Lichens are increasingly regarded as a complicated microcosm formed by a large number of lichen-associated fungi and non-photoautotrophic bacteria, in addition to the photobionts and mycobionts (Grube et al. Reference Grube, Cardinale and Berg2012). Some non-photoautotrophic bacteria harboured in lichen microbiomes are considered by some as integral players in the lichen symbiosis and have been referred to as bacteriobionts (Grube & Berg Reference Grube and Berg2009).
The first studies on lichen-associated bacteria using culture-independent molecular methods were conducted by Cardinale et al. (Reference Cardinale, Puglia and Grube2006). Subsequent research with molecular and microscopic methods clearly showed that Alphaproteobacteria is the predominant bacterial group in growing parts of lichens (Cardinale et al. Reference Cardinale, Müller, Berg, de Castro and Grube2008, Reference Cardinale, Steinova, Rabensteiner, Berg and Grube2012; Grube et al. Reference Grube, Cardinale, de Castro, Müller and Berg2009; Bates et al. Reference Bates, Cropsey, Caporaso, Knight and Fierer2011; Mushegian et al. Reference Mushegian, Peterson, Baker and Pringle2011; Schneider et al. Reference Schneider, Schmid, de Castro, Cardinale, Eberl, Grube, Berg and Riedel2011; Hodkinson et al. Reference Hodkinson, Gottel, Schadt and Lutzoni2012). In some cases, host species specificity of the lichen-associated bacteria has been shown (Grube et al. Reference Grube, Cardinale, de Castro, Müller and Berg2009; Bates et al. Reference Bates, Cropsey, Caporaso, Knight and Fierer2011; Bjelland et al. Reference Bjelland, Grube, Hoem, Jorgensen, Daae, Thorseth and Ovreås2011). These studies provide evidence that bacteriobionts are integral components in lichen symbioses. Rhizobiales (Alphaproteobacteria) was shown to be the predominant order among lichen bacteriobionts (Hodkinson et al. Reference Hodkinson, Gottel, Schadt and Lutzoni2012) and includes many nitrogen-fixing taxa forming plant-associated root nodules (Sy et al. Reference Sy, Giraud, Jourand, Garcia, Willems, de Lajudie, Prin, Neyra, Gillis and Boivin-Masson2001; Ngom et al. Reference Ngom, Nakagawa, Sawada, Tsukahara, Wakabayashi, Uchiumi, Nuntagij, Kotepong, Suzuki and Higashi2004). A recently discovered and uncultured lineage of this order, LAR1, has been found in many lichens (Hodkinson & Lutzoni Reference Hodkinson and Lutzoni2009; Bates et al. Reference Bates, Cropsey, Caporaso, Knight and Fierer2011; Hodkinson Reference Hodkinson2011; Hodkinson et al. Reference Hodkinson, Gottel, Schadt and Lutzoni2012). Furthermore, the bacterial nitrogen-fixing genes were also detected in chlorolichens using PCR (Grube et al. Reference Grube, Cardinale, de Castro, Müller and Berg2009). These studies suggest that bacteriobionts are potential nitrogen-fixers in the lichen symbioses.
Despite living in nutrient-poor conditions, most lichen mycobionts produce high levels of secondary compounds. For example, the usnic acid content in Alectoria ochroleuca (Hoffm.) A. Massal. is 8% of the dry weight of the lichen thallus, while lecanoric acid may constitute up to 23·5% of the thallus dry weight of Parmotrema tinctorum (Despr. ex Nyl.) Hale (Wei Reference Wei1998). More than 700 lichen compounds have been reported, almost all of which are insoluble in water (Huneck & Yoshimura Reference Huneck and Yoshimura1996; Dembitsky & Tolstikov Reference Dembitsky and Tolstikov2005). Most lichen compounds are aromatics (containing one or more benzene rings), while terpenoids are also very common in lichens (Huneck & Yoshimura Reference Huneck and Yoshimura1996; Dembitsky & Tolstikov Reference Dembitsky and Tolstikov2005).
Considering the high content of secondary compounds in lichen thalli and the enormous diversity of carbon sources utilized by bacteria, we first investigated the culturable bacteria from the chlorolichens Umbilicaria esculenta (Miyoshi) Minks and Parmelia omphalodes (L.) Ach., and the cyanolichen Lobaria retigera (Bory) Trevis., on media with acetone extracts of corresponding lichens as the nutrient source. However. some bacteria can use acetone as a carbon source (Platen & Schink Reference Platen and Schink1989). Therefore, we selected dimethyl sulfoxide (DMSO) to dissolve the acetone extracts, and DMSO media were used to clarify whether or not culturable bacteriobionts utilize DMSO as a substrate. All the lichen compounds extracted from these species are water-insoluble, but soluble in acetone and DMSO.
Specimens of U. esculenta and P. omphalodes were collected from Mt. Tulaopodingzi, Wangqing, Jilin, China. The specimens of L. retigera were collected from Mt. Jiaozixueshan, Luquan, Yunnan, China. Samples were placed in plastic bags, stored at 4 °C and processed within 7 days of collection. A specimen of each lichen species was used to isolate and culture bacteria, while the remaining specimens were used to prepare acetone extracts.
Air-dried lichen thalli (2 g) were cut into pieces (1×1 mm) and shaken in 100 ml acetone (24 h, 150 rpm) at room temperature. The acetone extracts were filtered using Whatman No. 1 filter paper and then concentrated in vacuo at 40 °C using a rotary evaporator. The dry extract was stored at 4 °C.
The isolation was performed as follows. For each lichen species five segments were cut from healthy growing parts of the thallus after washing in sterile water for 10 min. Each segment (about 5 mg) was treated to 10× 1 min. washes in 0·8% NaCl and crushed in 0·5 ml of sterile deionized water using a pestle and mortar. 0·1 ml of the resulting suspension was plated on the water medium plus DMSO and lichen extract (water medium: 0·4 g KH2PO4, 0·2 g MgSO4.7H2O, 0·1 g NaCl, 0·02 g CaCl2.2H2O, 0·01 g FeCl3.6H2O, 0·002 g Na2MoO4.H2O, 17 g agar, 1000 ml deionized water, pH 7·0–7·2). The DMSO solution of lichen acetone extract (5ml, 20mg ml–1) was filter sterilized and added to the medium after it was autoclaved and cooled. Plates were incubated at 20 °C and inspected every day until no new colonies appeared. For each lichen species, all the colonies with distinctive phenotypes were purified by the streak inoculation method to seed a single colony on a new plate filled with the same medium.
Strains of bacteria from a single colony were put into a sterile centrifuge tube (1·5 ml), washed three times using sterile deionized water, centrifuged and then mixed with 1·2 ml sterile deionized water after discarding the supernatant. 100 μl of bacterial suspension was plated on each of the isolation media, namely water medium plus DMSO and lichen extract, water medium and water medium plus DMSO(5 ml DMSO (5·5 g) added to the water media), and cultures incubated at 20 °C.
Total DNA from the bacteria was extracted using the DNA Extraction Mini Kit (SANGON). The primers 27F and 1492R (Dees & Ghiorse Reference Dees and Ghiorse2001) were used for PCR amplification of the 16S rDNA. The PCR temperature profile was as follows: initial denaturation at 95 °C for 3 min followed by 35 cycles of 30 s denaturation at 94 °C, 30 s annealing at 55 °C, 2 min extension at 72 °C and completed with a final 10 min extension at 72 °C. PCR products were purified with the Gel Extraction Mini Kit (SABC). Sequencing reactions were carried out by Shanghai SANGON Corporation. All the 16S rDNA sequences obtained are available in GenBank (KJ015997–KJ016230) and the phylogenetic analysis was performed as described by Wang et al. (Reference Wang, Jiang, Huang, Wang and Li2013). Primers F1 (5'-TAYGGNAARGGNGGNATYGGNAARTC-3') and nifH-r (5'-ADNGCCATCATYCTNCC-3') were used to amplify a partial stretch of the nifH gene.
Bacterial colonies appeared on the isolation plates after three days with no new colonies appearing after approximately two weeks. A total of 234 single bacterial colonies (72 from Umbilicaria esculenta, 93 from Parmelia omphalodes and 69 from Lobaria retigera) were obtained from the three lichens. After incubating for two weeks, it seemed that all 234 bacterial isolates grew better on the water medium plus DMSO and lichen extract than on the water medium plus DMSO (with more bacterial colonies on the plates), while none of the bacterial isolates were observed growing on the water only medium. Their 16S rDNA were then sequenced and the presence of the nifH gene was also detected (Figs 1–3).
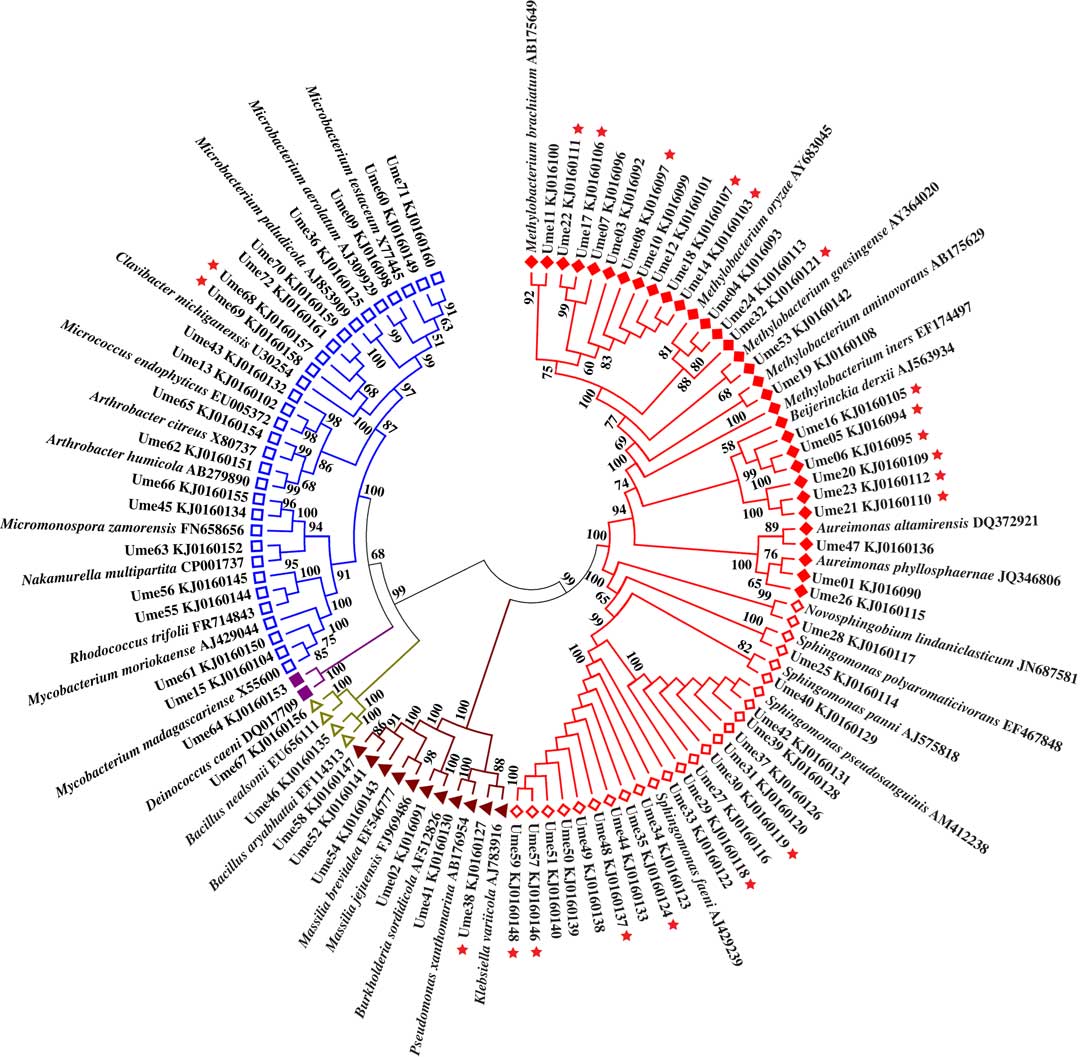
Fig. 1 Unrooted phylogenetic tree for bacterial isolates from Umbilicaria esculenta. ![]() =Rhizobiales;
=Rhizobiales; ![]() =Sphingomonadales;
=Sphingomonadales; ![]() =Gammaproteobacteria;
=Gammaproteobacteria; ![]() =Bacilli;
=Bacilli; ![]() =Deinococci;
=Deinococci; ![]() =Actinobacteria.
=Actinobacteria. ![]() =nifH gene positive. In colour online.
=nifH gene positive. In colour online.
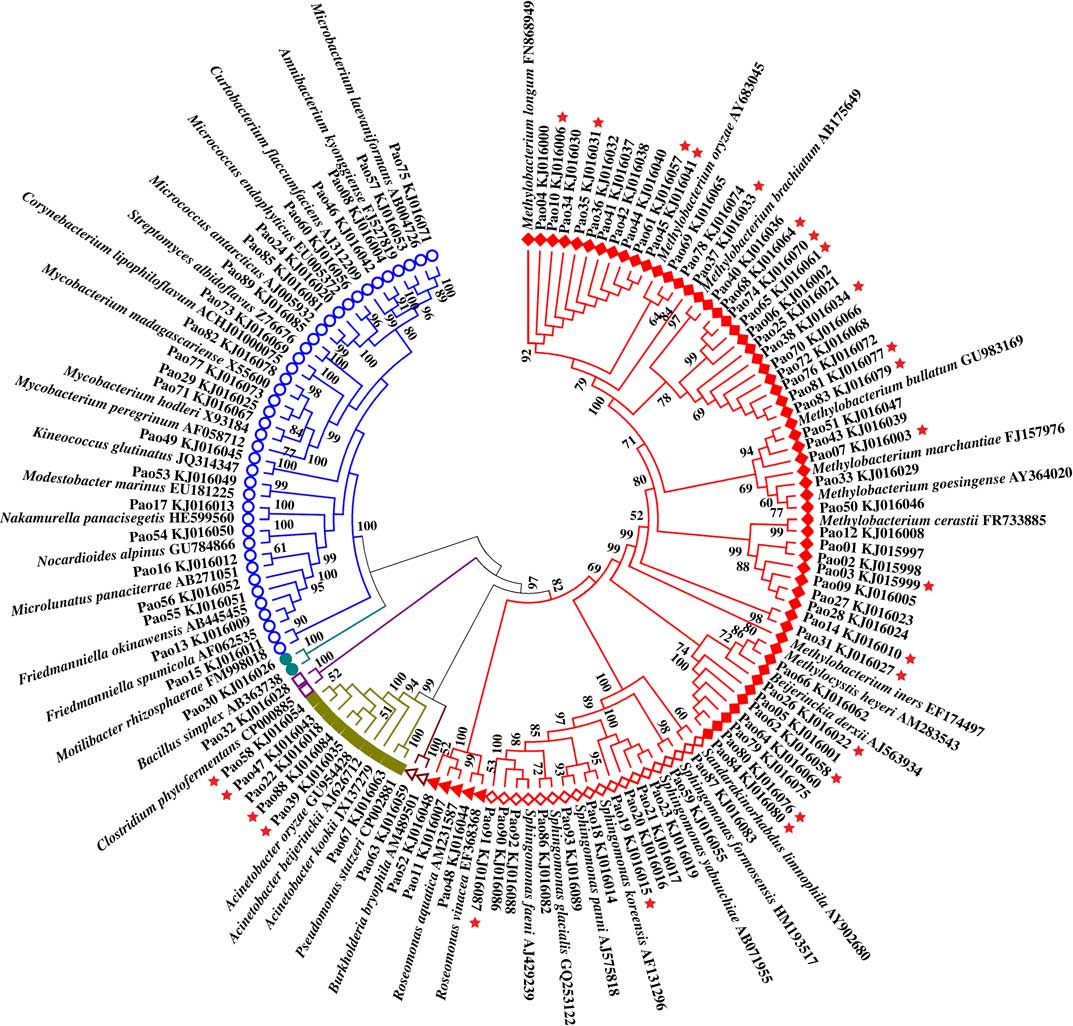
Fig. 2 Unrooted phylogenetic tree for bacterial isolates from Parmelia omphalodes. ![]() =Rhizobiales,
=Rhizobiales, ![]() =Sphingomonadales,
=Sphingomonadales, ![]() =Rhodospirillales,
=Rhodospirillales, ![]() =Betaproteobacteria,
=Betaproteobacteria, ![]() =Gammaproteobacteria,
=Gammaproteobacteria, ![]() =Clostridia,
=Clostridia, ![]() =Bacilli, and
=Bacilli, and ![]() =Actinobacteria.
=Actinobacteria. ![]() =nifH gene positive. In colour online.
=nifH gene positive. In colour online.

Fig. 3 Unrooted phylogenetic tree for bacterial isolates from Lobaria retigera. ![]() =Rhizobiales,
=Rhizobiales, ![]() =Rhodobacterales,
=Rhodobacterales, ![]() =Sphingomonadales,
=Sphingomonadales, ![]() =Betaproteobacteria,
=Betaproteobacteria, ![]() =Gammaproteobacteria,
=Gammaproteobacteria, ![]() =Deinococci, and
=Deinococci, and ![]() =Actinobacteria.
=Actinobacteria. ![]() =nifH gene positive. In colour online.
=nifH gene positive. In colour online.
Rhizobiales is the most predominant order among the isolates from U. esculenta, P. omphalodes, and L. retigera. In addition to the 13 known families, Rhizobiales includes LAR1 which was detected from lichens by part sequences of 16S rDNA (Grube et al. Reference Grube, Cardinale, de Castro, Müller and Berg2009; Hodkinson & Lutzoni Reference Hodkinson and Lutzoni2009; Hodkinson Reference Hodkinson2011; Hodkinson et al. Reference Hodkinson, Gottel, Schadt and Lutzoni2012). The phylogenetic tree (Fig. 4) based on the part sequences of 16S rDNA shows that several isolates of Rhizobiales are members of LAR1 which also includes many members detected from various hosts in addition to lichens. These isolates belong to two well-supported subclades of LAR1. In total, 67 isolates were positive for amplification of the nifH gene (U. esculenta: 21, Fig. 1; P. omphalodes: 25, Fig. 2; L. retigera: 21, Fig. 3) suggesting that these isolates are able to fix nitrogen. Since not all N2-fixing bacteria are positive for the nifH gene (Islam et al. Reference Islam, Sultana, Cho, Joe and Sa2012), there may be more than 67 N2-fixing bacteriobionts isolated from the three lichens.

Fig. 4 Phylogenetic tree for LAR1. ♦=samples from cyanolichens or chlorolichens with cephalodia. Outgroup is represented by 3 species of Methylobacteriaceae.
The better growth on the water medium plus DMSO and lichen extract than on the water medium plus DMSO reveals that, although bacterial isolates could utilize DMSO, the lichen extrolite extracts provided more nutrients. In addition, results for the water only medium indicate that agar did not provide any nutrition for bacteria. Culture-independent studies showed that Rhizobiales and Alphaproteobacteria are the predominant order and class among lichen bacteriobionts (Cardinale et al. Reference Cardinale, Müller, Berg, de Castro and Grube2008, Reference Cardinale, Steinova, Rabensteiner, Berg and Grube2012; Grube et al. Reference Grube, Cardinale, de Castro, Müller and Berg2009; Bates et al. Reference Bates, Cropsey, Caporaso, Knight and Fierer2011; Mushegian et al. Reference Mushegian, Peterson, Baker and Pringle2011; Schneider et al. Reference Schneider, Schmid, de Castro, Cardinale, Eberl, Grube, Berg and Riedel2011; Hodkinson et al. Reference Hodkinson, Gottel, Schadt and Lutzoni2012). Unfortunately, very few species in these groups have been successfully isolated and cultured from lichens on media with conventional carbon sources, regardless of whether the media were nitrogen-free or nitrogen-containing (Grube et al. Reference Grube, Cardinale and Berg2012). However, when lichen extrolites were used as the nutrient source, these predominant bacteriobionts were readily isolated from these lichen species, even including the previously uncultured lineage LAR1 which appears to commonly occur in lichens (Hodkinson & Lutzoni Reference Hodkinson and Lutzoni2009; Bates et al. Reference Bates, Cropsey, Caporaso, Knight and Fierer2011; Hodkinson Reference Hodkinson2011; Hodkinson et al. Reference Hodkinson, Gottel, Schadt and Lutzoni2012). The results of this study show that lichen compounds might be more appropriate than other carbon sources for isolating and culturing bacteriobionts.
Most of the nifH gene-positive isolates belong to Rhizobiales. These results suggest that many bacteriobionts have the potential for N2 fixation in the lichen thallus, consistent with previous reports (Liba et al. Reference Liba, Ferrara, Manfio, Fantinatti-Garboggini, Albuquerque, Pavan, Ramos, Moreira-Filho and Barbosa2006; Seneviratne & Indrasena Reference Seneviratne and Indrasena2006; Grube et al. Reference Grube, Cardinale, de Castro, Müller and Berg2009). A large number of N2-fixing isolates, including the members of LAR1, were isolated from all three lichen species. This demonstrates that both chlorolichens and cyanolichens harbour N2-fixing bacteriobionts, which possibly use lichen compounds as the carbon source, and that bacteriobionts with N2-fixing and lichen compound-digesting abilities might potentially help mycobionts transmute part of the spare carbon (lichen extrolites) into available nitrogen.
This project was supported financially by the Program for Scientific Research Innovation Team in Colleges and Universities of Shandong Province, the National Natural Science Foundation of China (31270059), the Science Foundation of Jinan (201202024), and the National Innovation Fund for college students (201310445082).






