Introduction
Minor physical anomalies (MPAs) are a range of subtle alterations of the head, hands, hair and feet which serve as indicators of altered morphogenesis during the first or early second trimester and act as timed, biological markers of developmental disturbance (Tarrant & Jones, Reference Tarrant and Jones1999). An increased prevalence of MPAs has been extensively documented in schizophrenia compared with healthy controls (Gualtieri et al. Reference Gualtieri, Adams, Shen and Loiselle1982; Guy et al. Reference Guy, Majorski, Wallace and Guy1983; O'Callaghan et al. Reference O'Callaghan, Larkin, Kinsella and Waddington1991; Lohr & Flynn, Reference Lohr and Flynn1993; Green et al. Reference Green, Salz and Christenson1994). However, the precise pattern of physical anomalies and their specificity for schizophrenia remains unclear.
The neurodevelopmental hypothesis of schizophrenia suggests that the aetiological origins of the condition are due to aberrant development of the brain in utero or early life (Weinberger, Reference Weinberger1987; Murray et al. Reference Murray, O'Callahan, Castle and Lewis1992) but there is much less evidence implicating deviant development in affective psychoses. Few studies have investigated the specificity of biological markers such as MPAs for schizophrenia, and those which have show conflicting results. Studies by Lohr & Flynn (Reference Lohr and Flynn1993), Green et al. (Reference Green, Salz and Christenson1994), Alexander et al. (Reference Alexander, Mukherjee, Richter and Kaufmann1994) and McGrath et al. (Reference McGrath, Van Os, Hoyos, Jones, Harvey and Murray1995) reported no significant difference in MPA scores between patients with mood disorders and control subjects. Conversely, Lohr et al. (Reference Lohr, Alder, Flynn, Harris and McAdams1997) and Trixler et al. (Reference Trixler, Tenyi, Csabi and Szabo2001) found increased rates of MPAs in affective disorders compared with controls. Furthermore, results of studies reporting the topographical distribution of MPAs in schizophrenia are highly variable, although there is a strong suggestion that the greatest number of MPAs occur within the oral cavity (Tarrant & Jones, Reference Tarrant and Jones1999).
We investigated the prevalence and the predictive power of MPAs in a large epidemiological sample of first-episode psychotic patients and controls. We postulated that by studying patients at the onset of their illness this would minimize the possible confounding effects of ageing and long-term antipsychotic treatment (Lloyd et al. Reference Lloyd, Doody, Brewin, Park and Jones2003; Waddington et al. Reference Waddington, Youssef and Kinsella1998). We applied a detailed anthropometric scale (Lane et al. Reference Lane, Kinsella, Murphy, Byrne, Keenan, Colgan, Cassidy, Sheppard, Horgan, Waddington, Larkin and O'Callaghan1997) that more thoroughly describes the nature of dysmorphology found in psychotic disorders compared with the widely used Waldrop Scale (Waldrop et al. Reference Waldrop, Pederson and Bell1968). Based on the neurodevelopmental model of schizophrenia, we hypothesized that patients with schizophrenia would have significantly elevated rates of MPAs compared with those with other psychoses and controls. We also examined the topographical profile of MPAs across diagnoses in order to determine whether a particular distribution of MPA is specific to schizophrenia in comparison to other psychoses.
Method and material
The AESOP study (Aetiology and Ethnicity of Schizophrenia and Other Psychoses) is an epidemiological, case-control study investigating the causes of high rates of psychosis including non-psychotic mania in certain ethnic minority populations in the UK. Ethical approval for the AESOP study was obtained from the Nottingham and London Local Research Ethics Committees. As part of the AESOP study, we identified everyone aged between 16 and 64 years living in defined catchment areas in Nottingham and south-east London who made contact with mental health services because of a first episode of probable psychosis. Cases from the Bristol arm of the study were not included in our sample as MPAs were not examined there. The search was deliberately broad to allow identification of all incident cases, and took place over 24 months in Nottingham and south-east London (September 1997–August 1999). Full details of the methodology employed in the AESOP study have been described elsewhere (Lloyd et al. Reference Lloyd, Kennedy, Fearon, Kirkbride, Mallett, Leff, Holloway, Harrison, Dazzan, Morgan, Murray and Jones2005; Kirkbride et al. Reference Kirkbride, Fearon, Morgan, Dazzan, Morgan, Tarrant, Lloyd, Holloway, Hutchinson, Leff, Mallett, Harrison, Murray and Jones2006) and a brief outline is provided below.
Subject ascertainment and assessment
The study sample comprised all patients who presented for the first time to any psychiatric service because of psychotic phenomena, including those with possible negative syndrome schizophrenia. An over-inclusive psychosis-screening instrument (Jablensky et al. Reference Jablensky, Sartorius, Ernberg, Anker, Korten, Cooper, Day and Bertelsen1992) was used to screen all the individuals identified in this case ascertainment procedure. Subjects with a functional psychotic illness (DSM-IV 296.x4, 295.70, 295.xx, 297.xx, 298.8, 298.9, 291.3, 291.5, 292.11, 292.12) (APA, 1994) became cases in our study and included individuals with a diagnosis of bipolar affective disorder who had experienced a previous non-psychotic depressive episode thereby making their current episode a first-onset psychosis. Patients with organic mental illness, a history of significant head trauma resulting in loss of consciousness for over 1 hour, disease of the central nervous system of aetiological relevance, or severe learning disability were excluded.
A group of healthy volunteers aged 16–64 were recruited from residents within the study areas. Subjects were screened for the presence of psychotic symptoms using the Psychosis Screening Questionnaire (Bebbington et al. Reference Bebbington and Nayani1995).
Subjects who gave informed consent underwent extensive assessment using established and standardized instruments including the Schedule for Clinical Assessment in Neuropsychiatry (SCAN) (WHO, 1994). Consensus diagnoses were made for each case by a group of senior clinicians (R.M., J.L., P.B.J., or G.H.) and diagnostic codes were assigned to each subject according to DSM-IV (APA, 1994) using all other information from the case notes, item ratings in SCAN and collateral histories. Inter-rater and inter-centre reliability for diagnosis was estimated as high (kappa scores ranged from 0·6 to 0·8).
The ethnicity status of the cases was determined by self-ascription. Cases and controls were asked to assign themselves to an ethnic group according to categories devised for the 1991 United Kingdom Census. Ten ethnic groups obtained using these methods were combined to create four groups for the present study: White, African-Caribbean, Black African and Other. People of Indian, Pakistani, or Bangladeshi descent and those of mixed ethnicity were combined with individuals of any other ethnicity owing to the small numbers in each of these groups, and came under the category of ‘Other’. People classified as Black, any other background, were grouped under the African-Caribbean category.
Anthropometric assessment
MPAs were evaluated as soon as possible after initial presentation using an abridged version of the Lane scale (Lane et al. Reference Lane, Kinsella, Murphy, Byrne, Keenan, Colgan, Cassidy, Sheppard, Horgan, Waddington, Larkin and O'Callaghan1997) containing 62 qualitative measurements of the head and face. The scale comprised anomalies of facial symmetry and details of eye, ear, nose, mouth, palate and hairline morphology. Individual dysmorphic features were operationlized as categorical or ordinal scores. A copy of this abridged version of the full scale, which takes approximately 45 minutes to apply, is available from the authors on request. Every attempt was made to ensure that raters were blind to diagnosis, although blinding may not have been complete due to the length of time spent with each subject. Inter-rater reliability between examiners on individual anomalies gave kappa scores of between 0·95 and 1·0.
Data analysis
To determine the frequency of any potential dysmorphogenesis in patients with psychosis, we compared total MPA scores in matched pairs (patients with psychosis versus controls). Since ethnicity, age and sex have been postulated as probable confounders in studies investigating anthropometric measures (Akabaliev & Sivkov, Reference Akabaliev and Sivkov2003; Lloyd et al. Reference Lloyd, Doody, Brewin, Park and Jones2003) individuals were matched for these characteristics. The initial analysis thus comprised a cohort of 126 case-control pairs matched according to gender, ethnic group and age (within 5 years). Subjects who could not be matched were excluded from this analysis.
For the analysis a binary variable was created for each anomaly such that the presence of a normal variant was assigned a score of 0 and the presence of any other variant was given a score of 1 (i.e. abnormal). An initial analysis was conducted comparing the cases and controls in the matched sample only. In this analysis a total MPA score was calculated by adding all the scores for individual anomalies on the scale such that no individual anomaly was assumed to have greater significance than any other. Mean total MPA scores for all matched cases and controls were compared and Student's t tests were used to assess differences.
To optimize the power to detect subgroup differences, we included all subjects except those with substance-induced psychosis in the assessment of diagnostic specificity (n=231). We divided subjects with psychoses into two groups comprising non-affective psychosis [schizophrenia (295.xx), delusions disorders (297.xx) and acute and transient psychotic disorders (298.8, 298.9)] and affective psychosis [bipolar I disorder including single manic episode with psychotic features (296.x4), depression with psychotic features (296.24, 296.34) and schizo-affective psychosis (295.70)]. As the diagnostic status of the subjects with substance-induced psychosis is unclear, we decided not to pool these individuals with the non-affective psychoses. The small sample size of this group (n=11) did not justify their inclusion as a separate sub-group and they were thus excluded from this analysis.
Data reduction was carried out using a non-linear version of categorical principal components analysis (Michailidis & Jan de Leeuw, Reference Michailidis and de Leeuw1998), and an analysis of variance with post hoc t tests was then undertaken on the object scores to test for the effects diagnosis. Individual items on the dimensions with significant group differences that had the highest loading were tested using χ2 tests. We tested for the effects of age, sex and ethnicity in this sample by entering each variable into a stepwise logistic regression analysis and but found no differences between patient and control groups for the effects of age, sex or ethnicity.
Results
We received informed consent from 245 cases (123 from London and 122 from Nottingham) who agreed to undertake the MPA assessments out of a total study population of 535 subjects. There were no differences between subjects who consented to the MPA assessments and those who refused in terms of age, sex and ethnicity. MPA schedules were thus completed on all consenting cases and 158 controls. Three subjects were excluded from the analysis as we did not have enough information on them to assign a diagnosis. Table 1 shows the baseline characteristics for the entire sample and the matched sample. In the total sample, cases were significantly different from controls in terms of age, ethnicity and IQ. Matched cases had a significantly lower mean IQ compared with controls but did not differ in any other measured characteristic. In the affective psychosis group, 25% had a diagnosis of bipolar I disorder, single manic episode, severe with psychotic features, 22% had bipolar I disorder, recurrent, most recent episode with psychotic features, 39% had a major depressive disorder with psychotic features and 14% had a schizoaffective disorder.
Table 1. Sample characteristics

IQ, National Adult Reading Test (NART) (Nelson & Willison, Reference Nelson and Willison1991).
a Schizophrenia category also includes other non-affective psychoses.
** p<0·01; *** p<0·001 for tests of within-analysis case-control differences (t test or χ2).
In our initial analysis on the matched sample MPA total score was normally distributed. Cases had a mean total MPA score of 12·21 and a standard deviation of 5·02. Controls had a compatively lower mean total MPA score of 7·98 and a standard deviation of 2·58. The difference between cases and controls was highly significant [mean difference 4·22 (95% C.I. 3·23–5·21); t=8·39; p<0·001].
The remaining analyses are based on all subjects with affective and non-affective psychosis. The non-linear version of categorical principal components analysis produced objects scores for a model with four dimensions and these were tested for the effects of diagnosis using an analysis of variance. This showed the object scores on the first and third dimension as having a significant effect for diagnosis. Object scores for patients with non-affective psychosis and affective psychosis significantly differed from controls on both these dimensions. Patients with non-affective psychosis and affective psychosis did not significantly differ from each other on any of the four dimensions. Table 2 shows the major item loadings on each of the four dimensions.
Table 2. Major item loadings on the four dimensions produced by categorical principal components analysis
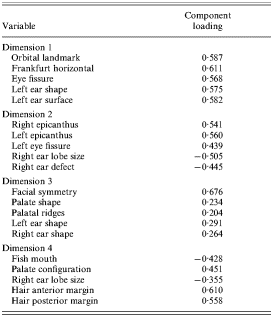
The first dimension (F=43·631; p<0·0001) had substantial positive loadings for anomalies relating to the eyes and ears and the third factor (F=8·543; p<0·0001) had positive loadings predominantly for variables relating to facial symmetry and the palate. The five items with the highest loading on these two dimensions were then tested individually using a χ2 test and the results are displayed in Table 3.
Table 3. A comparison of qualitative MPAs for 121 patients with non-affective psychoses (schizophrenia), 110 patients with affective psychoses and 158 controls (variables selected by principal components analysis and analysed by χ2 test)

*** p⩽0·01.
Overall facial symmetry, symmetry of the orbital landmarks and lowered frankfurt horizontals significantly differentiated patients with non-affective and affective psychosis from controls, as did a ‘V-shaped’ palate, reduced palatal ridges, asymmetry of the left and right ear contours and a flat and unfolded or wide surface of the left ear. In addition, the affective psychosis group had lowered eye fissures compared with controls.
Discussion
This study demonstrated that patients with psychosis had a significantly higher rate of MPAs compared with control subjects. More specifically, individuals with non-affective and affective psychosis had significantly more MPAs compared with controls. Both groups had reduced facial symmetry, abnormalities relating to the eyes and the orbit, ‘V’ shaped palates, and ears that were abnormal in terms of shape, surface and attachment compared with controls. Patients with substance-induced psychosis did not significantly differ from controls.
This study has significant strengths and investigates an important topic. We employed a scale for MPA assessment which was specifically designed for use in psychosis. The cases in this study were drawn from a population-based, first-episode psychosis sample and thus did not overrepresent patients with chronic persisting types of illness. We conducted an initial analysis which comprised a cohort of case-control pairs matched according to gender, ethnic group and age in order to rule out the possible confounding effects of these variables and we made corrections for multiple comparisons by employing a categorical principal components analysis in our investigation based on the entire sample.
There are a number of limitations to this study which warrant consideration. We have already reported an association between MPAs and psychosis using the same sample which focused on the prevalence of dysmorphology across different ethnic groups (Dean et al. Reference Dean, Dazzan, Lloyd, Morgan, Morgan, Doody, Hutchinson, Orr, Jones, Murray and Fearon2006). The primary aim of this study however, is very different to our previous paper as it investigates the topographical profile of MPAs across diagnoses in order to establish the specificity of MPAs in schizophrenia using a detailed scale incorporating individual MPA items. The scale does not include quantitative MPA measures, many of which have been identified as important in patients with psychosis (McGrath et al. Reference McGrath, El-Saadi, Grim, Cardy, Chapple, Chant, Lieberman and Mowry2002). The specific anomalies we found to be most prevalent in our cases are, however, consistent with those reported in previous studies. Although we made every attempt to ensure that our researchers carrying out the MPA assessments were blind to diagnosis we acknowledge that blinding may not have been complete due to the length of time (approximately 45 minutes) required to complete the assessment with a patient. Furthermore, the abridged version of the MPA scale used in this study did not include measures outside the head and face, which is likely to have an impact on the interpretation of our findings. In order to maximize our statistical power, we merged subjects into two diagnostic groups. We acknowledge that in doing this, important differences between specific diagnoses such as unipolar and bipolar affective disorder may have been lost, but our numbers in each individual diagnostic group were not large enough to justify a more detailed analysis.
Our findings are consistent with those of other studies which have investigated the pattern of dysmorphology in schizophrenia and found abnormalities of the mouth (Green et al. Reference Green, Satz, Gaier, Ganzell and Kharabi1989; McGrath et al. Reference McGrath, Van Os, Hoyos, Jones, Harvey and Murray1995; Lane et al. Reference Lane, Kinsella, Murphy, Byrne, Keenan, Colgan, Cassidy, Sheppard, Horgan, Waddington, Larkin and O'Callaghan1997), ears and eyes (Lane et al. Reference Lane, Kinsella, Murphy, Byrne, Keenan, Colgan, Cassidy, Sheppard, Horgan, Waddington, Larkin and O'Callaghan1997) to be particularly significant. Furthermore, this study extends the finding by showing that patients with affective psychosis also differ significantly from controls on all of these measures; this finding is at variance with the results of previous studies which indicate some diagnostic specificity of MPAs to schizophrenia compared with mood disorders (Lohr & Flynn, Reference Lohr and Flynn1993; Green et al. Reference Green, Salz and Christenson1994; Alexander et al. Reference Alexander, Mukherjee, Richter and Kaufmann1994; Trixler et al. Reference Trixler, Tenyi, Csabi and Szabo2001).
The increased frequency of MPAs in non-affective and affective psychosis found in this study suggests a common developmental pathway for these disorders and indicates possible shared aetiological antecedents that date to the perinatal period. This insult may be a result of genetic liability or an environmental teratogen. The results support existing research findings which demonstrate that schizophrenia and affective disorders share many risk factors. These include the excess of winter-spring births (Torrey et al. Reference Torrey, Rawlings, Ennis, Merrill and Flores1996, Reference Torrey, Miller, Rawlings and Yolken1997) and abnormal dermatoglyphics (Jelovac et al. Reference Jelovac, Milicic, Rudan, Turek, Ugrenovic and Milas1998). The clinical difference in expression between the disorders may be determined by the specific brain area involved, as well as by the individual's predisposing genes (Torrey, Reference Torrey1999).
The topographical distribution of MPAs in our study are consistent with a model of cerebrocranial dysmorphogenesis, developed by Waddington et al. (Reference Waddington, Lane, Larkin and O'Callaghan1999), to explain the neurodevelopmental basis of schizophrenia. They argue that midline craniofacial structures such as the palate develop over weeks 9–10 to 14–15 of gestation, and during the early phase of palatal morphogenesis, the process of narrowing and elongation of mid-face is prominent. Aetiological factors acting during the first trimester may affect this process of midline development of both the face and brain with prefrontal and temporoparietal areas being particularly affected as a result. The results of an earlier analysis which investigated the grey matter correlates of MPAs in a subgroup of the AESOP sample (Dean et al. Reference Dean, Fearon, Morgan, Hutchinson, Orr, Chitnis, Suckling, Mallet, Leff, Jones, Murray and Dazzan2006) provide further support for this model. In this analysis, we found a positive association between total MPA scores and grey matter reduction in the prefrontal cortex and precuneus and grey matter excess in the basal ganglia, thalamus and lingual gyrus. Thus the model proposed by Waddington and colleagues (Reference Waddington, Lane, Larkin and O'Callaghan1999) together with the findings of this study and our earlier work (Dean et al. Reference Dean, Fearon, Morgan, Hutchinson, Orr, Chitnis, Suckling, Mallet, Leff, Jones, Murray and Dazzan2006) strongly supports the notion of risk operating during organogenesis in the first trimester of pregnancy. Further investigation looking at clinical and aetiological factors associated with MPAs may tell us more about the causal pathways and pathogenesis of psychotic disorders.
Acknowledgements
We acknowledge the contributions of all the AESOP study team members in the three centres on whose behalf the paper was written. We are grateful to the Medical Research Council and the Stanley Foundation for financial support.
Declaration of Interest
None.





