I. INTRODUCTION
Green earths belong to the group of pigments used worldwide since antiquity. They were used by monks in the Ajanta Caves in India and by Amerindian artists (Feller, Reference Feller1986). They are often found in medieval paintings and frescoes. They were found among the very first collections of pigments in pots in the ruins of Pompeii and were extensively used in Pompeii wall paintings (Duran et al., Reference Duran, Castaing and Walter2008). What is surprising, green earths were found in decorations of Egyptian sarcophagi (Calza et al., Reference Calza, Anjos, Mendonca de Souza, Brancaglion and Lopes2007). In medieval times, Italian artists used green earths as a background for gilt ornaments. It was also Vermeer’s favourite green pigment and was present in some of his paintings.
Green earths were often applied due to their high colouring capacity, stability, and availability. In the past the pigments were obtained by mining. The most famous deposits of green earths could be found near Verona (Italy) as well as in Tyrol, Bohemia, Saxony, Poland, Hungary, France, Cyprus, and England.
Historical green earths are considered to be composed of one of the two clay micas: celadonite or glauconite. These minerals have complex chemical structures. In general their chemical formulae can be written as K(Mg, Fe2+)(Fe3+, Al)[Si4O10](OH)2 for celadonite and (K, Na)(Fe3+,Al,Mg)2(Si,Al)4O10(OH)2 for glauconite. Although the chemical compositions of these minerals are similar, they are formed under different geological conditions. Glauconite is found in the form of small green pellets in marine sedimentary rocks, whereas celadonite is found in amygdules or fractures in metamorphic rocks.
In the literature, one can find many variations of the chemical compositions of green earths. Both compounds crystallize in monoclinic system, and in Powder Diffraction File PDF-4+ (2008) one can find nine entries for celadonite and ten for glauconite.
Both minerals produce colors that vary from cold bluish greens to warmer yellow and olive hues. In many cases the color is determined by the specific ratio of divalent and trivalent irons in their structures. Besides celadonite and glauconite, smectite group minerals, chlorites, and serpentine group minerals can also be responsible for the green color of this pigment. In the case of smectites and chlorites the hue may be altered by minor goethite admixtures (Hradil et al., Reference Hradil, Grygar, Hradilova and Bezdicka2003).
While there have been numerous studies on clayey minerals, mineralogical studies are rare in the field of cultural heritage; light microscopy, a technique often used in the examination of art objects, is limited to identification of the generic class “green earths” without specific characterization of their mineralogical composition (Hradil et al., Reference Hradil, Grygar, Hruskova, Bezdicka, Lang, Schneeweiss and Chvatal2004). Although green earths were most popular in the past, they are still being used to create artworks and in the restoration of old masters’ paintings and they are still available on the market.
The aim of this study was to determine the mineralogical compositions of artistic, historical, commercially available pigments, mainly from Kremer pigments (and also from other suppliers), by means of X-ray powder diffractometry. We have studied the following pigments: Bohemian, Bavarian, German green earths, green earth from Thuringen, France, Terre Verte, and Verona green.
II. EXPERIMENTAL
Laboratory powder X-ray diffractometry is a commonly used technique for phase identification in geology, physics, chemistry, and materials sciences. This technique has proved
TABLE I. Powder diffraction data and mineralogical composition of Bohemian green earth.
useful in researching layers of painting on canvas, wall paintings, and polychromes on wood. X-ray diffraction, especially X-ray microdiffraction or synchrotron X-ray diffraction, is an indispensable method of distinguishing inorganic pigments of different natural provenances and revealing degradation products (Dooryhée et al., Reference Dooryhée, Anne, Bardies, Hodeau, Martinetto, Rondot, Salomon, Vaughan and Walter2005; Welcomme et al., Reference Welcomme, Walter, Bleuet, Hodeau, Dooryhee, Martinetto and Menu2007). One of the main advantages of this technique is its nondestructive nature and there is also no need for special sample preparation. For these reasons this technique is used more and more often in the field of cultural heritage investigations (Simova et al., Reference Simova, Bezdicka, Hradilova, Hradil and Grygar2005; Bugoi et al., Reference Bugoi, Constantinescu, Pantos and Popovici2008).
All measurements in the presented research were carried out with the use of an X’PERT MPD diffractometer; Cu Kα radiation at 40 kV and 30 mA, a graphite monochromator, and a scintillation or X’Celerator detectors were used. Pigments bought from Kremer or received from the collection of Jan Matejko Academy of Fine Art in Krakow were powdered in an agate mortar and then back loaded into a specimen holder. All measurements were performed at room temperature in 2θ range 5° to 80° with a step size of 0.02°.
III. RESULTS AND DISCUSSION
Powder diffraction patterns obtained in this study were interpreted with the use of diffractometer software and PDF-2 or PDF-4 databases. In the first stage of the analysis we looked for the presence of glauconite or celadonite, but, as is shown below, we have found many different minerals in the investigated pigments, and it is worth emphasizing that in some samples neither glauconite nor celadonite was found. Powder diffraction data for each investigated sample, with an indication of its mineralogical composition, are presented in Tables I–VII.
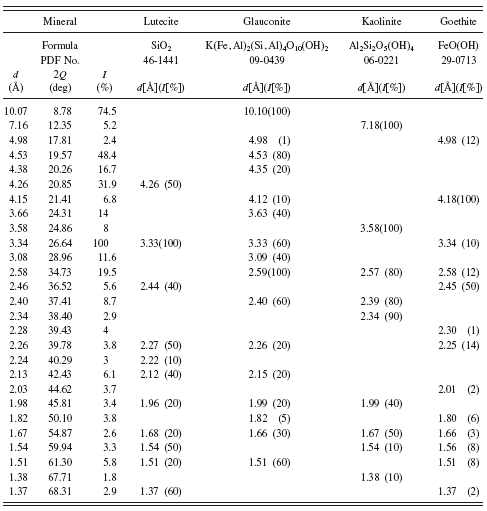
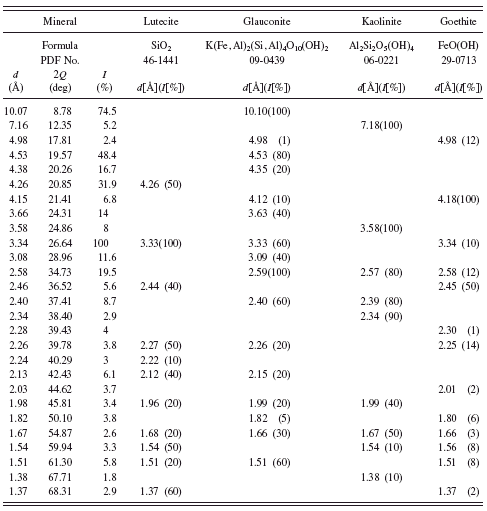
The results of XRD investigations of Bohemian green earth (Kremer catalogue No. 40 810) and green earth from Thuringen shown in Tables I and II indicate that these pigments contain glauconite. Minute amounts of celadonite (see Table III) were found in Bavarian green earth (Kremer catalogue No. 11 100).
Pale yellowish German green earth (Kremer catalogue No. 40 800) is a natural green earth according to the chemical description given by the supplier. Analysis of its powder diffraction pattern shows that among five minerals found in the sample, volborthite (copper vanadium oxide hydroxide hydrate, a mineral crystallizing in monoclinic system) is responsible for its greenish-yellow color (see Table IV). This pigment was investigated earlier by means of IR and Raman spectroscopies (Ospitali et al., Reference Ospitali, Bersani, Di Lonardo and Lottici2008). The authors report that the pigment consists of illite, goethite, haematite, quartz, and
TABLE II. Powder diffraction data and mineralogical composition of green earth from Thuringen.

TABLE III. Powder diffraction data and mineralogical composition of Bavarian green earth.
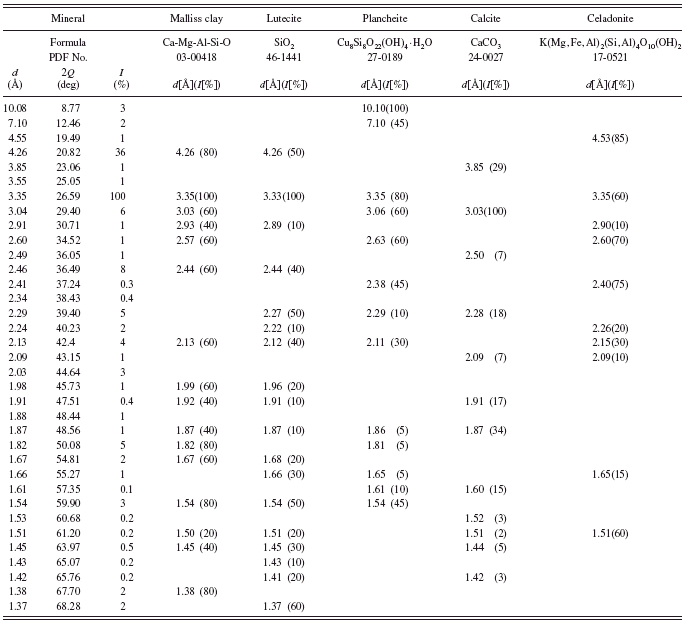
TABLE IV. Powder diffraction data and mineralogical composition of German green earth.

anatase. Our results confirmed the presence of illite and SiO2 but in the form of lutecite. It is worth noting that X-ray diffractometry is a technique, which easily enables detection of different polymorphs of the same compound. In the case of TiO2, in the investigated sample we found rutile not anatase. This fact indicates that X-ray powder diffractometry is a technique, which can be applied when differentiation between compounds with the same or similar chemical compositions is of importance in the elucidation of the history of an investigated object.
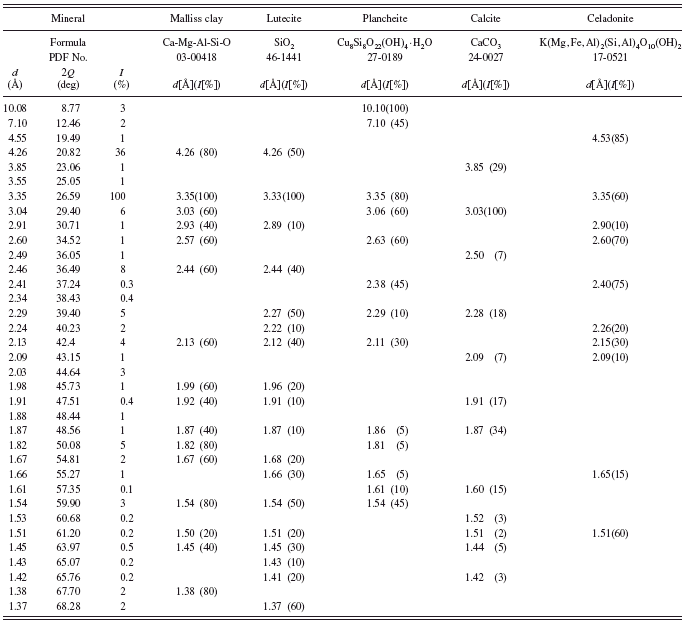
A pale green earth from France (Kremer catalogue No. 40 830) is also a natural green earth, also known as pigment green 23 according to Kremer’s chemical description. Analysis of its powder diffraction pattern (see Table V) shows that it consists of several minerals among which none is green. Raman and IR spectroscopies’ results reported by Ospitali et al. (Reference Ospitali, Bersani, Di Lonardo and Lottici2008) indicated that a synthetic organic colorant named Pigmosol is responsible for the green color of this pigment.
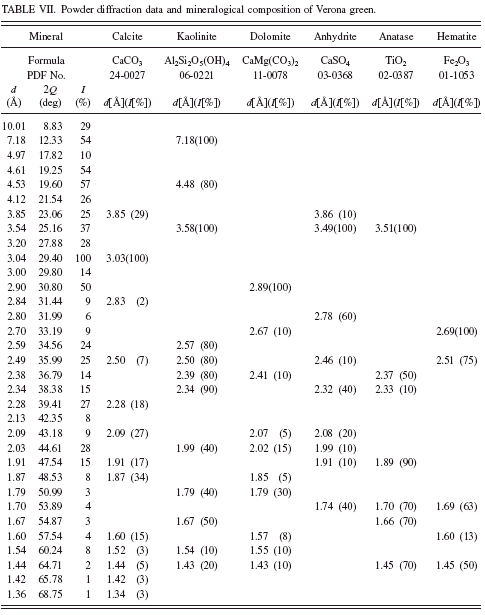
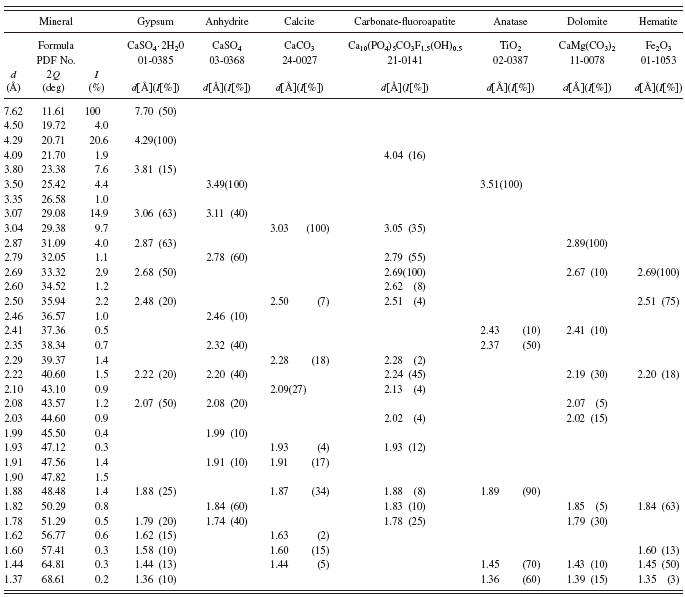
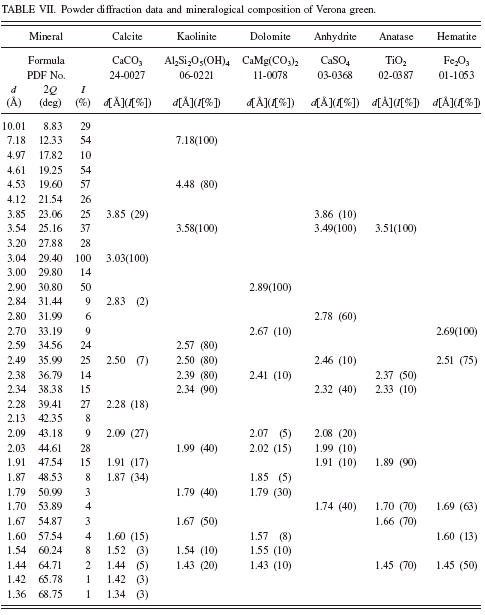
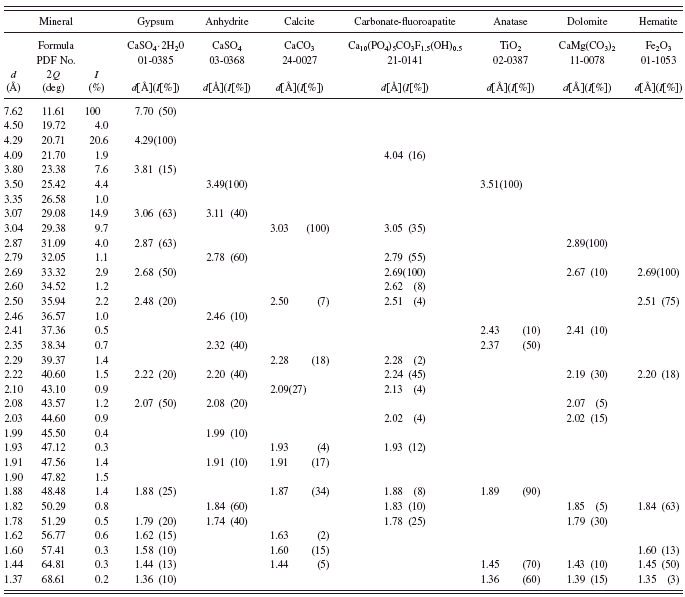
In green earth Terre Verte sold by Winsor & Newton andVerona green (Kremer catalogue No. 11 010) we have not found any minerals, which could be responsible for their green color (see Tables VI and VII). It is known from the literature that Terre Verte may contain some chromium oxide, cobalt-aluminum oxide, or chlorinated copper phthalocyanine which makes the pigment green (Hradil et al., Reference Hradil, Grygar, Hruskova, Bezdicka, Lang, Schneeweiss and Chvatal2004).
In the history of painting, green earth from Verona was considered as the best among green earths. The mine from which the pigment came from was operational until World War II. According to Pigment Compendium, celadonite was the main component of green earth from Verona (Eastaugh et al., Reference Eastaugh, Walsh, Chaplin and Siddall2008). Nowadays one can find numerous green pigments with “Verona” in the name, but their chemical composition may be far from the original green earth from Verona. In the investigated sample we have detected no celadonite or glauconite at all. Investigations of Verona green carried out by Hradil et al. (Reference Hradil, Grygar, Hruskova, Bezdicka, Lang, Schneeweiss and Chvatal2004) also failed to detect celadonite. In the investigated sample they identified plagioclase and Fe-rich smectite.
TABLE V. Powder diffraction data and mineralogical composition of green earth from France.
IV CONCLUSIONS
X-ray diffraction investigations of various green earth pigments enabled the establishment of their mineralogical compositions. The results show that among numerous pigments labeled by suppliers as green earths, only two (Bohemian green earth and green earth from Thuringen) contain glauconite. In Bavarian green earth small amounts of celadonite were detected. Other investigated pigments exhibit various mineralogical compositions but other minerals (e.g., volborthite) or, as is known from the literature, added synthetic colorants are responsible for their green color. Moreover, in the case of commercially available green earths, the geographical places in their names often have no relation with the mineralogical compositions of the green earth pigments from the historical localities. The presented results prove that X-ray powder diffractometry is a valuable complementary technique of chemical characterization of pigments. This technique can be also successfully applied in the investigation of other materials in area of art and archaeology.
TABLE VI. Powder diffraction data and mineralogical composition of green earth Terre Verte.

TABLE VII. Powder diffraction data and mineralogical composition of Verona green.