INTRODUCTION
Since 1997 subtype H5N1 highly pathogenic avian influenza A viruses of ‘Asian’ origin have caused epizootics amongst domestic poultry and wild aquatic birds throughout Europe, Asia and Africa, as well as causing more than 300 human deaths. These viruses, hereafter referred to as HP H5N1, can be traced back to infections of domestic geese in southern China between 1996 and 1999 (Wang et al. Reference Wang, Vijaykrishna, Duan, Bahl, Zhang, Webster, Peiris, Chen, Smith and Guan2008). Despite major outbreaks of HP H5N1 among Hong Kong poultry in 2001–02 (Guan et al. Reference Guan, Peiris, Lipatov, Ellis, Dyrting, Krauss, Zhang, Webster and Shortridge2002) and subsequent regional spread among poultry populations in late 2003 (Sims and Brown, Reference Sims, Brown and Swayne2008) there was little evidence of infection in wild birds until May 2005, when thousands of waterbirds – some of them infected with HP H5N1 – died at Qinghai lake in western China (Chen et al. Reference Chen, Smith, Zhang, Qin, Wang, Li, Webster, Peiris and Guan2005; Gauthier-Clerc et al. Reference Gauthier-Clerc, Lebarbenchon and Thomas2007). The virus appeared to spread westwards across Russia during 2005, reaching eastern Europe by October (OIE, 2005). Whether the virus was dispersed by human activity or migrating birds remains unclear (Gauthier-Clerc et al. Reference Gauthier-Clerc, Lebarbenchon and Thomas2007; Feare, Reference Feare2007).
The emergence of HP H5N1 among wild birds in Europe has been associated with numerous reports of swan mortality, particularly of mute swans (Cygnus olor). Swan deaths linked to HP H5N1 were initially noted in Europe in autumn 2005 in Romania (n > 140) and Croatia (n = 30). Early the following year many European countries reported H5N1-infected dead swans for the first time, often singly, but with notably larger epizootics in Greece and northern Germany (Feare, Reference Feare2007; Globig et al. Reference Globig, Staubach, Beer, Köppen, Fiedler, Nieburg, Wilking, Starick, Teifke, Werner, Unger, Grund, Wolf, Roost, Feldhusen, Conraths, Mettenleiter and Harder2009). Mute swans comprised more than 60% of infected dead birds discovered in Europe during spring 2006 (ECDC Influenza Team, 2006; Hesterberg et al. Reference Hesterberg, Harris, Stroud, Guberti, Busani, Pittman, Piazza, Cook and Brown2009) and the species is considered to be a sentinel for the presence of HP H5N1. However, the frequency of mortality in wild swan H5N1 outbreaks appears to be low and is in marked contrast to the high virulence of the virus in poultry flocks. For example, only 153 swans died from an Iranian flock numbering ∼3000, and in the Croatian epizootics just 30 of ∼1700 birds were reported dead (Feare, Reference Feare2007). Similarly low mortality rates of 1–5% have been reported for smaller incidents in Poland (4 of 116 birds; Smietanka et al. Reference Smietanka, Minta, Domańska-Blicharz, Tomczyk and Wijaszka2008), the Czech Republic (12 of 282 birds; Nagy et al. Reference Nagy, Machova, Hornickova, Tomci, Nagl, Horyna and Holko2007) and France (up to 54 of ∼800 birds; Hars et al. Reference Hars, Ruette, Benmergui, Fouque, Fournier, Legouge, Cherbonnel, Daniel, Dupuy and Jestin2008).
It is unclear whether the prominence of swans in reports of H5N1 wild bird deaths in Europe is simply a consequence of their large size and easy detection, or a reflection of biological factors, such as a greater susceptibility to infection or a higher case fatality rate. Further, the importance of swans in the international dissemination of HP H5N1 is unknown. Unfortunately these questions must be tackled indirectly as the detection of HP H5N1 viruses in live wild birds is very difficult (Hoye et al. Reference Hoye, Munster, Nishiura, Klaassen and Fouchier2010). Waterbirds are infected with a wide diversity of avian influenza virus (AIV) subtypes, most of which are of low pathogenicity (LP). Subtypes are classified according to the haemagglutinin (HA1-16) and neuraminidase (N1-9) surface proteins they encode and almost all combinations of these have been observed. Prior AIV infection can be detected using assays for specific, non-neutralising antibodies to the viral nucleoprotein, although these tests cannot distinguish among AIV subtypes.
Experimental infection with HP H5N1 viruses suggests that the mortality rate of immunologically-naïve juvenile swans is high (greater than that of geese) and that mute swans may be particularly competent transmitters of the strain because they can shed high levels of virus asymptomatically for several days before dying (Brown et al. Reference Brown, Stallknecht and Swayne2008). Adult mute swans fare little better if they too are immunologically-naïve; however, adult swans with evidence of previous exposure to naturally-acquired AIV have been reported to survive HP H5N1 infection and to shed virus for a reduced duration (Kalthoff et al. Reference Kalthoff, Breithaupt, Teifke, Globig, Harder, Mettenleiter and Beer2008; the sera of these birds contained antibodies to AIV nucleoprotein but did not neutralize the HP H5N1 inoculate). It is unknown whether exposure to heterologous AIV subtypes provides cross-protection in mute swans; if it exists, cross-protection might explain the relatively low mortality rate of avian influenza outbreaks in wild swan flocks. Similar experiments in wood ducks (Aix sponsa; Costa et al. Reference Costa, Brown, Howerth, Stallknecht and Swayne2011), Canada geese (Branta canadensis; Berhane et al. Reference Berhane, Leith, Embury-Hyatt, Neufeld, Babiuk, Hisanaga, Kehler, Hooper-McGrevy and Pasick2010) and Mallards (Anas platyrhynchos; Fereidouni et al. Reference Fereidouni, Starick, Beer, Wilking, Kalthoff, Grund, Häuslaigner, Breithaupt, Lange and Harder2009; Jourdain et al. Reference Jourdain, Gunnarsson, Wahlgren, Latorre-Margalef, Bröjer, Sahlin, Svensson, Waldenström, Lundkvist and Olsen2010) have shown that previous infection with homologous LP avian influenza viruses commonly prevents disease. The same studies indicate that some (but not all) heterologous exposures may provide partial protection (i.e. increased survival or reduced duration or intensity of infection).
In response to the 2006 HP H5N1 epizootics in Europe, some studies have measured naturally-acquired antibody responses to avian influenza viruses in free-living swan populations. Sero-prevalence to AIV nucleoprotein among mute swans in Torun, Poland, was reported to be >90% and more than 70% of samples tested positive against H5N2 and H5N1 subtype viruses in haemagglutination-inhibition tests (Smietanka et al. Reference Smietanka, Minta, Domańska-Blicharz, Tomczyk and Wijaszka2008). Similar tests indicated that 70% of swans sampled in 2007 in France had specific serum antibodies to AIV H5 antigens, including in a population that had not previously reported HP H5N1 infection (Niqueux et al. Reference Niqueux, Guionie, Schmitz, Hars and Jestin2010). Twelve mute swans sampled in May 2006 in northern Germany showed responses to six different LP avian influenza HA subtypes (Globig et al. Reference Globig, Staubach, Beer, Köppen, Fiedler, Nieburg, Wilking, Starick, Teifke, Werner, Unger, Grund, Wolf, Roost, Feldhusen, Conraths, Mettenleiter and Harder2009).
The United Kingdom had to wait until January 2008 for its first outbreak of HP H5N1 infection in wild swans with notable mortality. The incident took place on the Fleet Lagoon, a 12 km stretch of water behind Chesil Beach in Dorset, on the south coast of England. The Fleet's breeding swan population is concentrated around the Abbotsbury Swannery, found at the most western and least saline end of the lagoon. At first glance the outbreak seems entirely typical of previous events in mainland Europe: HP H5N1 was detected in ten dead mute swans out of a population of many hundreds. A brief summary of the event is given in the next section; further details are available in the official incident report, which focuses on surveillance responses and risks to commercial poultry (DEFRA, 2008).
What makes Abbotsbury unique among HP H5N1 outbreaks, and scientifically valuable, is that it occurred in a swan population that has been the subject of intense ornithological study for many years (e.g. Ogilvie and Perrins, Reference Ogilvie and Perrins1981; Perrins et al. Reference Perrins, McCleery and Ogilvie1994; McCleery et al. Reference McCleery, Perrins, Sheldon and Charmantier2008). Almost all swans that have bred or were reared in the colony have been ringed since the early 1970s. Furthermore, the composition and dynamics of other waterbird populations on the Fleet and surrounding areas are well known (Austin et al. Reference Austin, Collier, Calbrade, Hall and Musgrove2008). Here, we use these exceptional circumstances to investigate, for the first time, the effect of age on HP H5N1 epidemiology in a natural population. We also measure the age structure of sero-positivity to the AIV nucleoprotein in the population, before and after the introduction of HP H5N1. It has long been known that juveniles are more likely to be infected by avian influenza viruses (e.g. Hinshaw et al. Reference Hinshaw, Webster and Turner1980; Hoye et al. Reference Hoye, Fouchier and Klaassen2012) and it is suspected that viral transmission and dissemination vary among age classes, yet detailed information on host age structure is uncommon for natural populations (Hoye et al. Reference Hoye, Munster, Nishiura, Klaassen and Fouchier2010) and even rarer for those exposed to HP H5N1. Our study includes a novel analysis that combines virus molecular clock phylogenetics with bird count data in order to estimate the date and route of introduction of the virus. Similar approaches have proven very valuable in reconstructing the epidemic history of influenza virus transmission in humans and swine (e.g. Baillie et al. Reference Baillie, Galiano, Agapow, Myers, Chiam, Gall, Palser, Watson, Hedge, Underwood, Platt, McLean, Pebody, Rambaut, Green, Daniels, Pybus, Kellam and Zambon2012). We also hope our study addresses the concern that previous investigations of HP H5N1 incidents in wild birds have lacked sufficiently detailed ecological information and ornithological expertise (Yasue et al. Reference Yasue, Feare, Bennun and Fielder2006).
SUMMARY OF ABBOTSBURY SWAN FLOCK AND H5N1 OUTBREAK
Mute swans at the Abbotsbury Swannery breed colonially at the western end of the Fleet lagoon. The population is wild but partly habituated to human contact. In addition to a near-complete demographic record of breeding swans, information is also available for non-breeders and summer immigrants because a round-up of swans on the Fleet is undertaken every other July (including in 2007). Outside the breeding season some birds disperse eastwards along the 12 km length of the Fleet. Other swans (mostly from the River Frome, Weymouth, and surrounding countryside) move into and away from the Fleet at various times of the year. Most of these are either birds that arrive during the autumn (many of which leave again by the end of the year) or birds that arrive around June to moult, when they are flightless for about six weeks, and which leave again in August/September.
Apart from the mute swans, the commonest resident is the Canada Goose (up to ∼500 birds). Large numbers of waterbirds of several avian orders arrive in the autumn, starting in September and gradually increasing in number over the next two months. During 2002–07 approximately 15,000 waterbirds wintered on the Fleet each year and nearby waters (Austin et al. Reference Austin, Collier, Calbrade, Hall and Musgrove2008), the great majority of which are visitors from continental Europe and further East. This total does not include the thousands of migratory gulls (Larus spp.) that forage over surrounding areas, many of which roost on the Fleet at night. The main groups of wintering birds (approximate peak numbers on the Fleet in brackets) are Coot, Fulica atra (2000), Brent Goose, Branta bernicla (2000), diving ducks primarily Aythya spp (1500) and dabbling ducks primarily Anas spp (4000).
In total 11 HP H5N1-infected birds were discovered during the 2008 incident. During late December 2007 and early January 2008, three dead or severely ill mute swans were identified as being infected with HP H5N1. Subsequent surveillance resulted in the discovery of another seven HP H5N1-positive swans later in January 2007. The last HP H5N1-positive bird to be discovered was a Canada Goose found close to the Swannery in late February (this was the only Canada Goose tested). Of the ten positive swans, nine were found on the Fleet and had previously been seen at the Swannery. The remaining swan died at Radipole Lake, Weymouth, at least 3 km from the Fleet (detailed maps available in DEFRA, 2008). This bird hatched at Radipole in 1995 and had bred there for several years. However, in recent years it had left Radipole for much of the winter, including 2007/08, and only reappeared there a few days before being found moribund. Thus this swan could have been on the Fleet prior to its return to Radipole. Although this bird appears to be epidemiologically linked to the transmission on the Fleet, for the purposes of this study we do not consider it a member of the Swannery flock.
MATERIALS AND METHODS
Bird identification and demography
Comprehensive, long-term ringing records of the swan population on the Fleet are maintained by the Swannery staff and Professor Chris Perrins, Department of Zoology, University of Oxford. The records were used to identify the dead or moribund birds found during surveillance and to distinguish long-term Abbotsbury residents from immigrant or itinerant swans. The age distribution of the Fleet swan population in mid-2007 was reconstructed from swans caught in the July 2007 round-up (n = 806), plus the cygnets hatched in 2007 and some of their parents (n = 155) who were not caught because they were ashore. These values will closely approximate the population's age composition at the time of the HP H5N1 outbreak in January 2008, with the following minor differences: (1) some birds, mostly cygnets hatched in 2007, will have died between July and January, (2) a few immigrants will have arrived, predominantly young birds, and (3) some birds, mostly younger immigrants, will have left. We mitigated (1) by using cygnet count from September (rather than July), after which cygnet mortality is greatly reduced (McCleery et al. Reference McCleery, Perrins, Wheeler and Groves2002). Further estimates of bird numbers throughout the year (swans and other waterbirds) in and around the Fleet were obtained from The Wetland Bird Survey database (Austin et al. Reference Austin, Collier, Calbrade, Hall and Musgrove2008; S. Grove, personal communication).
Age structure of avian influenza seropositivity
Whole blood samples were collected in August 2008 (under UK Home Office licence PPL 30/2572) from 103 apparently healthy mute swans at Abbotsbury whose date of hatching was known. Birds were selected randomly within age-classes from a sample of >400 captured, with the aim of balancing sample sizes across age-classes as much as practically possible. Blood was drawn from either the brachial veins or superficial leg veins and transferred to BD Vacutainer Serum Separation Tubes. Serum was separated by centrifugation at 1000 g for 10 min. Untreated sera were tested for the presence of antibodies against the nucleoprotein of avian influenza A virus (AIV). We used the IDEXX FlockCheck Avian Influenza MultiS ELISA which detects all AIV subtypes, according to the manufacturer's instructions. Additionally we included 71 blood samples taken in July 2007 (i.e. before the outbreak) from Abbotsbury swans with known hatching dates (under UK Home Office licence PPL 80/1944).
Virus isolation, RNA extraction and sequencing
Post-mortem tissue samples from 38 mute swans and one Canada goose obtained during January and February 2008 were submitted for viral detection and isolation (17 from the Fleet, 21 from surrounding areas). The samples consisted of bulked viscera and intestines where possible and in most cases two samples per carcass were taken. Samples were subjected to virus isolation and detection tests using embryonated fowls’ eggs (OIE, 2008) and using real time PCR (M, H5, and N1 gene; see Slomka et al. Reference Slomka, Pavlidis, Coward, Voermans, Koch, Hanna, Banks and Brown2009, Reference Slomka, Irvine, Pavlidis, Banks and Brown2010 for details). Haemagglutinating agents derived from samples were further characterized according to the EU diagnostic manual for avian influenza (EU, 2006) to determine HA and neuraminidase subtype. Positive detections were subjected to amino acid sequencing of the HA cleavage site to confirm pathogenicity. As part of the active surveillance programme to investigate the source and transmission of the virus, buccal and cloacal swabs were taken from 60 live mute swans at the Swannery on 18th January 2008. Additionally, faecal samples from wild birds (mallards n = 56, and coots n = 44) were collected from around the Fleet on 21st January 2008.
Where available, RNA was extracted from either internal organs or allantoic fluid using the QIAamp Viral RNA Kit (Qiagen). HA gene sequences were reverse transcribed and amplified using the OneStep RT-PCR Kit (Qiagen). The following primers were used (F = forward, R = reverse): S1(F) = AGCAGGGGTATAATCTCTCA (H3 positions 7-26); B2a(R) = TTTTGTCAATGATTGAGTTGACCTTATTGG (1236-06); J3(F) = GATAAATTCTAGCATGCCATTCC (928-50); J1c(R) = AGTAGAAACAAGGGTGTT (1774-56); AS1(F)= AATGATGCGGCAGAGCAGAC (592-611); AS4R(R) = CCCCACAGTACCAAAAGATC (578-559); H5-5(F) = CATCAATTTTGAGAGTAATG (800-20);H5-6(F) = GGGTACCACCATAGCAACGAGCAGGG (1100-26); J2c(R) = GGTGTTTTTAACTACAATCTGG (1761-39). All RT-PCR products were excised from agarose gels and purified using the QIAquick Gel Extraction Kit (Qiagen). DNA was sequenced using the Prism BigDye Terminator v1.1 Cycle Sequencing Kit on the 3130 Genetic Analyzer (both Applied Biosystems). All commercial kits were used following manufacturer's instructions.
Molecular clock phylogenetic analysis
The nine available H5N1 HA gene sequences from the Abbotsbury outbreak were aligned using Se-Al v2.0 (http://tree.bio.ed.ac.uk), together with a set reference H5N1 sequences with known dates of sampling, resulting in an alignment of 68 taxa, 1704nt long. All reference sequences of HP H5N1 clade 2·2 that were closely related to the Abbotsbury strain were included. A molecular clock phylogeny, which places the history of transmission on a real time-scale, was estimated using the Bayesian Markov Chain Monte Carlo approach implemented in BEAST v1.5 (Drummond and Rambaut, Reference Drummond and Rambaut2007). Specifically, we used a HKY nucleotide substitution model with gamma-distributed among-site rate heterogeneity, together with a relaxed molecular clock (uncorrelated lognormal model) and a Bayesian skyline plot coalescent model. The relaxed clock model fitted the data significantly better than a simpler strict clock model (Bayes factor >100). A maximum clade credibility tree was summarized from the posterior distribution using TreeAnnotator (Drummond and Rambaut, Reference Drummond and Rambaut2007) and annotated using FigTree v1.3 (http://tree.bio.ed.ac.uk).
RESULTS
Bird identification and demography
All nine of the HP H5N1-infected mute swans found on the Fleet could be identified from the Swannery ringing records. Eight of the nine were hatched at Abbotsbury and are unlikely to have ever left (all were present on 21st July 2007). The ninth bird was first caught at Abbotsbury on 5th May 2007 when it was determined, from plumage characteristics, to have hatched the previous year; this bird was also present at Abbotsbury on 21st July 2007. Additionally, eight HP H5N1-negative mute swans that died between 27th December and the end of January 2008 were ringed and so could be aged; all were present on 21st July 2007. A further five partial swan carcasses were found without legs; their ringing status is thus unknown. Since all the infected mute swans (including the tenth bird found at nearby Radipole) were present in the area during summer 2007, five months prior to the outbreak, it seems highly unlikely that HP H5N1 was introduced by any of them.
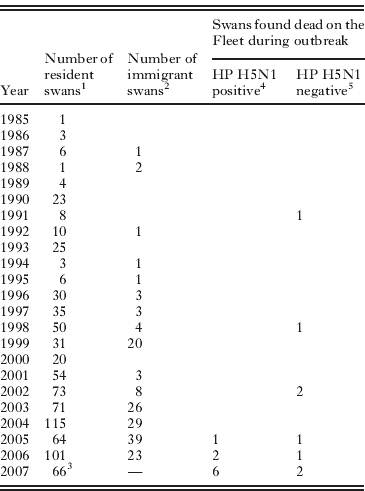
Table 1 gives the age-distribution of the infected birds and compares it to the distribution for resident and immigrant swans at Abbotsbury. Clearly, the dead birds that tested positive for HP H5N1 were disproportionally young; all were less than 3 years old and six of the nine were cygnets hatched in 2007. At the time of the outbreak, we estimate birds less than 3 years old comprised <29% of the Abbotsbury population (Table 1). The age distribution of the HP H5N1-infected birds is significantly different from that of the general Abbotsbury swan population (P < 0·0001; two-tailed Fisher's exact test; birds grouped into those hatched after 2004 versus the remainder). The age distribution of the HP H5N1 cases is also marginally different from that of the HP H5N1-negative dead birds found during the outbreak (P = 0·03; Fisher's exact test as above). However, small sample sizes mean the latter test is not robust. For example, inclusion of the older HP H5N1-positive mute swan found at Radipole is sufficient to render the test non-significant (P = 0·12; Fisher's exact test as above).
Table 1. Age distribution of mute swans on the Fleet
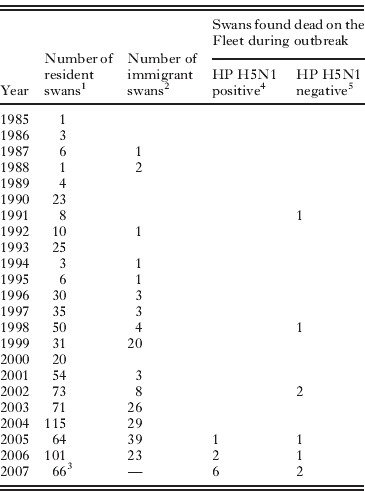
1 As of July 2007. Year represents the year of hatching of Abbotsbury-bred birds, and of immigrants young enough to be aged precisely when first caught (usually <15 months from hatching).
2 As of July 2007. Year represents year of first observation, hence these birds could have hatched earlier.
3 Number of surviving cygnets in September 2007.
4 See Methods for details of virus detection. Excludes the HP H5N1 positive swan found at Radipole lake.
5 Excludes 5 swan carcasses found without legs, whose ringing status is unknown.
The estimated rate of swan mortality due to HP H5N1 at Abbotsbury was only 1·1%, with only nine of an estimated 800 birds identified as succumbing to the disease. Overall mortality during the winter of 2007/08 was not noticeably higher than in other years (DEFRA, 2008), although direct statistical comparison is not possible because during and after the outbreak the colony and surrounding areas were searched for corpses more intensively and extensively than is normal. Indeed, if the age distribution of the flock in January 2008 was more accurately known then estimated mortality due to HP H5N1 would likely be even lower, because first year birds are more likely to die than older age classes (Perrins, Reference Perrins1991; McCleery et al. Reference McCleery, Perrins, Wheeler and Groves2002). It is likely that all mute swans that died after active surveillance began on 11th January 2008 were recovered (for details see DEFRA, 2008); before that date it is possible that dead swans were predated before being discovered, especially if they had dispersed along the Fleet away from the Swannery.
Age structure of avian influenza seropositivity
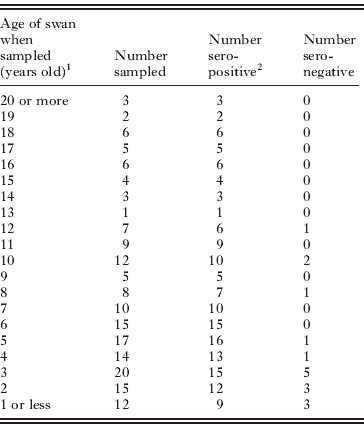
The percentage of Abbotsbury swans with detectable antibodies to AIV nucleoprotein was 85% for the 71 birds sampled in 2007 and 94% for the 103 birds sampled in 2008. Table 2 shows how AIV sero-positivity in the Abbotsbury population varies by age. Young birds, particularly those less than six years old, were significantly less likely to be AIV sero-positive (logistic regression; P < 0·01). Note that this assay does not distinguish between antibodies generated by recent versus past infection, nor is it specific to any particular AIV subtype.
Table 2 . Age distribution of sero-positivity to AIV nucleoprotein in the flock of Abbotsbury swans
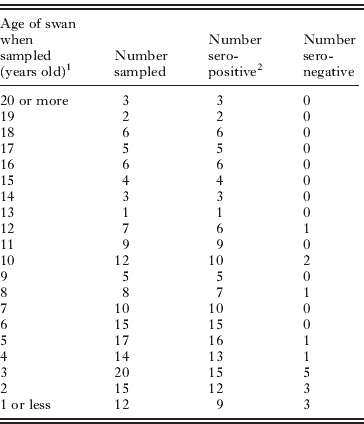
1 Swans sampled in either July 2007 or August 2008.
2 See Methods for detail.
Virus isolation, RNA extraction, and sequencing
HP H5N1 virus was detected in ten mute swans and one Canada Goose by real time PCR sequencing. Among these, virus could be isolated and cultured from samples derived from seven of the mute swans. All of the samples collected during or after the outbreak from live swans or wild birds (mallards and coots) were PCR-negative for H5N1. Furthermore, as noted previously, there was no evidence of H5N1 infection among the 13 people who had unprotected exposure to the infected swans (Wallensten et al. Reference Wallensten, Salter, Bennett, Brown, Hoschler and Oliver2010).
Table 3 provides details of the full HA gene sequences obtained from 10 mute swans and the Canada goose. The amino acid sequence of all samples at the HA cleavage site was identical (PQGERRRKKRGLF), indicative of a highly pathogenic strain (Senne et al. Reference Senne, Pederson, Suarez and Panigrahy2006). Systemic, highly pathogenic infection was corroborated by specific immunolabelling against influenza A: viral nucleoprotein was detected in the neurons and/or microglia of four mute swans (results not shown). There were only three amino acid variations among the outbreak HA sequences and none of these appeared to affect receptor binding or potential glycosylation sites. Seven of the HA sequences were identical at the amino acid level and five were identical at the nucleotide level (see Table 3 for sequence accession numbers).
Table 3. Virus isolation and sequencing details

Molecular clock phylogenetic analysis
The estimated maximum clade credibility phylogeny estimated from the HA gene sequences is presented in Fig. 1a. The Abbotsbury strains (green) form a single monophyletic cluster that is strongly statistically supported, demonstrating that the outbreak arose from a single source. The reference sequences most closely related to the Abbotsbury event were sampled from chickens in Turkey and the Ukraine in early January 2008. Thus these strains are unlikely to represent the direct source of the Abbotsbury epizootic but instead represent outbreaks that share a common ancestral strain with it.

Fig. 1. (a) Estimated molecular clock phylogeny of the Abbotsbury outbreak HA gene sequences (green) and closely related reference strains (black). The timescale of virus evolution is shown below. Statistical support for most clusters was high; red circles denote nodes with a posterior probability >0·95 and orange circles denote those with a posterior probability >0·85. The blue bars indicate the 95% credible regions for the estimated date of each internal node; the width of these indicates a high degree of temporal resolution. The estimated dates for two nodes are highlighted. The upper arrow points to the common ancestor of the Abbotsbury outbreak sequences. The lower arrow points to the common ancestor of the outbreak plus the most closely related reference strains (from Turkey and Ukraine). The 95% credible intervals for these dates are given in square brackets. Sequences are named using the following format: accession number / host / 3-letter country code of sampling location / date of sampling. Ck = chicken, Tk = turkey, Dk = duck. (b) Plot showing the average monthly count (2001–07) of waterbirds on the Fleet and nearby areas. The shaded areas illustrate the estimated ranges of dates for the introduction of HP H5N1 into the area in 2007, as obtained from the molecular clock analysis. The lighter area represents the most conservative (i.e. widest) range of dates that is compatible with the evolutionary analysis. The darker and narrower shaded area represents the estimated time between the two nodes highlighted by arrows in the phylogeny above (the common ancestor of the Abbotsbury outbreak, and the common ancestor of the outbreak plus closest reference strains). Modified from DEFRA (2008).
Further insights into virus transmission can be inferred from the time-scale of the molecular clock phylogeny (Fig. 1a). The estimated date of the common ancestor of the outbreak sequences is the 3rd week of November 2007, a month before the first sick swans were detected (the 95% credible region for this estimate ranges from the middle of October until the end of December). This estimate provides a lower limit for the date of virus arrival (provided that the outbreak had a single source). An upper limit on the date of virus arrival is provided by the estimated date of the common ancestor of the outbreak with related reference strains, which is the start of September (95% credible region: end-June to end-October 2007). Thus the virus is most likely to have arrived during September to mid-November 2007. During this time wildfowl and gulls start to arrive on the Fleet from continental Europe and the number of waterbirds in the area increases from ∼5000 to more than 12,000 (monthly averages for 2001–07; see Fig. 1b). The specific waterbird counts for 2007 were 3491 in September, rising to 8190 in November and subsequently declining to 3436 by March 2008 (Austin et al. Reference Austin, Collier, Calbrade, Hall and Musgrove2008). Mute swan numbers are more stable, and ranged between 820 and 651 in 2007. Although local swans have been known to move onto the Fleet during the autumn (see Summary of Abbotsbury Swan Flock section), there is no evidence that immigrant mute swans at Abbotsbury have crossed the English Channel. Further inspection of the molecular clock phylogeny shows that in the 12 months preceding the Abbotsbury incident the lineage to which this virus belongs had disseminated widely throughout Europe, including to Turkey, Germany and England.
DISCUSSION
The HP H5N1 outbreak in the Abbotsbury swan flock provided a unique ‘natural experiment’ in which to study the effects of age structure on the transmission of the virus in a natural population. Our findings help to understand the complex epidemiology of HP H5N1 in European swan populations; specifically, the occurrence of multiple, geographically widespread outbreaks, each of which is limited in duration and local dispersal, and which apparently exhibits low mortality.
The low overall mortality rate of the Abbotsbury outbreak, and the predominance of young birds among the dead, can both be explained by our observation of age-dependent sero-prevalence. Almost all the Abbotsbury mute swans had been previously exposed to AIV – the only age class with appreciable sero-negativity were those 3 or younger, of which ∼20–30% showed no detectable serological response to AIV nucleoprotein. If, as experimental work suggests, pre-existing antibodies to AIV HA or neuraminidase can partially protect against subtypes containing H5 or N1 (Kalthoff et al. Reference Kalthoff, Breithaupt, Teifke, Globig, Harder, Mettenleiter and Beer2008), then it is possible that much of the flock could have been exposed to, and have transmitted, the HP virus without overt signs of disease. Although experimental studies suggest that previous AIV exposure may reduce the duration and intensity of viral shedding upon re-infection, its effect on transmission in natural bird populations is unknown. An increasing sero-prevalence to LP AIV with age has recently been reported for free-living Bewick's swans (Cygnus columbianus bewickii; Hoye et al. Reference Hoye, Fouchier and Klaassen2012). Thus we speculate that the Abbotsbury flock, and wild swan populations in general, may be more resilient than populations of short-lived poultry to the HP H5N1 lineages currently in circulation.
Although this interpretation is consistent with the data there are three remaining areas of uncertainty. First, due to the limited size of the outbreak we cannot be confident that the age distribution of HP H5N1-positive dead birds was significantly different from that of virus-negative dead birds. The death rate over winter is highest among juveniles (McCleery et al. Reference McCleery, Perrins, Wheeler and Groves2002) and therefore it could be argued that HP H5N1-associated and background mortality were no different. However, both the HA cleavage site sequence of the Abbotsbury strains and our observation of viral nucleoprotein in nervous tissue demonstrate that the infections were systemic and highly pathogenic. We can safely reject the possibility that the juvenile birds were exposed to the HP H5N1 virus but the adults were not; all ages of the Abbotsbury population feed and forage together in dense flocks.
Second, our sero-prevalence results are not subtype specific and simply indicate past exposure; they do not demonstrate that protective immunity exists. Experimental challenge studies on other waterbirds suggest that exposure to H5 and N1 viruses provides better protection against subsequent HP H5N1 challenge than other subtypes (e.g. Fereidouni et al. Reference Fereidouni, Starick, Beer, Wilking, Kalthoff, Grund, Häuslaigner, Breithaupt, Lange and Harder2009; Berhane et al. Reference Berhane, Leith, Embury-Hyatt, Neufeld, Babiuk, Hisanaga, Kehler, Hooper-McGrevy and Pasick2010; Costa et al. Reference Costa, Brown, Howerth, Stallknecht and Swayne2011). Since the Abbotsbury flock feeds alongside thousands of migratory gulls and ducks that originate across much of Eurasia (as far as 80°E; e.g. Owen and Mitchell, Reference Owen and Mitchell1988) it is very likely that the swans are frequently exposed to a wide variety of LP AIV subtypes. Furthermore, there is evidence that mute swan populations in Europe carry antibodies to a range of avian influenza strains, including H5 and N1 viruses (Smietanka et al. Reference Smietanka, Minta, Domańska-Blicharz, Tomczyk and Wijaszka2008; Globig et al. Reference Globig, Staubach, Beer, Köppen, Fiedler, Nieburg, Wilking, Starick, Teifke, Werner, Unger, Grund, Wolf, Roost, Feldhusen, Conraths, Mettenleiter and Harder2009; Niqueux et al. Reference Niqueux, Guionie, Schmitz, Hars and Jestin2010). In future work we aim to use haemagglutination inhibition assays to characterize the antibody repertoire of the Abbotsbury flock; preliminary results have indicated responses to several different AIV subtypes. However, antibodies to HA and neuraminidase may not be the only factors contributing to protection against HP H5N1: cross-reactive cellular immunity in chickens induced by H9N2 has been shown to prevent disease caused by HP H5N1, even in the absence of neutralizing antibodies (Seo and Webster, Reference Seo and Webster2001).
Third, the cryptic circulation of HP H5N1 on the Fleet or surrounding areas for several weeks, as suggested by the molecular clock results, requires explanation because the incubation period of mute swans infected with HP H5N1 appears to be no longer than a week (Kalthoff et al. Reference Kalthoff, Breithaupt, Teifke, Globig, Harder, Mettenleiter and Beer2008). Although an outbreak origin in late December lies just within the 95% credible regions of the phylogenetic dating estimate, a more likely scenario is that the virus did not immediately transmit to the HP H5N1-positive birds but was instead present for some time among other species, or among swans away from the Swannery. This is consistent with the DEFRA surveillance report, which concluded that the source of the outbreak was most likely an incoming migratory bird (and not due to contaminated feed, local domestic poultry or infected poultry waste; DEFRA, 2008). The molecular clock best estimate of the date of outbreak origin is the 3rd week of November, coinciding with the greatest influx of migrant waterbirds (Fig. 1b). The ability of wild swans or other birds to move long distances whilst infected with HP H5N1 remains unclear and keenly debated (e.g. Weber and Stilianakis, Reference Weber and Stilianakis2007); one study has reported that the migration of Bewick's swans is hampered when infected with LP avian influenza viruses (van Gils et al. Reference van Gils, Munster, Radersma, Liefhebber, Fouchier and Klaassen2007). All available evidence suggests that the outbreak on the Fleet was of limited duration; sampling of live birds during active surveillance found no signs of virus circulation within the Abbotsbury flock (with a sample size that corresponds to a 95% probability of detecting 5% prevalence) and no further HP H5N1 cases were reported.
It is worth noting that molecular clock estimation is still possible – and reliable – when the outbreak sequences contain limited or no diversity. Given a rate of virus molecular evolution and an appropriate statistical model, the observation of zero mutations among outbreak sequences is consistent with only a narrow range of origin dates. Despite this, a more accurate date for the common ancestor of the outbreak would have been obtained if whole genome sequences were available for every isolate. The potential of whole genome data to provide detailed insights into viral transmission was demonstrated for human influenza during the 2009 H1N1 pandemic (e.g. Baillie et al. Reference Baillie, Galiano, Agapow, Myers, Chiam, Gall, Palser, Watson, Hedge, Underwood, Platt, McLean, Pebody, Rambaut, Green, Daniels, Pybus, Kellam and Zambon2012) and we encourage the application of these techniques to future investigations of AIV outbreaks.
The potential for long-lived birds such as swans to be exposed to multiple avian influenza subtypes during their lifetime raises interesting epidemiological questions. First, what determines the degree to which a bird species might possess complete or partial protection through previous exposure? Size is a likely predictor of survival of mild infections; a healthy swan can survive a week or more without food, whereas a small passerine, such as a tit (Parus) or sparrow (Passer) rarely has sufficient energetic reserves to survive a day without feeding. Second, does demography modulate the likelihood of protective immunity? We might expect only those species that have high adult survival rates to contain a significant number of multiply-exposed individuals. Birds with these characteristics include many of the larger waterfowl and gulls (e.g. Bennett and Owens, Reference Bennett and Owens2002). Third, will natural selection favour avian influenza virus variants that evade existing host immunity, thereby generating ‘antigenic drift’ similar to that which characterizes the evolution of human influenza? The potential for antigenic drift will depend on ecological as well as immunological factors. For example, long-term antigenic drift will be more likely if the virus circulates continuously among long-lived individuals that are repeatedly exposed, but less likely if viral persistence is maintained in short-lived (singly-exposed) species, with outbreaks in swans representing dead-end ‘spillover’ transmission from that reservoir. There is clearly much to learn about the ecology of avian influenza viruses and great potential for inter-disciplinary studies that can successfully combine field observation, immunological assays, viral genomics and mathematical modeling.
ACKNOWLEDGEMENTS
We are grateful to Mrs C. Townshend for permission to study the swans at Abbotsbury and to the Swannery staff for providing data and help. OGP is funded by The Royal Society. Professor A. Dawson kindly supplied the swan blood samples collected in 2007 and S. Grove supplied the Fleet counts. Many thanks to all the volunteers who helped with the swan round-ups, and to Ron Fouchier for discussion.