Introduction
In modern human in vitro fertilization (IVF) laboratories, many conditions must be strictly controlled to provide the best environment for embryos to increase the probability of successful uterine implantation. Perhaps none of which is more important than the culture medium provided to the embryos to sustain optimal growth. Although the introduction of sequential media has helped to drive the revolution to perform more embryo transfers at the blastocyst stage, evidence that sequential formulations are superior to one-step formulations is controversial (Khoury et al., Reference Khoury, Coffler, Potter, Frederick and Battaglia2012; Quinn, Reference Quinn2012; Summers et al., Reference Summers, Bird, Mirzai, Thornhill and Biggers2013). In the process of developing the best one-step medium, multiple substrates have been tested as an energy source for embryos. Previously, pyruvate was determined to be the only substrate required in early embryonic development until the 8-cell stage (Biggers et al., Reference Biggers, Whittingham and Donahue1967); however, the most recent studies have demonstrated that pyruvate uptake drastically decreases in embryos at the blastocyst stage (Lane and Gardner, Reference Lane and Gardner2000). Knowing the stage-dependent preferences for pyruvate and glucose, and understanding the regulatory effects of lactate on pyruvate uptake and oxidation at various stages of embryo development suggest that a lower lactate level could have a beneficial effect on embryo metabolism and growth.
In the last 5 years, the congruent use of one-step media with time-lapse monitoring (TLM) systems has been advantageous in allowing less interruption in early embryonic development (Cimadomo et al., Reference Cimadomo, Scarica, Maggiulli, Orlando, Soscia, Albricci, Romano, Sanges, Ubaldi and Rienzi2018). These TLM systems have revolutionized embryo culture by reducing the interruptions to the culture conditions, by allowing for a safer and more consistent environment for culture (Costa-Borges et al., Reference Costa-Borges, Bellés, Meseguer, Galliano, Ballesteros and Calderón2016) and by enhancing the embryo selection process with the use of many morphokinetic parameters (Racowsky et al., Reference Racowsky, Kovacs and Martins2015; Racowsky and Martins, Reference Racowsky and Martins2017). Several studies have investigated morphokinetic parameters using TLM including time of pronuclei appearance and disappearance, time of cell divisions at 2-cell, 4-cell, and 8-cell stages, and also time of morula, blastocyst formation (Patel et al., Reference Patel, Shah, Kotdawala, Herrero, Rubio and Banker2016). Subsequently, some groups have attempted to use some of these parameters to develop algorithms to differentiate aneuploid embryos from euploid embryos (Campbell et al., Reference Campbell, Fishel, Bowman, Duffy, Sedler and Hickman2013; Patel et al., Reference Patel, Shah, Kotdawala, Herrero, Rubio and Banker2016). More recently, higher implantation rates were reported using the KID score D5 algorithm in TLM in single euploid embryo transfer cases (Gazzo et al., Reference Gazzo, Peña, Valdéz, Chung, Bonomini, Ascenzo, Velit and Escudero2020). Although the implantation rates obtained from embryos incubated in TLM were not different from those incubated in a conventional incubator (Barberet et al., Reference Barberet, Chammas, Bruno, Valot, Vuillemin, Jonval, Choux, Sagot, Soudry and Fauque2018), there have been several studies published showing a higher blastulation rate and better embryo quality in TLM (Sciorio et al., Reference Sciorio, Thong and Pickering2018). However, the superiority of TLM systems over conventional incubators is still controversial.
In addition to effects on embryo growth and metabolism, culture media have been shown to have effects on embryos as well, such as ploidy status (Werner et al., Reference Werner, Hong, Franasiak, Forman, Reda, Molinaro, Upham and Scott2016). Considering the gap in the knowledge of optimum culture conditions in embryo development, we aimed in this study to determine if a lower lactate medium could affect embryo morphokinetic parameters using a TLM system and euploidy rates using NGS for preimplantation genetic testing for aneuploidy (PGT-A).
Materials and methods
Inclusion and exclusion criteria
Institutional Review Board approval for this study was obtained from the Eastern Virginia Medical School. Data for this study were collected from subjects undergoing fertility treatment at a private fertility clinic between 1 March 2018 and 12 November 2018. Data were collected from subjects and were included in an internal sibling oocyte quality control study comparing LifeGlobal Global Total medium (LGGT) as medium 1 (Cooper Surgical; https://fertility.coopersurgical.com/art_media/global-total/) to Continuous Single Culture®-NX Complete medium (CSCM-NXC) as medium 2 (Irvine Scientific; http://www.irvinesci.com/products/90168-continuous-single-culture-nx-complete). Inclusion criteria were as follows: (1) female partner aged between 18 and 42 years; (2) patients who had five or more metaphase II oocytes at time of denudation; (3) patients who had elected to have PGT-A performed; (4) fresh retrieved autologous or donor oocytes were used; (5) achieved fertilization with intracytoplasmic sperm injection (ICSI); (6) had some or all of their embryos incubated in an EmbryoScope™ (ES) time-lapse imaging incubator (Vitrolife). Only oocytes cultured in an EmbryoScope incubator were included in the study. Exclusion criteria were female partners over the age 42 years and patients using vitrified oocytes. Oocytes not cultured in an EmbryoScope incubator were excluded from the study.
Controlled ovarian stimulation for fresh and frozen cycles
All patients and oocyte donors underwent an antagonist stimulation protocol, with slight modifications. Cycles were monitored by follicular ultrasound and estradiol levels. After a minimum of 18 days on an oral contraceptive pill, patients were started on a daily protocol of Follistim and GonalF (Merck) for 7–12 days, until at least one dominant follicle >18 mm was observed. Final oocyte maturation and ovulation were then triggered using a combination of human chorionic gonadotropin and/or leuprolide. Ultrasound-guided oocyte retrieval was performed under general anaesthesia 36 h after trigger shot administration.
For the consecutive frozen cycle, a frozen embryo transfer was scheduled after PGT results were available. Patients were instructed to complete at least one menstrual cycle on oral contraceptives. At 4–5 days after the last contraceptive pill, patients were maintained on three times daily Estrace and Minivelle patches that were changed every 3 days. This was continued through 10–12 weeks’ gestation. Patients were started on daily intramuscular progesterone 5 days before scheduled embryo transfer and progesterone was continued for the first 10–12 weeks of pregnancy. Embryo transfer only proceeded if the endometrial thickness was >7 mm and progesterone levels were >20 μg/ml on the day of scheduled transfer. Finally, 2 days after embryo transfer was performed, patients were started on Lovenox (Pfizer) and continued for the first 10–12 weeks of pregnancy. Pregnancy was defined as biochemical when the serum beta human chorionic gonadotropin (β-hCG) was detected to be >5 IU/l at 7–10 days of post embryo transfer with no gestational sac visible on ultrasound, and as clinical when there was gestational sac using ultrasound at 21 days following embryo transfer.
pH adjustment of culture media
As the optimal pH of LGGT is between 7.2–7.4 and pH of CSCM-NXC is between 7.3–7.5, we considered the optimal pH of both media before starting the study and adjusted the CO2 % levels of incubators accordingly so that the pH of both media were optimized at ∼7.3 to avoid any bias. The pH of both media were measured under the same conditions and they were optimized to be similar in the same incubator as following:
-
7.34 for LGGT and 7.35 for CSCM-NXC in a big box incubator (Thermo Scientific, Heraeus) at 37°C with 6.5% CO2 and 6.0% O2;
-
7.37 for LGGT and 7.35 for CSCM-NXC in a tabletop incubator (ESCO Miri) at 37°C with 7.0% CO2 and 5.3% O2;
-
7.38 for LGGT and 7.36 for CSCM-NXC in an EmbryoScope (Vitrolife) incubator at 37°C with 6.8% CO2 and 5.7% O2.
Both media were equilibrated in the big box incubator (Heraeus) at 37°C with 6.5% CO2 and 6.0% O2 overnight.
Oocyte denudation, ICSI, culture and evaluation
After follicle aspiration, oocytes were recovered and washed in LifeGlobal total with HEPES buffer (LGTH) at 37°C. After all oocytes were recovered, they were washed in pre-equilibrated LGGT with bicarbonate buffer and incubated at 37°C, 7% CO2 and 5% O2 for 1 h in a tabletop incubator. Oocytes were then denuded in 40 IU/ml hyaluronidase (Life Global) with mechanical pipetting. Following denudation, oocytes were incubated in pre-equilibrated standard culture medium (LGGT) in the same tabletop incubator (ESCO Miri) until ICSI was performed in LGTH at ×400 magnification no later than 4 h post denudation.
Following ICSI, oocytes were placed in an EmbryoSlideTM dish (LifeGlobal) prepared and pre-equilibrated the day before. EmbryoSlide dishes have 12 wells that hold 25 μl of medium each. Each EmbryoSlide was prepared with six wells containing LGGT and six wells containing CSCM-NXC. The composition of both media can be found on the manufacturer webpages. Oocytes were then placed in each well of the EmbryoSlide after washing in the appropriate medium. An equal number of oocytes per patient was randomly allocated to the two media, the extra oocyte was randomly assigned to either medium if an odd number of oocytes were injected. EmbryoSlide dishes were placed in an EmbryoScope (Vitrolife) incubator kept at 37°C with 6.8% CO2 and 5.7% O2.
Assessment of fertilization and embryo development
Fertilization was assessed 16–19 h post insemination. As previously described by Meseguer et al. (Reference Meseguer, Herrero, Tejera, Hilligsøe, Ramsing and Remohí2011), exact times of embryonic development were calculated in hours from the time of insemination. Pronuclear appearance (tPNa) is the time when pronuclei could first be identified and pronuclear fading time (PNf) of a zygote denotes the time when both pronuclei disappear. The time when two, three, four, and five cells first appeared were defined as t2, t3, t4, and t5 respectively. The duration of time when morula first formed was defined as tM. The time spent at the 2-cell stage (i.e. time spent between divisions between two cells to three cells) is defined as cc2 (where cc2 = t3 − t2). The time spent at the 3-cell stage is defined as s2 (where s2 = t4 − t3). Finally, tSB, tB, tEB, and tHB were used to denote the times when blastulation first began, time of complete blastocyst formation, time of full expanded blastocyst development, and time at blastocyst hatching, respectively. Irregular divisions (IR) were defined as an embryo dividing directly from one to three cells or from two to five cells.
Morphological assessments were performed on days 3, 5, 6, and 7. Assessments for embryos cultured in an ES incubator were carried out using a single embryologist and observing digital images captured by the ES viewer. On day 3 the number of cells, percentage of fragmentation and symmetry were noted. All embryos were then laser-assisted hatched in anticipation of trophectoderm biopsy. On days 5, 6, and 7, embryo morphology was graded based upon the Gardner system (Gardner et al., Reference Gardner, Schoolcraft, Wagley, Schlenker, Stevens and Hesla1998).
Embryo biopsy, embryo freezing and thawing
According to our standard operation protocol for PGT-A, any embryos that reached the blastocyst stage by day 5, 6, or 7 and had a morphological score of 4BB or better (according to the Gardner system) were subjected to embryo biopsy. A trophectoderm sample of 3–5 cells from each biopsied embryo was taken using laser assistance and the samples were sent out to CooperGenomics for next generation sequencing (NGS) testing for aneuploidy (PGT-A).
Embryos that were biopsied were placed in a pre-equilibrated Corral dish containing the same medium in which they had been cultured. Within 1 h after completing trophectoderm biopsy, each embryo was vitrified using Vit-Kit Freeze (Irvine Scientific).
Based on the PGT-A results, frozen embryo transfer was scheduled for each patient. One embryo was thawed using RapidWarm medium (Vitrolife) at least 1 h before transfer. Embryo transfers were done under ultrasound guidance using SureView soft catheters (Wallace, CooperGenomics).
Statistical analysis
Statistical analyses were performed using GraphPad Prism v.9.1.2 (GraphPad Software, San Diego, CA, USA). In total, 404 oocytes (202 for each group) from 35 oocyte retrieval cycles and 34 different patients were analyzed, and patients with fewer than five mature oocytes were not included. The associations among media group morphokinetic parameters, fertilization, irregular division, degeneration, blastulation, euploidy, and pregnancy rates were assessed using univariate analyses. Embryology factors including normal fertilization, blastocyst formation, presence of irregular cleavage, degeneration rates and pregnancy outcomes were compared between two media groups using a simple t-test. Means of morphokinetic data (tPNa, tPNf, t2, t3, t4, t5, cc2, S2, tM, tSB, tB, tEB and tHB) were separated using paired t-test. Effectiveness of the pairing was tested by default option of the software by calculating correlation coefficient, r, and a corresponding P-value. The level of 0.05 was used to determine significance. Values were expressed as means ± standard error of the mean (SEM).
Results
Fertilization and ploidy status of embryos
Data were taken from an internal sibling oocyte quality control study whose aim was to compare embryo development characteristics between LGGT medium and the lower lactate CSCM-NXC medium. In total, 404 oocytes (202 for each group) from 35 oocyte retrieval cycles and 34 different patients were included. Pooled data from 34 patient cycles were assessed for morphokinetics parameters. The female patient ages ranged from 29 to 42 years with a mean of 35.35 ± 3.7 years. The number of oocytes recovered per retrieval ranged from 8 to 40 with a mean of 21.4 ± 7.9 oocytes. Fertilization rate was similar between the two groups with 81.8 ± 17.8% and 80.0 ± 17.0% of injected oocytes fertilizing normally (two pronuclei) in LGGT and CSCM-NXC, respectively (P = 0.40). The percentages of the degenerated embryos on days 3, 5, 6 and 7 after a successful fertilization were 22.82 ± 3.85% and 25.29 ± 4.27% in LGGT and CSCM-NXC respectively; these values were not different (P = 0.96).
Blastulation rate per metaphase II (MII) oocyte was also similar, at 57.9% in the LGGT group and 52.5% in the CSCM-NXC group (P = 0.31). Of those embryos that made blastocysts, 109 embryos cultured in LGGT and 98 cultured in CSCM-NXC developed to 4BB blastocysts or better and were biopsied. There was no significant difference observed in euploidy rates between the two culture groups with 36% and 42% of embryos from the LGGT and CSCM-NXC groups, respectively, having been determined to be euploid (P = 0.17). The number of aneuploid embryos was also not statistically different (P = 0.06) in these two media. The percentages of mosaic embryos were 17 and 22 (P = 0.17) and number of embryos with no result was two and six in LGGT and CSCM-NXC, respectively. These findings are summarized in Table 1.
Table 1. Fertilization rates and ploidy status of embryos cultured with LGGT or CSCM-NXC

Most embryos were biopsied on day 5 (72 and 65 for LGGT and CSCM-NXC, respectively), with 36 (LGGT) and 30 (CSCM-NX) being biopsied on day 6 and only two (LGGT) and one (CSCM-NXC) embryo biopsied on day 7. Embryos were biopsied on the first day that they reached 4BB or better, with no significant differences in either the embryo grades at time of biopsy or the day that biopsy was performed between the two groups (P > 0.05).
Analysis of morphokinetics data
Analysis of morphokinetics data showed that there were few significant differences in the timing of each developmental marker between the two culture conditions (Table 2). Cultured embryos reached the 2-cell (t2) and 3-cell (t3) stages significantly faster in LGGT than those in CSCM-NXC (t2 and t3, P = 0.008 and P = 0.01, respectively) (Fig. 1A,B). However, embryos cultured in CSCM-NXC caught up by morula formation, and had a tendency to develop through morulation and to the blastocyst stage faster than those embryos cultured in LGGT (significant only at tSB when P = 0.026) (Fig. 1C). Embryos were not biopsied until they were expanded blastocysts and there was no significant difference in either the number of embryos biopsied or the time to biopsy between the two culture groups.
Table 2. Morphokinetic parameters of embryos cultured with LGGT and CSCM-NXC

Average time in hours following ICSI before different developmental stages occurred in human embryos cultured in either CSCM-NXC or LGGT. All values are represented as mean ± standard error of the mean (SEM).
Abbreviations: tPB, time to second polar body extrusion, tPNa, time to pronuclei appearance; tPNf, time to pronuclei fade; t(2 − 5), time to (2 − 5) cells; cc2 = t3 − t2; s2 = t4 − t3; tM, time to morula; tSB, time to starting blastocyst; tB, time to blastocyst; tEB, time to expanded blastocyst; tHB, time to hatched blastocyst.
a Statistically significant when the P-value is <0.05.
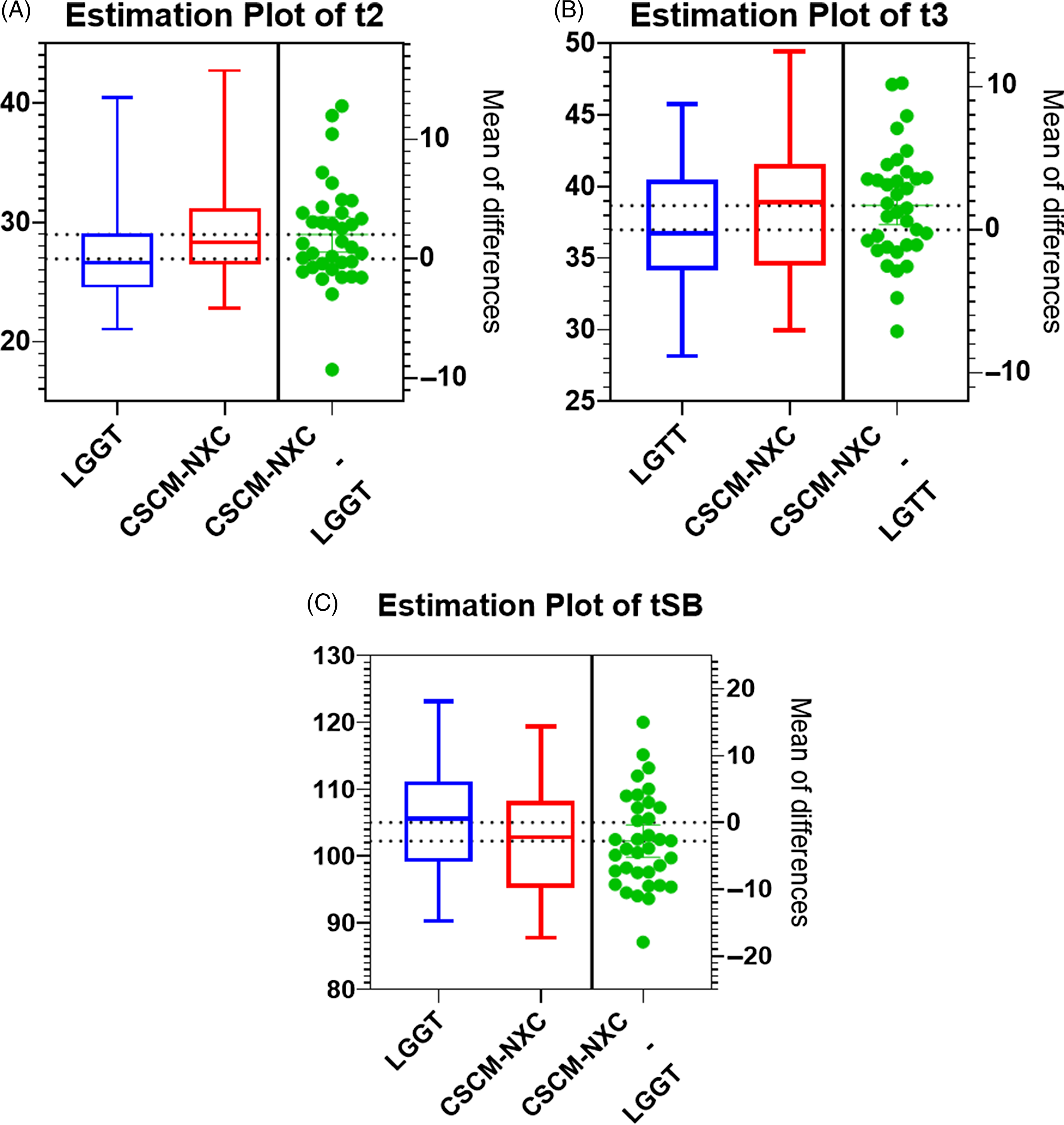
Figure 1. (A–C). Estimation plots for the time (h) of embryos reached 2-cell (t2), 3-cell (t3), and blastulation (tSB) stages. P < 0.05.
Single embryo transfer (SET) was conducted for these patients in their consecutive frozen cycle. Thirty-nine embryos were thawed and transferred, of which 21 had been cultured in CSCM-NXC and 18 were cultured in LGGT. The total number of transfers that resulted in a successful clinical pregnancy was 12 for CSCM-NXC (57%) and eight for LGGT (45%). However, there was no significant difference in either the number of embryos that were transferred or the clinical pregnancy rate between the two culture conditions (P = 0.74 and P = 0.09 respectively). However, there was a tendency towards a slightly increased pregnancy rate resulting from embryos cultured in medium with low lactate, CSCM-NXC.
Discussion
Typically, commercial media available for human IVF contain ∼5 mM lactate, which is approximately five times the amount found in CSCM-NXC (Morbeck et al., Reference Morbeck, Baumann and Oglesbee2017). Previous studies have shown an increase in blastocyst formation with mouse, bovine and human embryos using a low lactate medium (Gilbert et al., Reference Gilbert, White and Ni2016; White et al., Reference White, Popoff and Ni2017). Therefore, we aimed to determine if a medium containing a lower level of lactate could increase the blastulation rate and whether there was any influence on the ploidy status and morphokinetic parameters of those embryos biopsied.
In the current study, we used the same culture conditions for the sibling embryos, in an attempt to avoid any confounding variables due to oocyte source affecting the results. Although there was a trend for a higher percentage of euploid embryos in the CSCM-NXC medium, it did not achieve statistical significance (P > 0.05). As the euploid rates were similar in both media, it appeared the mitotic error rate was also similar and neither medium appeared to have an effect on aneuploidy (VerMilyea et al., Reference VerMilyea, Rios, Maizar, James, Picou, Werland, Marrs and Silverberg2018). Results showed a higher euploidy rate in embryos cultured in medium with low lactate compared with those cultured in a sequential medium. However, the study focused on embryos that were cultured in different media rather than embryos derived from sibling oocytes. It has been demonstrated that embryos exhibiting irregular divisions are at higher risk of aneuploidy (Vera-Rodriguez et al., Reference Vera-Rodriguez, Chavez, Rubio, Reijo Pera and Simon2015). We demonstrated that the irregular division rate was similar between the two media. It does not appear that the lower lactate medium had any influence on the euploidy rate. Looking at other morphokinetic data, it was observed that embryos grown in LGGT reached both the 2-cell and 3-cell stages significantly faster than those grown in CSCM-NXC. Conversely, those grown in the CSCM-NXC reached the starting blastocyst stage sooner. However, the full blastocyst stage was reached in a similar amount of time. Considering the similar blastulation rates and euploid rates produced by the two media, it is difficult to determine the significance of these differences. It may be that the lower lactate levels slowed growth at early stages, but at later stages increased the rate of growth. A shift from oxidative phosphorylation to glycolysis occurs at latter stages of embryo development, and influences the production of lactate in embryos (Gardner, Reference Gardner2015).
A randomized study compared the blastocyst utilization rate in two commercial single-step media and found no difference in cleavage rate, blastulation rate, and blastocyst utilization rate (Sfontouris et al., Reference Sfontouris, Kolibianakis, Lainas, Venetis, Petsas, Tarlatzis and Lainas2017). Another study showed no differences in fertilization rate, cleavage rate, and blastulation rate of cultured sibling oocytes in two different single-step media (Durand et al., Reference Durand, Sermondade, Herbemont, Benard, Gronier, Boujenah, Cédrin-Durnerin, Poncelet, Grynberg and Sifer2016). According to our study, we demonstrated a significant difference in first and second division time and start of blastulation time between the groups. It may be that the lower lactate level facilitated this natural shift and enhanced later embryo growth.
Although pregnancy rates of embryos cultured in CSCM-NXC and LGGT media were 57% and 45%, respectively, this difference was not statistically significant (P > 0.05). Researchers (Rodríguez-Alonso et al., Reference Rodríguez-Alonso, Maillo, Acuña, López-Úbeda, Torrecillas, Simintiras, Sturmey, Avilés, Lonergan and Rizos2020) presented that isthmic metabolites of the bovine oviduct had lower lactate and glycine, compared with those in the ampulla. Therefore, embryos may need lower lactate concentrations to achieve better division while entering the isthmus from the ampulla. This finding is consistent with our result that there was a significant difference at the 2-cell and start of blastulation stages between embryos cultured in the two media.
The strength of our study was to use sibling oocytes to avoid any bias. Rather than comparing two groups of patients, which could have important differences in demographics, a sibling oocyte study has each patient serving as their own control. This allowed a broad range of patients to be included in this study. It is also a strength of the study that sibling oocytes were cultured in the same incubator and in the same dish, which means that the culturing conditions were not biased. Furthermore, because both media were monophasic formulations they were handled and used in a very similar fashion. As far as the limitations of this study, our experimental design was not controlled, and the number of subjects was limited. There was no controlled grouping done for the embryo transfers. In some cases, embryos to be transferred were selected based on the patients’ sex preference rather than its quality. Therefore, embryos that were cultured in both media were not transferred equally. As our facility is a multiphysician centre, the embryo transfers were done by different physicians. Although our embryo transfer protocol is the same for all physicians, there might have been a minimal variation in their techniques. Because all patients did not receive an embryo transfer at time of writing, power was not adequate to determine significance in pregnancy rates.
In conclusion, while the lower lactate-containing medium, CSCM-NX, proved to be a viable alternative to a medium with a conventional lactate level, it did not appear to enhance euploidy or pregnancy rates. While there were observable differences in morphokinetic data between the two media, it is difficult to discern the implications and significance, if any, of this finding. According to our knowledge, this is the only study testing different lactate concentrations in sibling oocytes. Previous studies have failed to reach a clear consensus on the potential benefits of high compared with low lactate culture media, and our study seems to corroborate with this. Although there were some slight differences in the dynamics of embryo development, these differences did not lead to an effect on embryo quality, ploidy or resultant pregnancy progression, indicating that neither high nor low lactate culture media are strongly preferred by the developing embryos. However, a randomized controlled study with a larger sample size will be necessary to ascertain any positive effects of using a culture media with low lactate.
Acknowledgements
We would like to thank Jacob F. Mayer (Director) and Helena I. Russell (Associate Director) for their guidance and help in the process of this research study as a part of a thesis submitted to the MSc Biomedical Sciences Clinical Embryology and Andrology Programme at Eastern Virginia Medical School (EVMS).
Author contributions
SD designed the study design, and drafted and revised the manuscript, and participated the laboratory work. MU did the literature search, and participated the laboratory work and drafted the manuscript. FL and RU data analysis and revised the manuscript. HNC, FNS, and AH critically revised the manuscript. All authors approved the submitted version of this manuscript.
Declarations of interest
There is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported. The authors declare that they have no competing interests.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethics approval and consent to participate
This study was approved by the IRB board (IRS# 19-04-XX-0116) of EVMS and was determined as ‘exempt’ for patient informed consent.





