INTRODUCTION
The nematode genus Syphacia Seurat, Reference Seurat1916 (Oxyuridae Cobbold, Reference Cobbold1864: Syphaciinae Railliet, 1916) members of which are colloquially often referred to as pinworms (Adamson, Reference Adamson1994; Grear and Hudson, Reference Grear and Hudson2011), is a cosmopolitan genus parasitizing rodents. Hugot (Reference Hugot1988) separated the genus into three subgenera including Syphacia (Syphacia) comprising 14 species, all occurring in Cricetidae Fisher, 1817 or Muridae Illiger, 1811. Since that time more than 25 new species have been described in this subgenus, particularly from Southeast Asia, Indonesia, South America and Australia (see, e.g. Hasegawa and Tarore, Reference Hasegawa and Tarore1996; Weaver and Smales, Reference Weaver and Smales2010; Rojas et al. Reference Rojas, Elba, Mino, Notarnicola and del Rosario Robles2011, Smales, Reference Smales2011; Dewi et al. Reference Dewi, Mitsuhiko and Fitrana2014) such that the subgenus now encompasses more than 39 species. It is generally assumed that species of Syphacia are narrowly host specific, partly because the French school considered them a priori to have co-evolved with their host rodents (Hugot, Reference Hugot1988). Syphacia species may have exacting dietary requirements which restrict them to one or a few congeneric hosts, although under intensive mixed husbandry of rodents, cross-infections can occur, for example, the occurrence of S. obvelata (Rudolphi, Reference Rudolphi1802) in laboratory rats and S. muris (Yamaguti, Reference Yamaguti1935) in laboratory mice (Hussey, Reference Hussey1957). It has also been possible to experimentally infect hosts that do not normally harbour particular species in the wild (Syphacia stroma in laboratory mice, Lewis, Reference Lewis1968). Nevertheless, while most rodents in nature harbour only their own specific species of Syphacia, casual infection by more generalist species (ecological fitting) may also have occurred (Araujo et al. Reference Araujo, Braga, Brooks, Agosta, Hoberg, von Hartenthal and Boeger2015; Weaver et al. Reference Weaver, Monks and Gardner2016).
Species of Syphacia are perhaps best known as unwanted contaminants of laboratory rodents (Taffs, Reference Taffs1976) that have been bred for medical research, under husbandry conditions specifically designed to eliminate entirely, or at the very least to minimize the possibility of infection with specific pathogens (referred to as specific pathogen-free mice and rats). Control of these nematodes is made difficult as a result of their extremely efficient transmission strategies; for example, egg laying on the perianal surface of their hosts during sleep, very short life cycles including rapid embryonation of eggs and rapid maturation in the host, and eggs that are resilient but sticky and capable of adhering to host hair, dust and materials in the bedding area (see references in Lewis, Reference Lewis1968; Lewis and D'Silva, Reference Lewis and D'Silva1986; Kerboeuf and Lewis, Reference Kerboeuf and Lewis1987; Lewis, Reference Lewis1987; Adamson, Reference Adamson1994; Grear and Hudson, Reference Grear and Hudson2011; Meade and Watson, Reference Meade and Watson2014).
In the Euro-Siberian region of the Palearctic, wood mice (Apodemus sylvaticus) and yellow-necked mice (Apodemus flavicollis) are parasitized by two Syphacia species. Perhaps the best known of these is S. stroma (von Linstow, Reference von Linstow1884) Morgan, Reference Morgan1932, which has been reported throughout the range of Apodemus spp. in Europe [e.g. Ireland (Loxton et al. Reference Loxton, Lawton, Stafford and Holland2016), Portugal (Eira et al. Reference Eira, Torres, Vingada and Miquel2006) and Fauna Europaea (http://www.fauna-eu.org) lists 16 countries for this species]. Syphacia stroma is an unusual and atypical member of the genus, because in contrast to all other known members of the genus, this species lives mainly in the small intestine of its host, although migrating patent females may be found in the caecum and colon, and in heavy infections there can be a spill over of worms from the small into the large intestine. All other known species of Syphacia are parasites of the large intestine, living predominantly in the caecum of their rodent hosts but also in the colon, environments that are rich in the bacteria upon which these nematodes feed. In our experience, and that of many other workers, infections with S. stroma in wood mice can be huge, exceeding many hundreds and even thousands of worms per host, and prevalence has generally been recorded as high, so clearly this is one of the dominant parasitic nematodes infecting wood mice in the region (Thomas, Reference Thomas1953; Behnke et al. Reference Behnke, Lewis, Mohd Zain and Gilbert1999; Abu-Madi et al. Reference Abu-Madi, Behnke, Lewis and Gilbert2000).
The second species of Syphacia infecting Apodemus spp. in Europe is S. frederici Roman, Reference Roman1945, which is a more recently recognized caecal dwelling species that has been widely recorded throughout the range of its hosts in Europe [e.g. in former Czechoslovakia (Tenora et al. Reference Tenora, Quentin and Durette-Desset1974); Italy (Milazzo et al. Reference Milazzo, Di Bella, Casanova, Ribas and Cagnin2010); Portugal (Eira et al. Reference Eira, Torres, Vingada and Miquel2006); Romania (Mészáros and Murai, Reference Mészáros and Murai1979); Serbia (Čabrilo et al. Reference Čabrilo, Jovanović, Bjelić-Čabrilo, Budinski, Blagojević and Vujošević2016) and 10 countries listed in Fauna Europaea (http://www.fauna-eu.org)], but to our knowledge has apparently never been reported in wood mice from the British Isles [personal Communication; Eileen Harris and records at the Natural History Museum in London (NHM)]. Arguably, the rodent–helminth fauna of the British Isles ranks among the better studied rodent–helminth compound communities in the world (Elton et al. Reference Elton, Ford, Baker and Gardiner1931; Thomas, Reference Thomas1953; Sharpe, Reference Sharpe1964; Lewis, Reference Lewis1968; Canning et al. Reference Canning, Cox, Croll and Lyons1973; Murúa, Reference Murúa1978; Montgomery and Montgomery, Reference Montgomery and Montgomery1990; Abu-Madi et al. Reference Abu-Madi, Behnke, Lewis and Gilbert1998; Reference Abu-Madi, Behnke, Lewis and Gilbert2000), and it seems to have been well accepted by workers in this field that the only Syphacia endemic in wood mice in the British Isles is S. stroma (Lewis, Reference Lewis1987).
However, while conducting surveys of helminth communities in the last decade in various sites in the British Isles, we have encountered wood mice harbouring Syphacia infections restricted to the caecum and colon of their hosts and suspected that these might be S. frederici. Intrigued by the lack of any earlier reports of S. frederici in the UK, we conducted a thorough investigation of the suspected worms. In this paper we compare their morphology to published reports of S. frederici, and provide novel data on their genetic signature, showing how they relate to other common Syphacia species parasitizing murid rodents in the British Isles. For comparison we draw on additional Syphacia spp. worms recovered from murid hosts from some other locations in Europe. We also emphasize the key morphological features that can be used easily in quantitative studies to distinguish between S. stroma and S. frederici.
MATERIALS AND METHODS
Sources of worms
The sources of worms used for genetic analysis are shown in Table 1, and the approximate locations are illustrated on a map of Europe in Fig. 1. Rodents were trapped using Longworth, Sherman and other humane live capture traps. Traps were set at dusk and collected soon after dawn. Animals were inspected, culled by cervical dislocation and the entire intestinal tract was preserved in 80% ethanol, for subsequent dissection in our laboratory. In the laboratory, the intestinal tracts were divided into stomach, small intestine (three sections corresponding approximately to duodenum, jejunum and ilium), caecum and colon, and the contents of each section were examined carefully in separate Petri dishes ensuring no contamination with material from other sections. All recovered worms were transferred to 80% ethanol in 1·5 mL tubes and frozen at −80 °C until further processing for DNA extraction. All tubes were given a unique identifier reference (Table 1) and a range of pertinent details of each host was recorded on our database.

Fig. 1. The location of rodents that were sampled in the current study. 1 = Nottingham, England; 2 = Anglesey, Wales; 3 = Mazury, Poland; 4 = Pancas, Portugal; 5 = Warsaw, Poland; 6 = Wrocław, Poland; 7 = Limerick, Ireland; 8 = Brittany, France; 9 = Jersey, British Isles; 10 = Norfolk, England; 11 = Edinburgh, Scotland; 12 = Friesland, Netherlands; 13 = Isle of May, Scotland; 14 = Berkshire, England; 15 = Dorset, England; 16 = Durham, England.
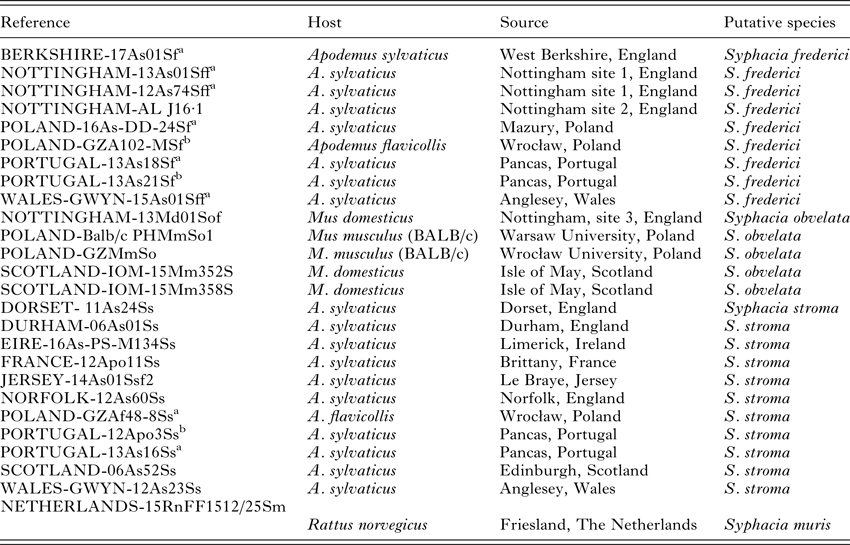
Table 1. Details of the worms that were sequenced (CO1 and/or rDNA) for the current study and/or examined morphologically
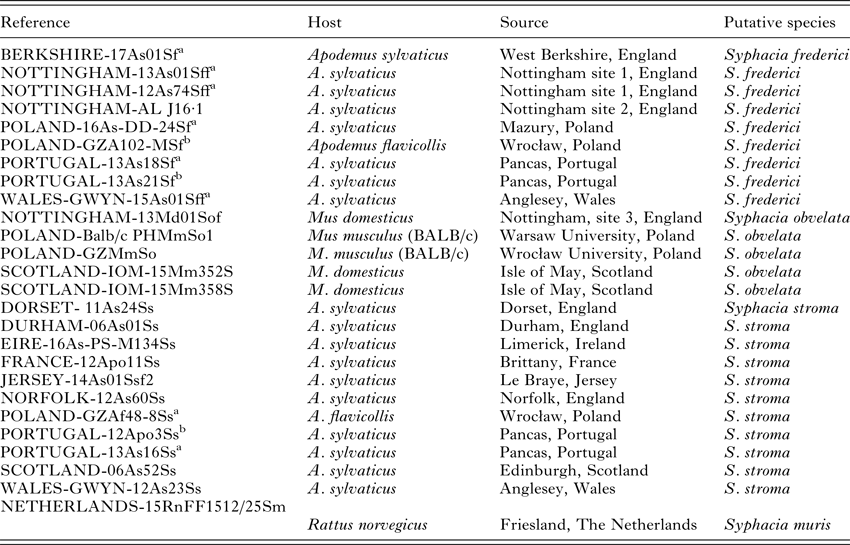
All specimens of S. stroma were recovered from the small intestine of the wood mice. All S. frederici and S. obvelata were recovered from the caecum of wood mice and S.muris from the caecum of rats.
The reference column gives the identity of the animal in our database and frozen helminth library, but for this study we only provide the genetic sequence of one worm extracted from each of these hosts.
Nottingham site 1 = suburban garden in West Bridgford, Nottingham.
Nottingham site 2 = suburban garden in Beeston, Nottingham.
Nottingham site 3 = Farm in Tollerton, Nottingham.
a Specimens also examined morphologically.
b Only examined morphologically.
Some worms were obtained from our collaborators and the details of processing varied. In Ireland, mice were first euthanized with an overdose of isoflurane, following which their digestive tracts were immediately removed and frozen at −20 °C for subsequent dissection and recovery of worms. In the Netherlands, rats were anaesthetized with isoflurane and were euthanized by cardiac puncture. Upon death, the intestinal tract including the stomach was taken from each rat for parasitological examination and was stored either at +4 °C for immediate evaluation or at −20 °C until further use (Franssen et al. Reference Franssen, Swart, van Knapen and van der Giessen2016).
Molecular genetic comparison of species
DNA was isolated from individual worms using Extracta™ DNA prep kits (Quantabio) or DirectPCR lysis buffer (Viagen Biotech) according to the manufacturer's instructions. The cytochrome C oxidase 1 (CO1) gene was amplified using primers (forward: 5′-TGGTCTGGTTTTGTTGGTAGTT-3′, reverse: 5′-AACCACCCAACGTAAACATAAA-3′; Okamoto et al. Reference Okamoto, Urushima, Iwasa and Hasegawa2007). The ribosomal DNA (rDNA) region consisting of internal transcribed spacer (ITS)-1, 5·8S gene and ITS-2 (~700–750 bp) was amplified in separate reactions using the universal NC5 forward (5′-GTAGGTGAACCTGCGGAAGGATCATT-3′) and NC2 reverse primers (5′-TTAGTTTCTTTTCCTCCGCT-3′; Newton et al. Reference Newton, Chilton, Beveridge, Hoste, Nansen and Gasser1998, Table 2). PCR reactions contained: 12·5 µL of BioMix Red (Bioline), 0·5 µ m of the forward and reverse primers, <250 ng of template DNA and nuclease-free water to a total volume of 25 µL. AccuStart II Taq Polymerase (Quantabio) was used in place of BioMix Red to improve amplification of the CO1 gene, according to the manufacturer's instructions. Thermal cycling conditions for CO1 were: denaturation for 3 min at 94 °C, then 35 cycles of 1 min at 94 °C, 1 min at 52 °C and 1 min 30 s at 72 °C, with a final extension time of 7 min at 72 °C before being held at 4 °C. Thermal cycling conditions for the rDNA region were: denaturation for 1 min at 94 °C, then 30 cycles of 30 s at 94 °C, 30 s at 60 °C, 30 s at 72 °C, with a final extension of 72 °C for 5 min before being held at 4 °C. All PCR reactions were conducted in a Biorad PCT-200 thermocycler. Amplification in all PCR reactions was confirmed by visualization on a 1× SYBRSafe™, 1·5% agarose gel. PCR products were purified using ExoSAP (Affymetrix) and the final DNA concentration estimated by Nanodrop before dilution with nuclease-free water to the required concentration for sequencing. Sequencing primers, identical to the amplification primers, were diluted to the required concentration with nuclease-free water and supplied to Source Bioscience, along with PCR products, for Sanger sequencing. Chromatograms were inspected visually for ambiguities.
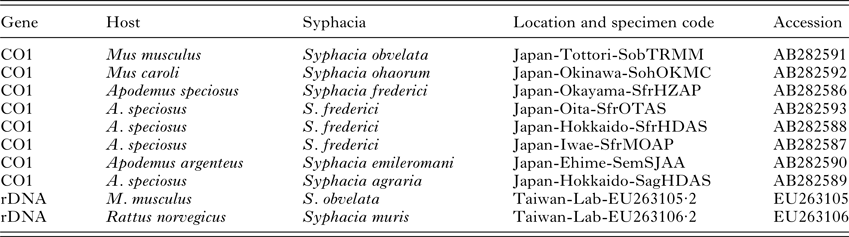
Table 2. Sequences used in the current study taken from GenBank
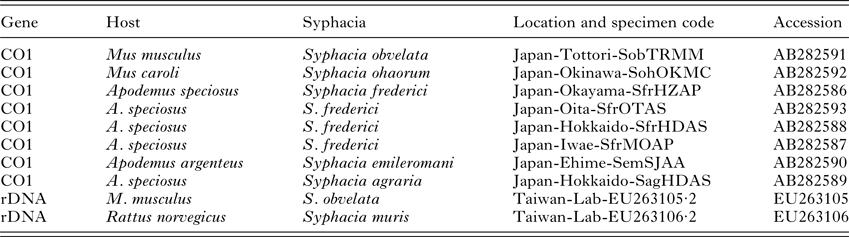
Sequence alignments were produced within the Mega 6·0 package using ClustalX followed by visual inspection. Phylogenetic analysis was performed with RAxML (v8·2·9) via the CIPRES Science Gateway using the maximum likelihood algorithm. Analysis of the rDNA ITS and 5·8S regions was carried out using the full sequence including indels. The Syphacia spp. DNA sequences acquired from Apodemus spp. and Mus spp. by Okamoto et al. (Reference Okamoto, Urushima, Iwasa and Hasegawa2007) were included in the CO1 alignments (Table 2). The S. obvelata and S. muris sequences produced by Parel et al. (Reference Parel, Galula and Ooi2008) were included in the rDNA alignment. Voucher sequences, including all sequences generated by this study and included in the current paper, have been deposited in GenBank (CO1 – MF142419 to MF142433; rDNA – MF142434 to MF142456).
Methods utilized for morphological comparison of species
Individual worms, voucher specimens taken from the samples of worms used for genetic analysis, were cleared in lactophenol and examined microscopically as temporary wet mounts. Measurements in micrometres, unless otherwise stated, were taken using an eyepiece micrometre and light micrographs using an Olympus photomicrographic system. Specimens of S. stroma from the NHM registration numbers 1934·7·19. 10–15; 1956·8·16·3–6; 1970·55–62. 63–68; 1979. 175–194; 983·3928–3929 were also examined. Specimens of S. stroma from Portugal (SAM AHC 47961 and 47962), and S. frederici from Portugal (SAM AHC 47963 and 47964), from Poland (SAM AHC 47965), from Wales (SAM AHC 47966) and from Nottingham (SAM AHC 47967) have been deposited in the South Australian Museum, Adelaide, with registration numbers shown in parenthesis. Additional specimens were deposited in the NHM with voucher numbers as follows: NORFOLK 12As60Ss, S. stroma, NHMUK 2017·5·19·1–6; WALES-GWYN-12As23Ss, S. stroma, NHMUK 2017·5·19·7–12; NOTTINGHAM-12As74Sff, S. frederici, NHMUK. 2017·5·19·13–18; WALES-GWYN-15As01Sff, S. frederici, NHMUK.2017·5·19·19–24.
RESULTS
Molecular genetic comparison of worms
The rDNA primers amplified 709–796 bps of DNA with a large number of nucleotide insertion/deletion events (indels) giving rise to the differences in length between clades. To the best of our knowledge, there are no published sequences for this target region in either S. stroma or S. frederici. As a result of the indels the S. stroma clade was uniformly 753 bp while S. frederici was 710 bp, the result of 69 nucleotide indels between the two species. Within clades, the nucleotide sequence was highly uniform with the S. stroma clade containing only two single nucleotide polymorphisms (SNPs) and S. frederici containing only one.
For S. obvelata and S. muris, we were able to compare our rDNA sequences to published data from GenBank. For S. obvelata there were three SNPs in the ITS-1 region; one [a site 28 bp (site 28) from the start of the primer binding region: T/G] in the Scotland (IOM-16Mm352S, IOM-16Mm358S) and Nottingham (13Md013Sf) isolates, another in the Scotland isolates only (site 62: T/A) and a third in the Nottingham isolate only (site 240: G/T). A fourth SNP occurred in the Nottingham (13Md01Sf) ITS-2 region (site 618: C/A). Therefore, the sequence for S. obvelata whether from laboratory mice from Poland or from wild caught mice in the UK was very similar to the published sequence for this species; although interestingly British isolates appear to have diverged slightly from European and laboratory isolates. Likewise our sequence for S. muris from wild caught brown rats from the Netherlands was 100% identical across a 796 bp region to laboratory worms from Taiwan published in GenBank (Table 2 and Parel et al. Reference Parel, Galula and Ooi2008).
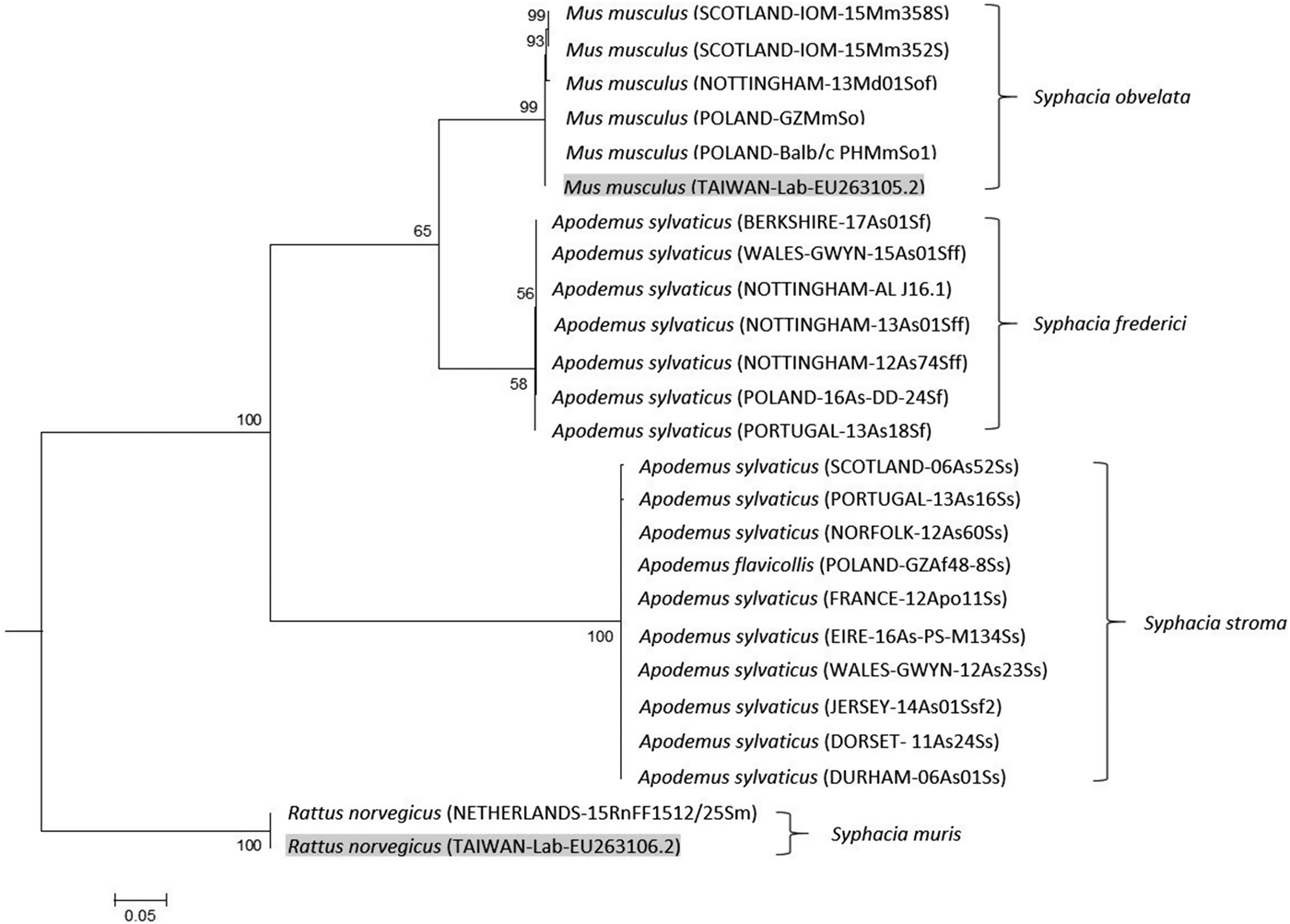
Our genetic data unambiguously confirm that S. stroma and S. frederici are indeed two distinct species and that molecular methods may be easily employed to distinguish between them. Importantly, the rDNA maximum likelihood tree (Fig. 2) confirmed our notion that S. frederici does exist on the British Isles. First, worms recovered from British A. sylvaticus formed separate deeply divided clades with 100% bootstrap support, separated from the S. obvelata clade, confirming two separate species. Our isolates of putative S. frederici, whether from Nottingham, Berkshire or Wales, formed a tight clade with worms known to be S. frederici from Poland and Portugal (see Materials and Methods). The rDNA sequence of the worm from Portugal differed by just a single SNP. Hence, known samples of European S. stroma and S. frederici clustered within the same clade as the worms from British hosts that are suspected of belonging to the same species. In the case of worms, we had assigned to S. stroma based on location in their hosts and morphological criteria, there was little variation in amplified rDNA sequences, one SNP in each of the Portugal (13As16Ss) and Scotland worms (06As52Ss) in the ITS-2 region. Second, as hypothesized, worms recovered from the small intestine fell within the S. stroma clade, while worms confined to only the caecum formed a clade containing only S. frederici.
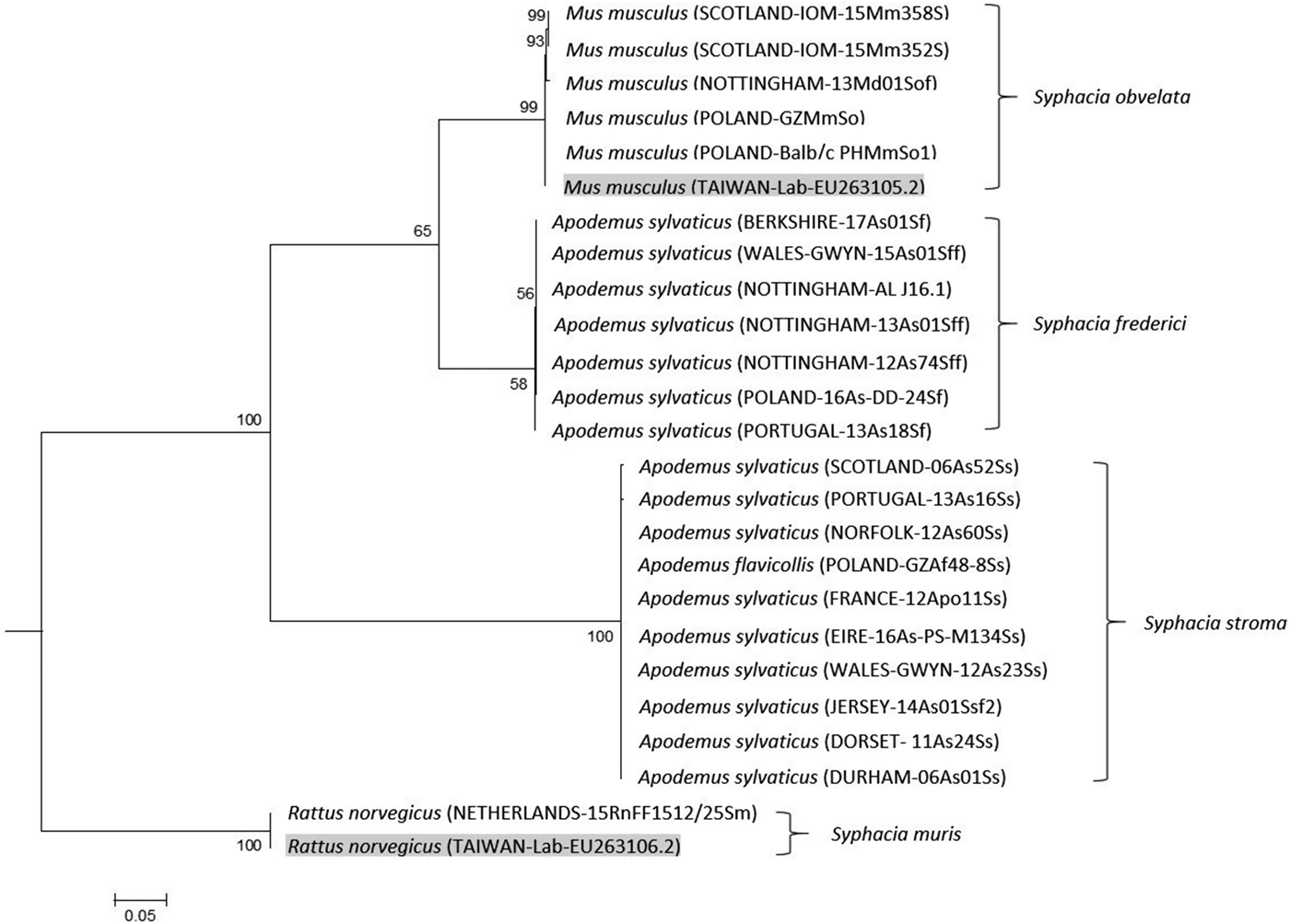
Fig. 2. Molecular phylogenetic tree of Syphacia from murid hosts (Apodemus spp. and Mus spp.) based on the ribosomal DNA following maximum-likelihood analysis with 100 bootstrap replicates implemented via the RAxML package. Scores at nodes represent bootstrap support for that node. Scale bar is proportional to the genetic distance in substitutions per site. Highlighted isolates are based on sequences from GenBank.
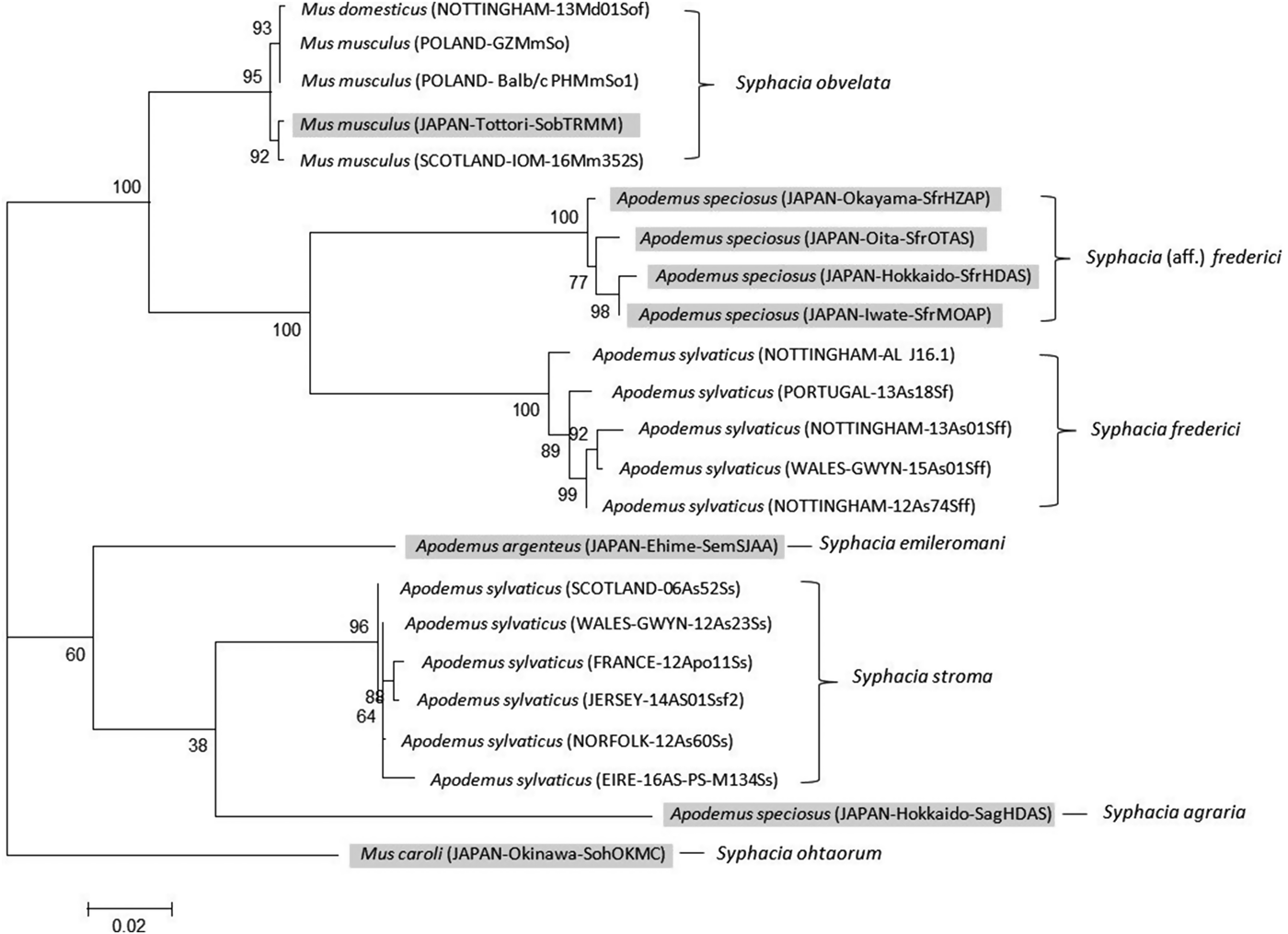
The CO1 primers amplified a 792 bp region across all species differing only by SNPs. In cases where the ‘reference identification’ is the same between trees, the gene was amplified from cDNA belonging to the same worm. Amplification of this region was not as reliable as the rDNA region resulting in a different set of isolates used for the phylogenetic analysis, although consistency was maintained where possible. The maximum likelihood phylogeny produced four distinctive clades (Fig. 3) containing S. stroma, S. obvelata, S. frederici from A. sylvaticus, with the fourth clade containing S. frederici from Apodemus speciosus (Okamoto et al. Reference Okamoto, Urushima, Iwasa and Hasegawa2007). Importantly, this tree supports the rDNA phylogeny, although with a greater level of within species variation, and demonstrates that S. stroma and suspected British S. frederici form their own deep individual clades, with S. frederici also grouping with a known European sample. Interestingly, the Japanese S. frederici forms its own distinctive clade from that of European S. frederici with 100% bootstrap support, the result of 45 unique SNPs between the two clades across the 792 bp region. GenBank sequences for three other species, Syphacia emileromani, Syphacia ohtaorum and Syphacia agraria, indicated that these species are unrelated to the S. stroma, S. obvelata and S. frederici clades.
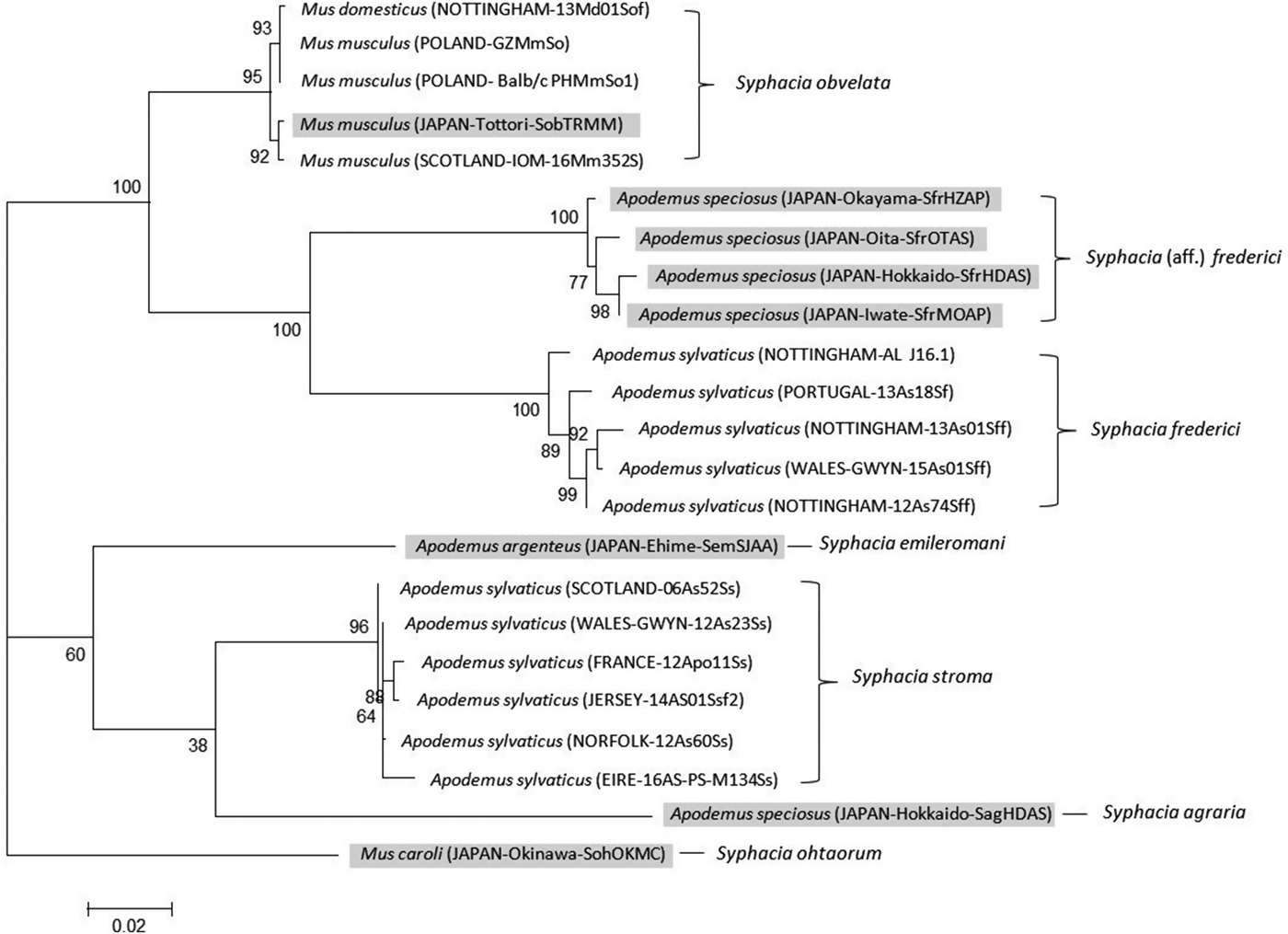
Fig. 3. Molecular phylogenetic tree of Syphacia from murid hosts (Apodemus spp. and Mus spp.) based on the mitochondrial cytochrome C oxidase 1 gene (CO1) following maximum-likelihood analysis with 100 bootstrap replicates implemented via the RAxML package. Scores at nodes represent bootstrap support for that node. Scale bar is proportional to the genetic distance in substitutions per site. Highlighted isolates are based on sequences from GenBank and all others are new sequences generated in the course of this study. Syphacia ohtaorum is described in Hasegawa (Reference Hasegawa1991), Syphacia emileromani in Chabaud et al. (Reference Chabaud, Rausch and Desset1963) and Syphacia agraria in Sharpilo (Reference Sharpilo1973).
Morphological comparison of worms
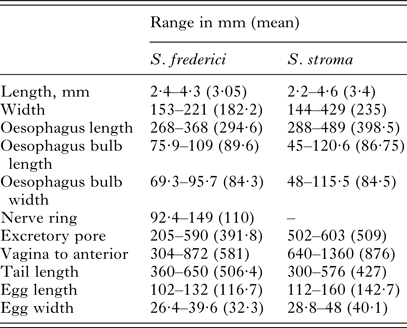
Key specimens were studied morphologically as highlighted in Table 1, with comparative measurements given in Table 3. A consistent difference in external morphology between S. stroma and S. frederici is in the shape of the tail of female worms. As illustrated in Figs 4 and 5, the end of the tail in female S. stroma is not as fine and attenuated as that of S. frederici, being rather broader and tapering to an end more sharply. In S. frederici, the tapering is more gradual, resulting in a longer, thinner and finer tip to the tail. Moreover, the tail in female S. frederici is often bent or twisted as shown in Figs 4B, 4C and 5. The morphology of the tail of female S. stroma worms was well illustrated by Morgan (Reference Morgan1932), while the comparison of both species is illustrated in Tenora and Mészáros (Reference Tenora and Meszaros1975), and the twisted fine tapering tail of S. frederici was emphasized by Ryzhikov et al. (Reference Ryzhikov, Gvozdev, Tokobaev, Shaldybin, Matsaberidze, Merkusheva, Nadtochi, Khokhlova and Sharpilo1979) and illustrated also in Sharpilo (Reference Sharpilo1973). Other differences that can be observed in female worms using light microscopy include the presence of cervical and lateral alae in S. frederici but only cervical alae in S. stroma, and the vulva nearer the anterior end in S. frederici. On the whole, S. frederici also has smaller eggs although there is some overlap between the species and authors differ about the degree of overlap. Based on our measurements, eggs <112 µm in length are likely to be S. frederici, and those >112 µm are likely to be S. stroma. This is in general agreement with egg measurements recorded by Morgan (Reference Morgan1932), Roman (Reference Roman1945), Sharpilo (Reference Sharpilo1973), Baruš et al. (Reference Baruš, Tenora and Wiger1979), Sharpilo (Reference Sharpilo1973) and Ryzhikov et al. (Reference Ryzhikov, Gvozdev, Tokobaev, Shaldybin, Matsaberidze, Merkusheva, Nadtochi, Khokhlova and Sharpilo1979). Baruš et al. (Reference Baruš, Tenora and Wiger1979) also detail some morphological differences between the eggs of these and other species of Syphacia that can be seen under scanning electron microscopy (SEM), but these are not suitable for quantitative studies. It is worth pointing out here that our observation of the presence of cervical alae on S. stroma differs from Wiger et al. (Reference Wiger, Baruš and Tenora1978) who concluded from a scanning electron microscopy (SEM) examination of S. stroma that this species has no cervical alae. However, Bernard (Reference Bernard1966) described cervical alae for S. stroma, and was cited by Quentin (Reference Quentin1971), and Morgan (Reference Morgan1932) illustrated cervical alae on S. stroma although he did not refer to them as such.

Fig. 4. The distal ends of female worms showing the difference in tails. (A) Syphacia stroma; (B) Syphacia frederici; (C) two S. frederici on the left and S. stroma on the right. The scale bar is 1 mm.

Fig. 5. The distal ends of three female Syphacia frederici from isolate WALES-GWYN-15As01Sff, to show the location of the point at which the tail bends. The bent tail in A and C are clearly apparent, and in B while the tail is straighter, most likely a fixation artefact, the point of flexion is clearly visible and suggests that an investigation of the ultrastructure of this feature may be revealing. All images were taken at the same magnification with ×20 objective. Scale bar in B is 10 µm.
Table 3. Comparative measurements of females of Syphacia frederici and Syphacia stroma from Apodemus sylvaticus from localities in Poland, Portugal and the UK
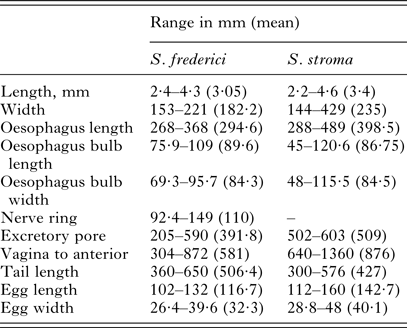
These measurements are based on all the worms that were measurable from among the isolates that were examined morphologically (for S. frederici: five worms from NOTTINGHAM-12As74Sff, 10 worms from WALES-GWYN-15As01Sff, five worms from PORTUGAL-13As21Sf, three worms from PORTUGAL-13As18Sf and five from POLAND-16As-DD-24Sf and for S. stroma eight worms PORTUGAL-12Apo3Ss, and worms at the BMNH registration numbers 1934·7·19·10–15; 1983. 3928–3929; 1956·8·16. 3–6; 1970. 63–68; 1970. 55–62;1979 175–194).
There are also differences between the males with S. federici having shorter spicules (55–65 µm in S. frederici and 71–87 µm in S. stroma based on Ogden, Reference Ogden1971; Tenora and Meszaros, Reference Tenora and Meszaros1975; Ryzhikov et al. Reference Ryzhikov, Gvozdev, Tokobaev, Shaldybin, Matsaberidze, Merkusheva, Nadtochi, Khokhlova and Sharpilo1979). Unfortunately since males are not often found in infections with Syphacia, this may not be a useful discriminatory character. Quentin (Reference Quentin1971), in his analysis of the species of Syphacia based on the morphology of the cephalic plate, placed S. frederici in Group VI (with a laterally thinner cephalic plate) and S. stroma in Group IX (with an oval cephalic plate), and these differences are easily recognizable in lateral view at higher magnifications. Wiger et al. (Reference Wiger, Baruš and Tenora1978) using SEM were able to document another difference between the species. Syphacia frederici has a row of denticles on each of the three teeth, S. stroma does not. Similarly these characters are best viewed in en face preparations, at higher magnifications or by SEM and are not suited for quantitative studies. Wiger et al. (Reference Wiger, Baruš and Tenora1978) also reported that S. frederici has longitudinal septa on the body surface, S. stroma does not and again these are best observed by SEM.
DISCUSSION
The primary objective of the study reported herein was to ascertain whether S. frederici exists in the wood mouse population of the British Isles, this species never having been recorded previously in wood mice from this region, and on the evidence we have presented we can confirm that this species does indeed parasitize wood mice in the British Isles. Over several decades of working on helminths in wild British rodents, we had occasionally observed wood mice with Syphacia sp. confined to the caecum, a characteristic of S. frederici, rather than the widely reported S. stroma which lives predominantly in the upper small intestine (Lewis, Reference Lewis1968). Our molecular data show clearly that the Syphacia we included in the study fall into very distinct genetic clades, whether assessed by rDNA (ITS-1, 5·8S, ITS-2) or by the mitochondrial gene CO1 and further demonstrate that both our British suspected S. frederici isolates and worms known to be S. frederici from the European mainland constitute a single clade.
It is also of some interest that the CO1 gene, as sequenced from our specimens of S. frederici from the British Isles and Europe clearly differed from sequences published for S. frederici from A. speciosus in Japan (Okamoto et al. Reference Okamoto, Urushima, Iwasa and Hasegawa2007 and GenBank, see Table 2), and these two formed two distinct clades with a similar evolutionary distance between them as, for example, S. stroma, S. agraria and Syphacia emilromani (Fig. 3). However, Hasegawa (Reference Hasegawa1981) initially concluded that the female worms he recovered from A. speciosus in Japan were morphologically identical to the descriptions given by Roman (Reference Roman1945, Reference Roman1951) and Quentin (Reference Quentin1971) for worms from A. sylvaticus in Europe. Subsequently, Hasegawa et al. (Reference Hasegawa, Asakawa, Yagi and Takao1994) concluded that the morphology of Japanese ‘S. aff. frederici’ does not correspond precisely with that of the European type material; indeed Hasegawa et al. (Reference Hasegawa, Asakawa, Yagi and Takao1994) suggested that the Japanese material should belong in a different subgenus to that conventionally assigned to European specimens. The eastern Asian nematode fauna differs in many respects to that found in western Europe (e.g. the genus Aspiculuris, see Behnke et al. Reference Behnke, Stewart, Bajer, Grzybek, Harris, Lowe, Ribas, Smales and Vandegrift2015, or Heligmosomoides, see Zaleśny et al. Reference Zaleśny, Hildebrand, Paziewska-Harris, Behnke and Harris2014), and there is no a priori reason to suspect that Syphacia from Japan and western Europe should be conspecific. Moreover, A. speciosus, the host of S. aff. frederici in Japan, is a member of the subgenus Apodemus, whereas the type host of S. frederici, A. sylvaticus, is a member of the subgenus Sylvaemus; these subgenera diverged c. 8 million years ago (Michaux et al. Reference Michaux, Reyes and Catzeflis2001), giving ample time for speciation of their parasites to have occurred. A thorough morphological comparison of worms from Europe and Japan, especially at the SEM level, with molecular analysis of nuclear loci, is therefore necessary to resolve the specific identity of the Japanese worms.
Taken together, our genetic data are consistent with the idea that S. frederici, which was originally described from French wood mice (Roman, Reference Roman1945), exists in the UK population of A. sylvaticus. Based on both CO1 and rDNA, the gene sequences were either identical or very similar to those of worms from continental mice, from a region where S. stroma does not exist (Urwitałt in the Mazury region of Poland – JMB & AB pers. obs.) and from other locations where S. frederici is known to exist (Portugal; see Eira et al. Reference Eira, Torres, Vingada and Miquel2006). All the S. frederici isolates used for genetic analysis, whether from British wood mice or from abroad, were female worms recovered from the caeca of their respective hosts.
Morphological inspection confirmed that the S. frederici identified in this study conformed to the morphological descriptions of the species provided by authors in the past. Individuals of S. frederici can be readily distinguished from S. stroma as described above. Where specimens are in good condition, cervical and lateral alae can be detected easily. Moreover, the differences in the cephalic end of each species can also be seen easily. The morphology of the female tail is particularly useful in this regard allowing the screening of large numbers of individual worms, at relatively low microscopical magnification, for identification, even in circumstances where mixed infections are present.
Thus, on three lines of evidence (location in the caecum and not small intestine, phylogenetics, and morphology), we can confirm that S. frederici does indeed parasitize wood mice in the British Isles, as it does on the European mainland. The interesting question that arises now is why S. frederici has never previously been reported from wood mice in the British Isles, despite the many thorough studies of helminth communities in the region?
One obvious explanation is that morphologically at low magnification S. frederici and S. stroma are actually superficially quite similar to one another in appearance, and to distinguish between them based simply on morphology takes some experience and is time consuming. Moreover, much of the taxonomy of Syphacia spp. is based on male worms, which dominate in available keys to the genus (see, e.g. Tiner's, Reference Tiner1948 and Khera's, Reference Khera1956 keys to the genus), primarily because females, which together with juveniles usually form the vast majority of the worm burden in infected animals, are relatively poor in characters suitable for species discrimination, difficult to distinguish between and in some species even indistinguishable morphologically. Hence understandably, female worms have been given far less attention in keys. However, male worms which are believed to die soon after mating are very small, very rare and in some species unknown (Morgan, Reference Morgan1932; Lewis, Reference Lewis1968; Abdel-Gaber, Reference Abdel-Gaber2016).
Syphacia stroma was the first described of the two species. In the early literature of the genus, the specific status of S. stroma was not accepted (e.g. in Seurat, Reference Seurat1916, the first paper using the name Syphacia, S. stroma is clearly considered a junior synonym of S. obvelata). This led to confusion that persisted throughout the earlier reports on wood mouse helminths in which wood mice were reported to be infected with S. obvelata, the typical Syphacia sp. of house mice (Seurat, Reference Seurat1915; Elton et al. Reference Elton, Ford, Baker and Gardiner1931; see Ogden, Reference Ogden1971). Infections with S. stroma can be very intense, running to many hundreds and even thousands of worms, and often swamp numerically other concurrently infecting helminths (Lewis, Reference Lewis1968; Behnke et al. Reference Behnke, Lewis, Mohd Zain and Gilbert1999; Abu-Madi et al. Reference Abu-Madi, Behnke, Lewis and Gilbert2000). To check each worm microscopically is a hugely onerous task, although several authors working on the European continent have successfully differentiated these two species quantitatively in Apodemus spp. populations that were exposed to both (Eira et al. Reference Eira, Torres, Vingada and Miquel2006; Milazzo et al. Reference Milazzo, Di Bella, Casanova, Ribas and Cagnin2010; Čabrilo et al. Reference Čabrilo, Jovanović, Bjelić-Čabrilo, Budinski, Blagojević and Vujošević2016). In very heavy infections, S. stroma can also spill into the caecum of its host, and even in low-intensity infections, female worms migrate through the caecum to lay their eggs on the perianal surface of their host (Kerboeuf and Lewis, Reference Kerboeuf and Lewis1987).
As stated earlier, S. frederici was described more recently, and may therefore be less well known. Published during wartime, the original description is brief (eight lines) and deals only with the mature female and meristic characters of the eggs, as the male at that time was unknown. This original description is very poor providing the reader with little to go on in terms of distinction from other species. It was not until the reviews by Quentin (Reference Quentin1971), and Tenora and Meszaros (Reference Tenora and Meszaros1975), and chapters in books by Sharpilo (Reference Sharpilo1973) and Ryzhikov et al. (Reference Ryzhikov, Gvozdev, Tokobaev, Shaldybin, Matsaberidze, Merkusheva, Nadtochi, Khokhlova and Sharpilo1979) that some of the key differences between S. frederici and S. stroma were more clearly reported, but even from the information provided in these it is not easy to extract the essential, workable differences between the species, and translations of the latter two Russian texts (both are in Cyrillic) are lacking. Syphacia are in any case character-poor, and many morphological differences identified between S. stroma and S. frederici are too difficult to implement in quantitative studies (e.g. those based on SEM) in which hundreds/thousands of worms may be recovered from individual hosts and need to be identified to species level. Two differences, however, are easier to implement in quantitative studies; the location of the majority of the worms in the caecum in the case of S. frederici and in the small intestine in the case of S. stroma and, as we have shown here and with some experience, the fine morphology of the tails in female worms can be distinguished even under low magnification microscopy. Further, if conducting phylogenetic analysis of this genus for species determination, we recommend the rDNA region, which is easier to amplify in a standard PCR.
To date, no S. frederici have been detected in Ireland, based on the molecular analysis of Syphacia in this study and lack of observations of Syphacia infections solely occurring in the caecum in previously reported helminth surveys and current ongoing studies (personal Communication; I. Montgomery, P. Stuart and K. Loxton). However, despite no earlier reports of its presence in Britain, it is possible that S. frederici has parasitized British wood mice for as long as these rodents have existed on the British mainland, but has simply not been recognized as a different species to S. stroma. Alternatively, S. frederici may have been introduced to the British Isles more recently, although given that some ecological studies of wood mouse helminths in Britain were conducted as recently as the 1990s without reporting S. frederici (Abu-Madi et al. Reference Abu-Madi, Behnke, Lewis and Gilbert2000; Behnke et al. Reference Behnke, Lewis, Mohd Zain and Gilbert1999), it seems unlikely that this species could have spread as widely in the last 3–4 decades as between the locations referred to in this paper (i.e. Nottingham in the centre and Berkshire in the central south of the British Isles, and Anglesey in the north-west tip of Wales). It may of course be more widespread, or indeed show a patchy distribution across the British Isles since Apodemus populations in the British Isles and Ireland originated in a variety of ways, including from potential southern refugia (Montgomery et al. Reference Montgomery, Provan, McCabe and Yalden2014) and from synanthropic transfer with colonizing or invading humans (Berry, Reference Berry1973; Yalden, Reference Yalden1999), and their parasites originated with them; Heligmosomoides polygyrus of two quite distinct mitochondrial haplotypes occur within the British Isles, for example, one widespread in western Europe, while the other has been found only in Britain, Ireland and Denmark (Nieberding et al. Reference Nieberding, Libois, Douady, Morand and Michaux2005; Cable et al. Reference Cable, Harris, Lewis and Behnke2006; Zaleśny et al. Reference Zaleśny, Hildebrand, Paziewska-Harris, Behnke and Harris2014). A heterogeneous distribution of Syphacia species might therefore be expected, but further relevant fieldwork must be conducted to elucidate these patterns. In this context, the distribution of small indels in the sequence of ITS-2 of Syphacia species may prove invaluable in tracking the fine-grained phylogeography of their hosts.
Finally, this study highlights further the potential cryptic or ‘near-cryptic’ diversity of parasitic nematodes, even within such well-studied host groups as European rodents. By highlighting the two easily examined key differences between S. frederici and S. stroma, it is hoped that this study will make it much easier for future studies in the British Isles to confirm with confidence the presence/absence of S. frederici in wood mice from England and Wales, and to ascertain whether wood mice in Ireland and Scotland also carry this species.
ACKNOWLEDGEMENTS
We thank Dr D. de Pomerai for providing wood mice from Brittany in France, Denise McGowan (Department of the Environment, Jersey) for providing wood mice from Jersey in the British Isles, Dr Alexander Mozeika for translation of key Russian texts, and we thank Professor I. Montgomery, Professor Celia Holland and Dr K. Loxton for advice and information about Syphacia sp. in wood mice from Ireland. We are also grateful to Professors P. Harris, J. M. Kinsella and E. Harris for their helpful advice on earlier versions of the manuscript.
FINANCIAL SUPPORT
We are grateful to the Universities of Nottingham and Warsaw for financial support for travel and consumables. Our work was supported additionally by travel grants from the Royal Society (JMB), the British Ecological Society (JMB), the Grabowski Fund (JMB), the Polish State Committee for Scientific Research (KBN, AB), and by the KBN and British Council's Young Scientist Programme (AB). PS was supported by a Government of Ireland Postdoctoral Fellowship awarded by the Irish Research Council.