Special thanks to Madhumita Panigrahi for helping in field data collection. A.P. Zaibin and Deepak Veerappan provided comments in earlier drafts of the manuscript. Ashok Kumar Mallik, Saunak Pal and Vivek Philip Cyriac helped in species identifications. David Bickford provided constructive comments and suggestions to improve the flow of the paper. We appreciate the help of field assistants and local communities during the field study.
Financial support
Department of Biotechnology (DBT), Ministry of Science and Technology, Govt. of India (Grant No. BT/PR-13769/BCE/08/803/2010).
Competing interests
The authors declare that they have no known conflicts of interest or competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.









