Approximately 0.4–5% of infants are born with CHD.1 Those with moderate-to-severe illness will undergo surgery in childhood, often requiring the use of cardiopulmonary bypass.Reference Hoffman and Kaplan1 Cardiac surgery on cardiopulmonary bypass remains a high-risk operation, with an overall mortality of ~3% and major complications in up to 38% for the most complex operations.Reference Jacobs, He and Mayer2,Reference Jacobs, O’Brien and Jacobs3 Morbidity and mortality are highest in children who develop post-operative low cardiac output syndrome, which occurs in up to 25% of infants.Reference Smith-Parrish, Schumacher and Gruppen4,Reference Parr, Blackstone and Kirklin5 Post-operative low cardiac output syndrome may be prevented and treated with vasoactive and inotropic medications.Reference Smith-Parrish, Schumacher and Gruppen4
Vasoactive and inotropic medications are used in 90% of post-operative admissions to the paediatric cardiac ICU.Reference Loomba and Flores6,Reference Roeleveld and de Klerk7 The most frequently used drugs include epinephrine, dopamine, dobutamine, milrinone, and vasopressin; a median of three vasoactives are typically used per patient and admission.Reference Loomba and Flores6,Reference Roeleveld and de Klerk7 While studies have shown the value of certain inotropes in specific populations, no inotropes or vascoactive medications are labelled by the United States of America Food and Drug Administration or the European Medicines Agency for the prevention or treatment of low cardiac output syndrome in children.Reference Torok, Li and Kannankeril8 Instead, the choice of which inotrope or vasoactive, as well as the dose, timing, and duration of administration of these medications are highly variable and mostly driven by the provider and institutional preference.Reference Burstein, Rossi and Jacobs9 In addition, adjunct medications that modulate targets upstream or downstream of inotrope and vasoactive receptors are sometimes used to reduce vasoactive exposure despite limited evidence of efficacy (Table 1).Reference Ferrer-Barba, Gonzalez-Rivera and Bautista-Hernandez10 The purpose of this systematic review is to summarise the existing literature on clinical trials with endpoints related to post-operative administration of inotropes and vasoactives in children after cardiopulmonary bypass surgery to help inform both clinical practice and the design and conduct of future trials.
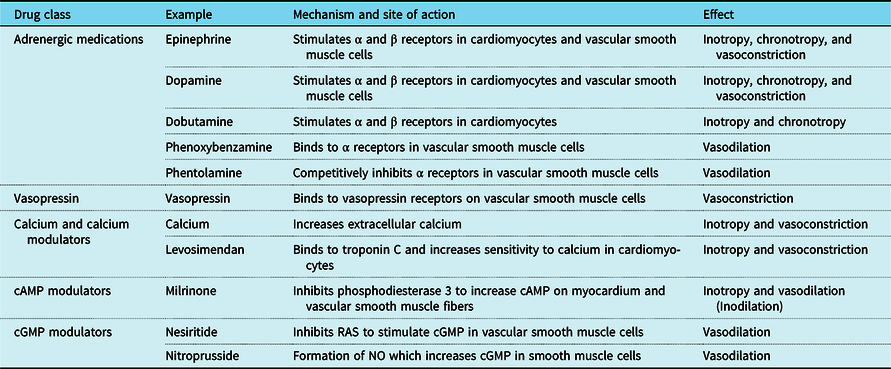
Table 1. Drugs included in this review and summary of their molecular mechanisms and net physiologic effects.
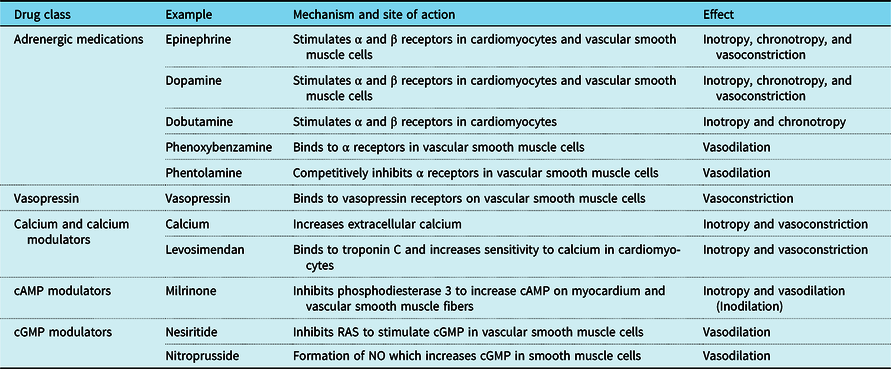
cAMP = cyclic adenosine monophosphate; cGMP = cyclic guanosine monophosphate
Materials and methods
Search strategy
PubMed and EMBASE were searched to identify studies that had the primary goal of investigating medications used for vasoactive support for paediatric patients after cardiac surgery with cardiopulmonary bypass. Studies from the years 2000 to 2020 were included. The patient population was defined as birth to 18 years of age, and identified using a controlled vocabulary and keywords related to “pediatrics.” The patient population was further refined using keywords related to “postoperative care,” “cardiac surgery,” and “cardiopulmonary bypass.” This population was then searched for vasoactive medications using the keywords “vasodilator OR vasorelaxant” and “cardiotonic agents OR inotrope OR cardiac stimulant.” Animal studies; pre- or intra-operative medication administration; studies other than English; and case reports, letters, editorials, and comments were excluded. The search strategies are shown in the Appendix. The literature search included multiple classes of medications. Primary reviewers selected those pertaining to inotropic and vasoactive medications for screening for this paper. A total of 420 studies were identified.
Study selection
Identified articles were imported into EndNote. The title of each study was screened. Studies were included if they focused on vasoactive support for a medication administered in the post-operative period and excluded if they focused on a medication from a different class or if the medication was administered pre- or intra-operatively. Referenced articles were also screened and included if they met the search and selection criteria.
Two reviewers independently reviewed the abstracts of 51 studies to determine final eligibility. Papers were rejected if they did not report a primary endpoint related to vasoactive support for a medication administered in the post-operative period. A total of 37 papers were included in the final analysis (Fig 1).
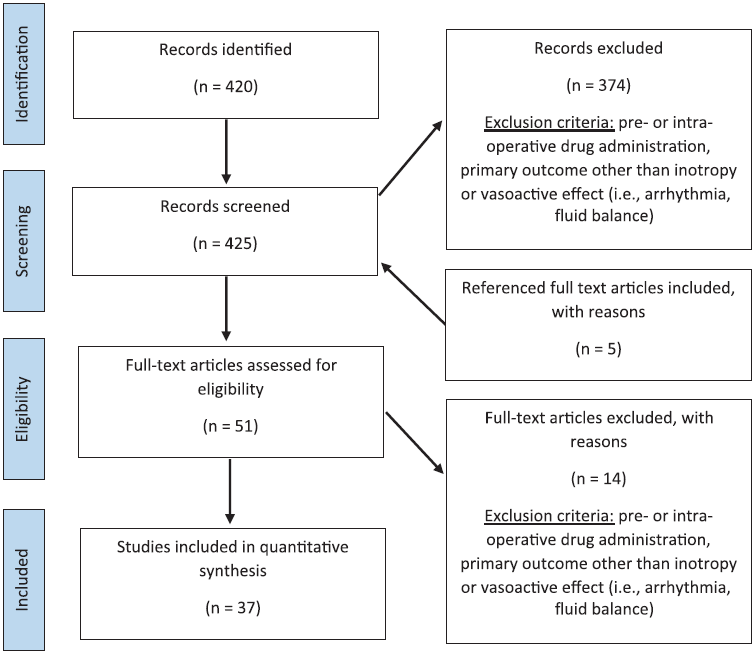
Figure 1. Summary of literature search strategy and results.
Search parameters: all trials from 2000 to 2020 are in English with a medication (inotrope, vasoactive) and cardiac surgery and post-operative care and paediatrics.
Data extraction and study classification
A standardised data collection form was used to extract data from each eligible study. The following data were collected: study characteristics (including years of study and study design), study population characteristics (including age and cardiac defects), intervention (including medication administered), and study endpoints and results.
All studies included were primary research studies. Studies were further classified as prospective or retrospective, single or multicentre, randomised or non-randomised, placebo-controlled or not placebo-controlled, and blinded or non-blinded. For each medication, the dose, timing of administration, primary outcomes, and secondary outcomes were compiled and analysed.
Results
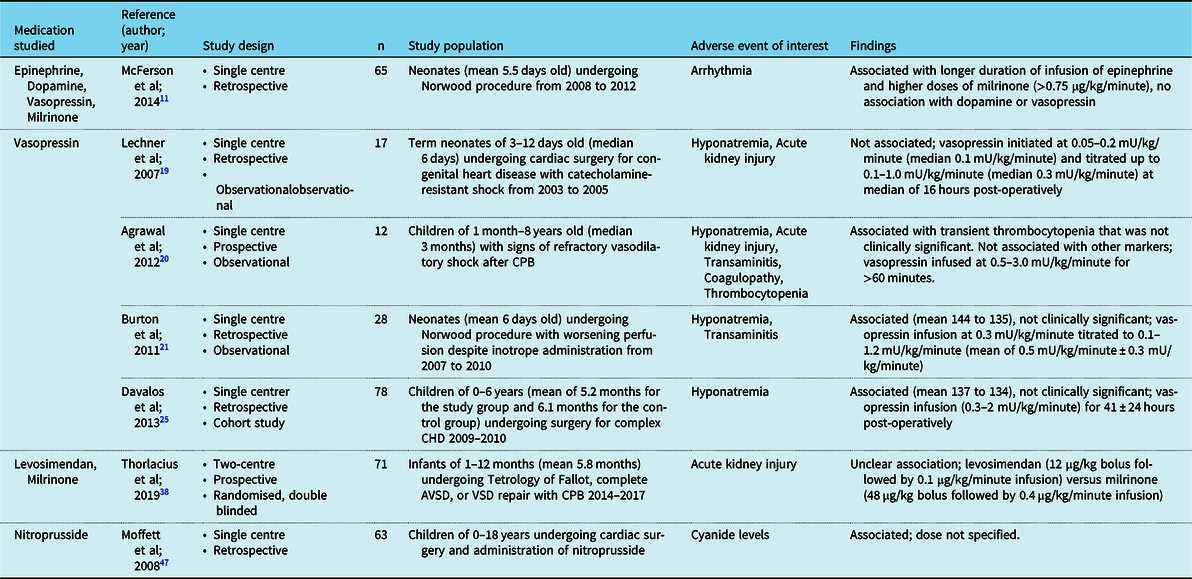
A total of 37 studies met our selection criteria: 20 studies were prospective, 17 were retrospective, 9 were placebo-controlled, 2 were multi-arm clinical trials, 32 (Table 2)11–47 had a measure of efficacy as the primary outcome, including 2 studies that evaluated mortality as a primary outcome. Five studies focused on safety and side effects (Table 3).Reference McFerson, McCanta and Pan11,Reference Lechner, Hofer, Mair, Moosbauer, Sames-Dolzer and Tulzer19–Reference Burton, Kaufman, Goot and da Cruz21,Reference Davalos, Barrett and Seshadri25,Reference Thorlacius, Suominen and Wahlander38,Reference Moffett and Price47
Table 2. Selected inotropic and vasoactive drug trials in children after cardiac surgery.

ASD = aortic septal defect; AVP = arginine vasopressin; BP = blood pressure; CHD = congenital heart disease; CI = confidence interval; CPB = cardiopulmonary bypass; ECHO = echocardiogram; ECMO = extracorporeal membrane oxygenation; HR = heart rate; ICU = intensive care unit; IQR = interquartile range; LCOS = low cardiac output syndrome; LOS = ; MAP = mean arterial pressure; OI = ; PAP = pulmonary artery pressure; POB = phenoxybenzamine; PVR = pulmonary venous return; RACHS = risk adjustment in congenital heart surgery score; SBP = systolic blood pressure; SvO2 = mixed venous oxygen saturation; TAPVR = total anomalous pulmonary venous return; UOP = urine output; VSD = ventricular septal defect
Table 3. Adverse events were reported in the reviewed studies.
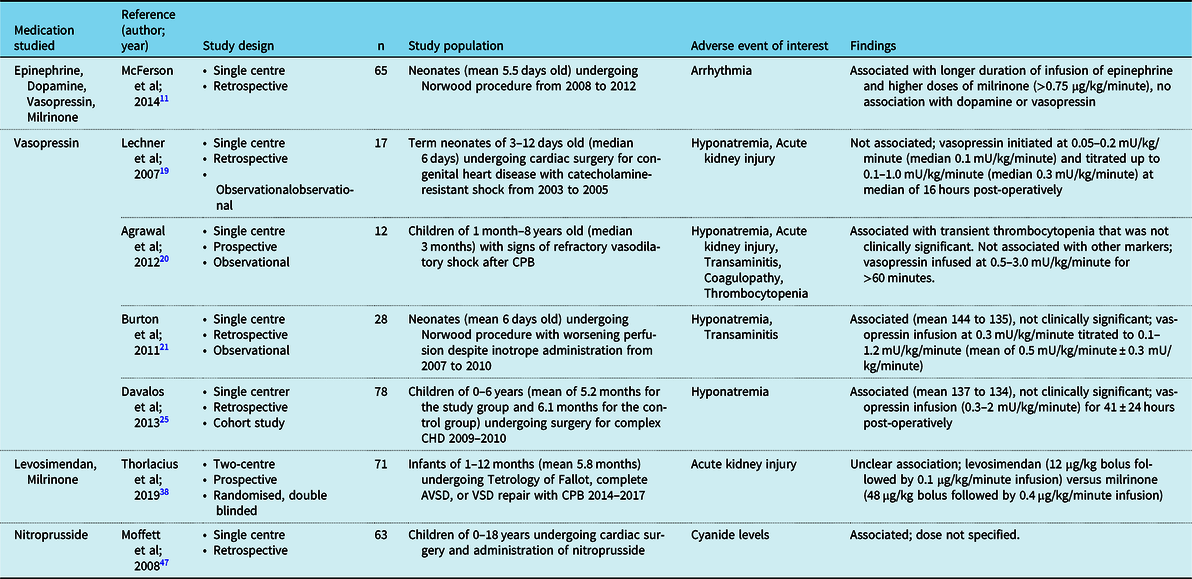
AVSD = atrioventricular septal defect; CHD = congenital heart disease; CPB = cardiopulmonary bypass; VSD = ventricular septal defect
Collectively, selected studies reported on 12 medications in 2856 children: 15 studies focused on neonates or infants, and included 969 patients; 12 studies specified a surgical repair or congenital heart defect as part of the study population, including 5 studies that included only Norwood patients. All medications were given between the end of a bypass through the first 72 hours post-operatively. There was a wide variance in specific timing, dosing, and duration of treatment.
Adrenergic pathway targeting agents
Epinephrine, norepinephrine, and dopamine are alpha (α) and beta-(β) receptor agonists that promote inotropy and peripheral vasoconstriction in a dose-dependent manner.Reference Loomba and Flores6,Reference Pekkarinen and Hortling48–Reference Mueller52 These drugs are commonly used in the post-operative cardiac care of children,Reference Loomba and Flores6,Reference Roeleveld and de Klerk7 but only 1 study to date investigated the efficacy of epinephrine12 and no studies were found on the efficacy of norepinephrine or dopamine. In a study of 39 children, epinephrine dosages of 0.01–0.23 µg/kg/minute was not shown to prevent post-operative low cardiac output syndrome.Reference Oualha, Urien and Spreux-Varoquaux12 Docarpamine, an oral dopamine precursor which degrades to stable dopamine levels in the serum,Reference Watarida, Shiraishi and Sugita13 was found to be safe in 11 post-operative children, but there was no control arm to evaluate efficacy and the drug is only available in Japan.Reference Watarida, Shiraishi and Sugita13
Dobutamine is a synthetic β agonist, which promotes inotropy and chronotropy.Reference Ferrer-Barba, Gonzalez-Rivera and Bautista-Hernandez10,Reference Steen, Tinker, Pluth, Barnhhorst and Tarhan51,Reference Nakazawa, Takahashi, Aiba, Okuda, Ohta and Takao53,Reference Mills, Costello and Almodovar54 A total of 3 prospectives, including 2 with randomisation between arms, clinical trials of dobutamine enrolled a combined total of 110 children 0–18 years of age.Reference de Souza, de Carvalho, Maluf and Carvalho14–Reference Ebade, Khalil and Mohamed16 None of these studies demonstrated a clinical benefit of dobutamine using dosages of 6–10 µg/kg/minute. Dobutamine was not superior to milrinone in preventing low cardiac output syndrome in 50 children undergoing elective cardiac repair,Reference Cavigelli-Brunner, Hug and Dave15 and was inferior to levosimendan in increasing cardiac index in 50 children with elevated pulmonary arterial pressures undergoing atrial septal defect or ventricular septal defect repair.Reference Ebade, Khalil and Mohamed16 Furthermore, dobutamine showed no significant increase in splanchnic perfusion as measured by gastric tonometry in 10 post-operative children.Reference de Souza, de Carvalho, Maluf and Carvalho14
Phenoxybenzamine and phentolamine, irreversible and reversible α blockers, respectively, were used for afterload reduction after the Norwood operation.Reference Fowler, Holmes, Gaffney, Privitera and Grupp55–Reference Collier, Nachev and Robinson58 In a single trial of 105 infants undergoing stage 1 surgical palliation for single-ventricle CHD, patients were consecutively recruited to receive phenoxybenzamine or not. There was a decrease in the sudden circulatory collapse in the group of infants who received phenoxybenzamine compared to the negative control.Reference De Oliveira, Ashburn and Khalid17 Phentolamine was studied against nitroprusside in 146 post-operative Norwood infants and showed lower mean arterial blood pressure and coronary perfusion pressure; however, deep hypothermia was used in the nitroprusside group only and may have confounded the results.Reference Furck, Hansen, Uebing, Scheewe, Jung and Kramer18
One study looked at the association of tachyarrhythmias and adrenergic agent use in 65 Norwood patients. The prolonged use of epinephrine in the post-operative period was associated with an increased risk of tachyarrhythmias.Reference McFerson, McCanta and Pan11 Dopamine, norepinephrine, and vasopressin were included as covariates in the study analysis, but the overall association was only reported for epinephrine.
Vasopressin
Vasopressin activates V1 receptors in vascular smooth muscle to activate protein kinase C, increasing intracellular calcium, and producing smooth muscle contraction.Reference Silva and Rosenberg59,Reference Kaplan-Albuquerque and Di Salvo60 Vasopressin may have fewer proarrhythmic effects compared to catecholamines and may be more efficacious in catecholamine-refractory shock.Reference Barrett, Singer and Clapp61 Seven studies of vasopressin met our inclusion criteria, of which 1 was a prospective trial. The dosing of vasopressin ranged from 0.05 to 3 mU/kg/minute. Vasopressin reproducibly increased blood pressure in 127 post-operative cardiac infants and children with refractory shock.Reference Lechner, Hofer, Mair, Moosbauer, Sames-Dolzer and Tulzer19–Reference Burton, Kaufman, Goot and da Cruz21,Reference Lu, Wang, Yang, Li and Yan23 In four out of the five studies, other inotropes were able to be weaned without any adverse effects.Reference Lechner, Hofer, Mair, Moosbauer, Sames-Dolzer and Tulzer19–Reference Burton, Kaufman, Goot and da Cruz21,Reference Lu, Wang, Yang, Li and Yan23,Reference Mastropietro, Davalos, Seshadri, Walters and Delius24 Administration of vasopressin was also correlated with signs of improved systemic perfusion including decreased lactate, increased pH, and decreased fluid requirement.Reference Burton, Kaufman, Goot and da Cruz21,Reference Lu, Wang, Yang, Li and Yan23 In a retrospective study of 37 consecutive infants undergoing the Norwood or arterial switch operation, vasopressin was started de facto rather than as rescue therapy, and was likewise associated with improvements in systemic perfusion including lower lactates, higher cerebral oxygen levels, and decreased fluid requirements compared to historical controls.Reference Alten, Borasino, Toms, Law, Moellinger and Dabal22 No study reported clinically significant hyponatremia with the administration of vasopressin.Reference Lechner, Hofer, Mair, Moosbauer, Sames-Dolzer and Tulzer19–Reference Burton, Kaufman, Goot and da Cruz21,Reference Davalos, Barrett and Seshadri25
Calcium, calcium sensitisers, and cAMP pathway targeting agents
A total of 22 studies investigated calcium or modulators of the calcium cyclic adenosine monophosphate pathway. Calcium infusion, directly and indirectly, increases blood pressure, by way of increasing serum calcium and by cyclic adenosine monophosphate excretion.Reference Murray, Alford and Haney26,Reference Salsburey and Brown62,Reference Hvarfner, Mörlin, Wide and Ljunghall63 A retrospective cohort study on calcium administration in 82 infants found that the administration of parenteral calcium was associated with a decrease in post-operative cardiac arrest compared with historical controls.Reference Murray, Alford and Haney26 The mean ionised calcium level was 1.33 mmol/L in the study group compared to 1.24 mmol/L in the control group.
Levosimendan is a calcium sensitiser, binding to the cardiac troponin C protein and preventing binding of troponin I for sustained cardiac myocyte contraction.Reference Sorsa, Heikkinen and Abbott64–Reference Bowman, Haikala and Paul66 We examined 13 studies on 867 patients focused on the administration of levosimendanReference Ferrer-Barba, Gonzalez-Rivera and Bautista-Hernandez10,Reference Ebade, Khalil and Mohamed16,Reference Amiet, Perez and Longchamp28–Reference Giordano, Palma and Palumbo30,Reference Wang, Cui and Fan32–Reference Basto-Duarte, Flores-Rodriguez, Bermon and Mendoza-Crespo34,Reference Jadhav, Bobhate and Garekar36–Reference Thorlacius, Suominen and Wahlander38 : 7 studies were retrospective, and 6 were prospective randomised controlled trials. The dosing range was 0.05–0.2 µg/kg/minute for 48–72 hours with or without a loading dose of 12–15 µg/kg. The median dose was 0.1 µg/kg/minute. There was mixed data on the effects of levosimendan on heart rate, mean arterial blood pressure, cerebral oxygenation, and lactate level. Overall, three studies showed that levosimendan may be effective at preventing low cardiac output syndrome when used as an adjunct therapy to other vasopressors.Reference Ricci, Garisto, Favia, Vitale, Di Chiara and Cogo27,Reference Amiet, Perez and Longchamp28,Reference Giordano, Palma and Palumbo30
Milrinone inhibits phosphodiesterase type III which increases levels of cyclic adenosine monophosphate and calcium uptake into cells, leading to inotropy and peripheral vasodilation.Reference Farah and Frangakis67–Reference Silver, Lepore and O’Connor70 Milrinone has been studied in 12 prospective and 3 retrospective studies, both as a primary agent and as rescue therapy in children after cardiopulmonary bypass, totaling 1476 patients. Dosing was variable, with a range of 0.25–1.0 µg/kg/minute with or without a loading dose of 20–50 µg/kg. Two studies suggested that milrinone may prevent low cardiac output syndromeReference Duggal, Pratap, Slavik, Kaplanova and Macrae39,Reference Hoffman, Wemovsky and Atz40 ; however, another study measured milrinone levels and found that 16% of treated patients had supratherapeutic levels, which was associated with higher rates of low cardiac output syndrome.Reference Garcia Guerra, Joffe, Senthilselvan, Kutsogiannis and Parshuram41 Milrinone has also been studied in children with pulmonary hypertension, and was found to decrease pulmonary arterial pressure in 2 studies.Reference Barnwal, Umbarkar, Sarkar and Dias42,Reference Chu, Lin and New43
Nine trials on milrinone and levosimendan reported rates of low cardiac output syndrome as an endpoint. In a randomised, double-blind, placebo-controlled trial of 238 post-operative children, milrinone was shown to significantly decrease the risk of low cardiac output syndrome.Reference Hoffman, Wemovsky and Atz40 Milrinone also significantly increased biventricular function in 15 post-operative children who were at risk or developed low cardiac output syndrome.Reference Duggal, Pratap, Slavik, Kaplanova and Macrae39 Levosimendan showed no decrease in low cardiac output syndrome or mortality in a randomised, double-blind, placebo-controlled trial of 187 post-operative children.Reference Wang, Cui and Fan32 However, 6 studies compared levosimendan to milrinone and showed mixed data regarding superiority.Reference Momeni, Rubay and Matta33–Reference Thorlacius, Suominen and Wahlander38 Three studies were randomised controlled trials and 3 were retrospective for a total of 236 patients. There were no consistent differences in inotropic requirements or rates of low cardiac output syndrome between the levosimendan group and milrinone groups, and there was mixed data comparing rates of renal failure with levosimendan versus milrinone.Reference Lechner, Hofer, Mair, Moosbauer, Sames-Dolzer and Tulzer19,Reference Basto-Duarte, Flores-Rodriguez, Bermon and Mendoza-Crespo34,Reference Lechner, Hofer and Leitner-Peneder37,Reference Thorlacius, Suominen and Wahlander38 Nevertheless, five out of the six studies suggested that levosimendan is as safe and effective as milrinone. These studies had wide age variance, as well as variation in cardiac defects.
There is mixed evidence as to whether milrinone is associated with arrhythmias. A double-blind placebo-controlled study of 238 children found no association with arrhythmias, even with a loading dose followed by a high continuous infusion of milrinone at 0.75 µg/kg/minute.Reference Hoffman, Wemovsky and Atz40 Yet, a larger observational study of 603 paediatric patients after cardiac surgery found that milrinone administration is an independent risk factor for arrhythmias.Reference Smith, Owen, Borgman, Fish and Kannankeril44
Nitric oxide and cyclic guanosine monophosphate pathway targeting agents
Modulators of the cyclic guanosine monophosphate pathway have been studied both independently and in comparison to cyclic adenosine monophosphate modulators. Nesiritide is a synthetic brain natriuretic peptide that inhibits the renin–angiotensin aldosterone system and stimulates cyclic guanosine monophosphate, causing vasorelaxation.Reference Song, Kohse and Murad71,Reference Zhou and Fiscus72 There were 2 studies of nesiritide in 106 children. Dosing was variable, with a loading dose followed by infusion up to 0.03 µg/kg/minute. A loading dose of nesiritide decreased mean arterial pressure by 7% in a study of 17 children after cardiac surgery,Reference Simsic, Scheurer and Tobias46 and was found to be equivocal when compared to milrinone in preventing low cardiac output syndrome in 106 children.Reference Costello, Dunbar-Masterson and Allan45 Nitroprusside releases nitric oxide, which increases the formation of cyclic guanosine monophosphate, causing vasorelaxation.Reference Gmeiner, Riedl and Baumgartner73–Reference Kamisaki, Waldman and Murad75 There were two retrospective studies on nitroprusside. Dosing was not prescribed but the mean starting dose was between 1 and 2 µg/kg/minute in these studies. One study found no increase in outcomes of complications compared to phentolamine.Reference Furck, Hansen, Uebing, Scheewe, Jung and Kramer18 In terms of safety, one study found that 11% of patients treated with nitroprusside were found to have toxic levels of cyanide.Reference Moffett and Price47
Discussion
Current knowledge gaps
We compiled data on 37 drug trials in 2856 children across 12 vasoactives and inotropes from 2000 to 2020. Our review found that overall evidence supporting the use of these drugs in children in the post-operative setting, including for the prevention or treatment of low cardiac output syndrome, is limited. The majority of studies were small sample size and underpowered for effect size, less than half were randomised, and safety and efficacy endpoints differed widely, limiting the ability to combine data for meta-analyses.
Only 2856 children were enrolled across all studies, and only 2 studies were multicentre trials. These findings are despite the fact that according to the Society of Thoracic Surgeons database, >22,000 children undergo cardiopulmonary bypass surgery each year, and that by previously published estimates, 90% of post-operative paediatric patients receive inotropes or vasoactives.Reference Loomba and Flores6,Reference Kartha, Jacobs and Vener76 While reasons for the relative lack of studies are likely multifactorial, low consent rates, cost, and current study designs that interfere with the complex and high stakes clinical care delivered in the post-operative setting are likely key drivers.Reference Torok, Li and Kannankeril8,Reference Zimmerman, Gonzalez, Swamy and Cohen-Wolkowiez77 Parental, as well as provider, stress, and anxiety, coupled with children being at significant medical and surgical risk while undergoing invasive procedures, is also likely to reduce consent rates.Reference Thomas and Menon78,Reference Kleiber, Tromp, Mooij, van de Vathorst, Tibboel and de Wildt79 Even for those patients who do get enrolled in a trial, study designs with extensive protocol-specific procedures may result in a large number of protocol deviations, study drop-outs, and decreased participation.Reference Torok, Li and Kannankeril8
When studies are conducted, elements of their design and endpoint selection may contribute to the inability to identify significant efficacy or safety signals. Only 11 of the included studies were designed with a randomised controlled arm, limiting their ability to draw definitive conclusions about efficacy.Reference Fanaroff, Califf and Harrington80 Two studies employed serial recruitment of each cohort, such as the studies investigating the use of afterload reduction in post-operative infants after the Norwood procedure, with time as an inherent confounder in these studies.Reference De Oliveira, Ashburn and Khalid17,Reference Furck, Hansen, Uebing, Scheewe, Jung and Kramer18,Reference Wernovsky, Kuijpers and Van Rossem81 Furthermore, in an attempt to overcome limited enrollment, studies often included different cardiac lesions with variable physiologic states, as was the case for 25/37 studies included in our review. While information from combined populations may be helpful to guide overall practice, significant physiologic differences (e.g., between infants with systemic right versus left ventricles) may induce biases that, if left unadjusted, obscure drug efficacy or safety signals.Reference Miyamoto, Stauffer and Polk82,Reference Garcia, Nakano and Karimpour-Fard83
The selection of consistent, meaningful endpoints remains elusive. Heart rate and blood pressure changes were used as primary endpoints in 12 of the 37 studies. While improvements in these biomarkers are likely of clinical significance, their relatively downstream position in the cardiovascular function cascade may obscure the important effects of studied drugs. Ultimately, clinical endpoints are needed to confirm the efficacy of interventions, but often require very large sample sizes to identify treatment effects in complex populations.84 Under these circumstances, pooling data across studies and conducting meta-analyses may provide additional evidence. Low cardiac output syndrome is an endpoint strongly correlated with clinical outcomes, and was used as a primary or secondary endpoint in 16 of the 37 studies. Unfortunately, these studies used 7 different surrogate markers of low cardiac output, again making it difficult to compare results across studies.Reference Smith-Parrish, Schumacher and Gruppen4
Future directions
Vasoactive and inotropic support are essential components of post-operative care for paediatric patients after cardiopulmonary bypass surgery. Underpowered studies have led to a history of negative trials,Reference Torok, Li and Kannankeril8,Reference Zimmerman, Gonzalez, Swamy and Cohen-Wolkowiez77 and there is a paucity of data for the selection and dosing of vasoactives and inotropes for these patients. As a result, institutional preference rather than evidence-based medicine underlies many of the treatment decisions.Reference Torok, Li and Kannankeril8,Reference Zimmerman, Gonzalez, Swamy and Cohen-Wolkowiez77 Innovations in clinical trials and paediatric drug development programs in other therapeutic areas may hint at solutions to address this knowledge gap.
First, pragmatic trial designs may limit interference with clinical care.Reference Skarsgard84,Reference Kelly, Dyson and Butcher85 The post-operative period is typically a highly monitored environment, in which large amounts of clinical data are collected per routine medical care.Reference Peters, Argent and Festa86 This creates an opportunity for leveraging standard of care physiologic monitoring, laboratory results, and cardiac function assessments (e.g., echocardiograms) in study designs. Wide assessment time windows rather than fixed time points, use of local clinical laboratories for biospecimen quantification, and data collection mechanisms that allow for uploading of care notes, imaging studies and interpretations, and other clinical data may all facilitate leveraging clinical data and minimise the need for study-specific procedures.Reference Lasky, Carleton and Horton87 To alleviate concerns about site-based differences in assessments and result interpretations, a review of diagnostic studies and adjudication of events based on clinical documentation can be performed centrally under strict protocol guidance.Reference Torok, Li and Kannankeril8,Reference Choong, Duffett, Cook and Randolph88 Ultimately, harnessing the vast amounts of data captured in the electronic health record will create opportunities for study designs with drastically reduced and simplified data acquisition and collection mechanisms. With large national efforts underway, clinical trials in children after cardiopulmonary bypass surgery may be an ideal target for such innovative approaches. Similar efforts leveraging data collected from a nationwide clinical registry (as opposed to an electronic health record), sometimes referred to as “trials within the registry,” are currently being conducted in children after cardiac surgery.Reference Hill, Baldwin and Bichel89
In addition to pragmatic trials, strategically designed registries may help support drug development. Again, leveraging existing data collection, or linking electronic health records across institutions into registries, as is done with the National Patient-Centered Clinical Research Network and similar initiatives, may limit the burden of such efforts.Reference Pletcher, Forrest and Carton90–Reference Huser, Kahn, Brown and Gouripeddi95 A comprehensive database of clinical data, drug utilisation, and outcomes would help identify potential signals of efficacy and safety, aid in the design of clinical trials, and, for pragmatically designed trials, could serve as a data collection platform. Efforts to ensure rigorous and high-quality data collection, including adherence to regulatory guidance, where applicable, are essential to maximise the benefit of registries to drug development.Reference Klonoff96 Importantly, the paediatric heart disease community has extensive experience and a track record of success leveraging registries, from the Society of Thoracic Surgeons Congenital Databases to wide-ranging efforts capturing all phases of cardiac care, through most recent efforts to organise and align registries as done through Cardiac Networks United.Reference Jacobs, O’Brien and Pasquali97,Reference Gaies, Anderson and Kipps98
Finally, careful attention to individual clinical trial considerations, including simplified informed consent and electronic consent forms, incorporation of screening efforts to facilitate early consenting, and extensive education and training of site staff may improve enrollment rates and facilitate trial execution.Reference Torok, Li and Kannankeril8,Reference Thomas and Menon78 Standardised endpoints and definitions, such as for low cardiac output syndrome, will improve the ability for meta-analysis. Most significantly, efforts to engage patients and their families in the design and conduct of clinical trials can significantly impact both study quality and participation.Reference Thomas and Menon78
In conclusion, knowledge gaps remain in the use of vasoactives and inotropes in post-operative paediatric cardiac care, but numerous recent innovations create opportunities to rethink the conduct of clinical trials in this high-risk population.
Acknowledgements
The authors are grateful for the support received from the Duke University Medical Center Library while performing the literature search.
Financial support
Dr King was supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award Number T32GM086330. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflicts of interest
CE King reports no relevant disclosures.
EJ Thompson reports no relevant disclosures.
HP Foote reports no relevant disclosures.
KO Zimmerman reports no relevant disclosures.
KD Hill reports no relevant disclosures.
R Chamberlain reports no relevant disclosures.
CP Hornik receives salary support for research from the National Institute for Child Health and Human Development (NICHD) (1K23HD090239), the National Heart Lung and Blood Institute (NHLBI) (R61/R33HL147833), the United States of America Food and Drug Administration (1R01-FD006099, PI Laughon; and 5U18-FD006298, PI: Benjamin), the United States Government for his work in paediatric clinical pharmacology (Government Contract HHSN275201800003I, PI: Benjamin under the Best Pharmaceuticals for Children Act), the non-profit Burroughs Wellcome Fund, and other sponsors for drug development in adults and children (https://dcri.org/about-us/conflict-of-interest/).
Appendix
Search strategy:
Databases: EMBASE, EndNote
((((randomized controlled trial[pt] OR controlled clinical trial[pt] OR randomized[tiab] OR randomised[tiab] OR randomization[tiab] OR randomisation[tiab] OR placebo[tiab] OR drug therapy[sh] OR randomly[tiab] OR trial[tiab] OR groups[tiab] OR Clinical trial[pt] OR “clinical trial”[tiab] OR “clinical trials”[tiab] OR “evaluation studies”[Publication Type] OR “evaluation studies as topic”[MeSH Terms] OR “evaluation study”[tiab] OR evaluation studies[tiab] OR “intervention study”[tiab] OR “intervention studies”[tiab] OR “case-control studies”[MeSH Terms] OR “case-control”[tiab] OR “cohort studies”[MeSH Terms] OR cohort[tiab] OR “longitudinal studies”[MeSH Terms] OR “longitudinal”[tiab] OR longitudinally[tiab] OR “prospective”[tiab] OR prospectively[tiab] OR “retrospective studies”[MeSH Terms] OR “retrospective”[tiab] OR “follow up”[tiab] OR “comparative study”[Publication Type] OR “comparative study”[tiab] OR systematic[subset] OR “meta-analysis”[Publication Type] OR “meta-analysis as topic”[MeSH Terms] OR “meta-analysis”[tiab] OR “meta-analyses”[tiab]) NOT (Editorial[ptyp] OR Letter[ptyp] OR Case Reports[ptyp] OR Comment[ptyp]) NOT (animals[mh] NOT humans[mh])) AND (”2000/01/01”[PDat] : “3000/12/31”[PDat]) AND English[lang])) AND ((((((drug therapy[sh] OR diuretics[mesh] OR diuretics [Pharmacological Action] OR diuretic[tiab] OR diuretics[tiab] OR anticoagulants[mesh] OR Anticoagulants [Pharmacological Action] OR anticoagul*[tiab] OR “thrombin inhibitors”[tiab] OR “thrombin inhibitor”[tiab] OR “Immunosuppressive Agents”[mesh] OR immunosuppression[mesh] OR “Immunosuppressive Agents”[Pharmacological Action] OR immunosuppressive OR immunosuppressants[tiab] OR immunosuppressant[tiab] OR immunosuppression[tiab] OR immunosuppressions[tiab] OR steroids[mesh] OR steroid*[tiab] OR analgesics[mesh] OR analgesics[Pharmacological Action] OR analgesic[tiab] OR analgesics[tiab] OR anesthetics[mesh] OR “Anesthetics”[Pharmacological Action] OR anesthetic[tiab] OR anesthetics[tiab] OR “Vasodilator Agents”[Mesh] OR “Vasodilator Agents”[Pharmacological Action] OR vasodilator[tiab] OR vasodilators[tiab] OR vasorelaxants[tiab] OR vasorelaxant[tiab] OR “vasoactive antagonists”[tiab] OR “vasoactive antagonist”[tiab] OR “Cardiotonic Agents”[Mesh] OR “Cardiotonic Agents”[Pharmacological Action] OR cardiotonic[tiab] OR inotrope*[tiab] OR “cardiac stimulants”[tiab] OR “cardiac stimulant”[tiab] Or cardiotonic*[tiab] OR “myocardial stimulant*”[tiab] OR “cardioprotective agent*”[tiab] OR “Hypoglycemic Agents”[mesh] OR “Hypoglycemic Agents”[Pharmacological Action] OR “hypoglycemic agent*”[tiab] OR antihyperglycemic*[tiab] OR “hypoglycemic drug*”[tiab] OR antidiabetic*[tiab])) AND ((”Cardiopulmonary Bypass”[Mesh]) OR (”Cardiac Surgical Procedures”[Mesh] OR “cardiac surgery”[tiab] OR “heart surgery”[tiab] OR “cardiopulmonary bypass”[tiab]))) AND (”postoperative period”[mesh] OR “Postoperative Care”[mesh] OR “postoperative complications”[mesh] OR postoperative[tiab])) AND ((”Pediatrics”[Mesh] OR pediatric[tiab] OR pediatrics[tiab] OR paediatric[tiab] OR paediatrics[tiab] OR juvenile[tiab] OR juveniles[tiab] OR “Infant”[Mesh] OR infant[tiab] OR infants[tiab] OR infantile[tiab] OR “Child”[Mesh] OR child[tiab] OR children[tiab] OR childhood[tiab] OR preadolescent[tiab] OR preadolescents[tiab] OR prepubescent[tiab] OR “Adolescent”[Mesh] OR adolescent[tiab] OR adolescents[tiab] OR youth[tiab] OR youths[tiab] OR teenager[tiab] OR teenagers[tiab] OR teenaged[tiab] OR teen[tiab] OR teens[tiab]) NOT (”Adult”[Mesh] NOT (”Adolescent”[Mesh] OR “Child”[Mesh] OR “Infant”[Mesh])))) AND (“2000/01/01”[PDat] : “3000/12/31”[PDat])AND English[lang])
Search strategy:
Databases: PubMed (MEDLINE)