Introduction
Facial expressions are essential for social communication as they provide information concerning emotional states and intentions of others. Depression has been considered a disorder of emotion and its regulation (Gotlib et al. Reference Gotlib, Sivers, Gabrieli, Whitfield-Gabrieli, Goldin, Minor and Canli2008). A high prevalence of depressive and anxiety disorders has been observed in primary care settings (Hirschfeld, Reference Hirschfeld2001). Moreover, 63% of patients with panic disorder and 35% of patients with social phobia were reported to have at least one episode of major depression (Stein et al. Reference Stein, Tancer, Gelernter, Vittone and Uhde1990).
Both depression and anxiety disorders have been associated with neurophysiological abnormalities regarding emotion perception (Bishop, Reference Bishop2007; Fitzgerald et al. Reference Fitzgerald, Laird, Maller and Daskalakis2008). In depressed patients, stronger amygdala activation has been reported in response to negative emotional stimuli (Sheline et al. Reference Sheline, Barch, Donnelly, Ollinger, Snyder and Mintun2001; Anand et al. Reference Anand, Li, Wang, Wu, Gao, Bukhari, Mathews, Kalnin and Lowe2005; Fales et al. Reference Fales, Barch, Rundle, Mintun, Snyder, Cohen, Mathews and Sheline2008; Savitz & Drevets, Reference Savitz and Drevets2009). It has also been suggested that depressed patients have a sustained amygdala response during processing of negative emotional stimuli, compared to healthy volunteers (Siegle et al. Reference Siegle, Steinhauer, Thase, Stenger and Carter2002). In response to positive stimuli, ventral striatum hypoactivation was reported in depressed patients compared to controls, and it was suggested that lack of ventral striatum activation may reflect anhedonia, that is the reduced capacity to experience pleasure (Epstein et al. Reference Epstein, Pan, Kocsis, Yang, Butler, Chusid, Hochberg, Murrough, Strohmayer, Stern and Silbersweig2006).
With regard to anxiety disorders, amygdala hyper-responsiveness has been reported to negative facial expressions (Stein et al. Reference Stein, Goldin, Sareen, Zorrilla and Brown2002; Campbell et al. Reference Campbell, Sareen, Paulus, Goldin, Stein and Reiss2007; Etkin & Wager, Reference Etkin and Wager2007), whereas other studies reported amygdala hyperactivation to neutral (Birbaumer et al. Reference Birbaumer, Grodd, Diedrich, Klose, Erb, Lotze, Schneider, Weiss and Flor1998; Cooney et al. Reference Cooney, Atlas, Joormann, Eugne and Gotlib2006) or positive (Straube et al. Reference Straube, Mentzel and Miltner2005) facial expressions. Therefore, it is not clear whether amygdala hyper-responsiveness in anxiety disorders is specific to threat-related stimuli and/or to positive and ambiguous facial expressions.
In addition to these abnormalities observed during emotion processing, Mayberg (Reference Mayberg1997) hypothesized that hypoactivation in dorsal neocortical areas (anterior and posterior cingulate, prefrontal, premotor and parietal cortex) and hyperactivation in ventral paralimbic areas (subgenual cingulate, anterior insula, hypothalamus and caudate) may characterize depression. Phillips et al. (Reference Phillips, Drevets, Rauch and Lane2003) proposed a model that is consistent with that of Mayberg (Reference Mayberg1997) but is more comprehensive, involving deficient corticolimbic interactions in depression. Specifically, depression is assumed to be associated with hyperactivation of limbic regions responsible for emotion identification and generation of emotional behavior, including the subgenual cingulate gyrus, ventrolateral prefrontal cortex (PFC), amygdala, anterior insula, ventral striatum and thalamus, and hypoactivation of dorsal regions, important for emotion regulation, including the dorsomedial PFC and dorsolateral PFC (DLPFC) (Phillips et al. Reference Phillips, Drevets, Rauch and Lane2003). In anxiety disorders, Etkin & Wager (Reference Etkin and Wager2007) concluded that hyperactivation of the amygdala and insula may constitute a common pathway in social anxiety disorders and specific phobia.
Emotion-dependent abnormal amygdala activation has thus been reported in both depression and anxiety disorders, although mainly in reaction to syndrome-specific emotional stimuli. Taken together, it seems that there are both distinct and common neural substrates underlying processing of various emotional information in depression and anxiety disorders. We would therefore expect the presence of depression and anxiety diagnoses to have a differential impact on the neural response to emotional stimuli. However, no study to date has focused on emotion processing in depression and anxiety, while explicitly controlling for their co-morbidity.
The present study is part of the Netherlands Study of Depression and Anxiety (NESDA), a multisite cohort study aimed to provide an insight into the long-term course of depression and anxiety disorders in patients selected from primary care and mental health organizations (Penninx et al. Reference Penninx, Beekman, Smit, Zitman, Nolen, Spinhoven, Cuijpers, De Jong, Van Marwijk, Assendelft, Van Der Meer, Verhaak, Wensing, De Graaf, Hoogendijk, Ormel and Van Dyck2008). Hence, the aim of the present study was to identify the areas involved in perception of facial expressions of emotion in large community-based samples of out-patients with depression and/or anxiety relative to healthy participants using a dimensional approach. We further tested for differences in the temporal amygdala response to facial expressions between groups. Out-patients with anxiety-depression co-morbidity were included to investigate the possible implications of co-morbidity on the neural mechanisms involved in emotion perception.
Based on the literature, we hypothesized that amygdala hyperactivation would occur in response to negative emotional expressions in depressed out-patients compared with healthy controls. In anxiety disorders, amygdala hyperactivation would be expected in response to angry, fearful and neutral faces.
Method
Participants
The present work is a multicenter study that involved University Medical Center Groningen (UMCG), Amsterdam Medical Center (AMC) and Leiden University Medical Center (LUMC). This study was approved by the Ethical Review Boards of each participating center. Participants were selected from the NESDA (Penninx et al. (Reference Penninx, Beekman, Smit, Zitman, Nolen, Spinhoven, Cuijpers, De Jong, Van Marwijk, Assendelft, Van Der Meer, Verhaak, Wensing, De Graaf, Hoogendijk, Ormel and Van Dyck2008) . After receiving written information, each participant gave written informed consent. Participants did not receive any compensation for their participation in this study.
Exclusion criteria were: a diagnosis of DSM-IV (APA, 1994) Axis I disorders other than major depression disorder (MDD), social phobia, panic disorder and generalized anxiety disorder, such as psychotic disorder or dementia, current alcohol or substance abuse, presence or history of major internal and neurological disorder with potential central nervous system sequelae; current use of beta-blockers; hypertension >180/130 mmHg; age over 57 years; and magnetic resonance imaging (MRI)-incompatible implants or tattoos.
We included 68 out-patients with MDD, 61 out-patients with anxiety disorders (Anx; with panic disorder with/without agoraphobia, general anxiety disorder and/or social phobia), 78 out-patients with depression-anxiety co-morbidity (DAC) and 60 healthy controls (HC). All diagnoses were made prior to the scanning session by trained clinical staff on the basis of the CIDI, lifetime version 2.1 (Andrews & Peters, Reference Andrews and Peters1998), in accordance with DSM-IV criteria. The HC had never met the criteria for any DSM-IV disorder. Functional MRI (fMRI) data from four Anx patients, nine MDD patients, 12 DAC patients and four HC participants were discarded because of technical problems during scanning, such as head movement artifacts (>3 mm on any axis) or incomplete coverage of the temporal lobe. Table 1 presents the demographic characteristics of the samples. The groups were matched on age [F(3, 234)=1.71, p=0.15], gender [χ2(3)=2.94, p=0.40] and handedness [χ2(3)=0.08, p=0.99] but not on years of education [F(3, 234)=13, p<0.05]. Post-hoc tests using Bonferroni correction (αcrit=0.0167) indicated that HC had significantly longer education than MDD [t(113)=4.76, p<0.001], Anx [t(111)=3.45, p=0.001] and DAC [t(120)=6.322, p<0.001] patients.
Table 1. Demographic and clinical characteristics of the groups and behavioral data

n, Number of participants; HC, healthy controls; MDD, major depressive disorder; Anx, anxiety disorder; DAC, depression-anxiety co-morbidity; SSRI, selective serotonin reuptake inhibitor; BAI, Beck Anxiety Inventory; FQ, Fear Questionnaire; MADRS, Montgomery–Asberg Depression Rating Scale; s.d., standard deviation.
Fifty-four patients were using selective serotonin reuptake inhibitors (SSRIs): citalopram 20–60 mg (16 patients), paroxetine 20 mg (30 patients), sertraline 50 mg (two patients), fluoxetine 20 mg (three patients) and fluvoxamine 50–100 mg (three patients). Ten patients were using the serotonin–norepinephrine reuptake inhibitor (SNRI) venlafaxine 75–225 mg. Three patients used benzodiazepines infrequently (three times two tablets weekly, or within 48 h prior to the scanning): oxazepam 40 mg (two patients) and diazepam 20 mg (one patient).
On the day of scanning, and before the scanning session, all participants were evaluated by means of a battery of standardized questionnaires and structured interviews, including the Montgomery–Asberg Depression Rating Scale (MADRS; Montgomery & Asberg, Reference Montgomery and Asberg1979), the Beck Anxiety Inventory (BAI; Beck et al. Reference Beck, Epstein, Brown and Steer1988) and the Fear Questionnaire (FQ; Marks & Mathews, Reference Marks and Mathews1979).
Faces paradigm
The paradigm used in the present study was based on the event-related emotional paradigm used by Wolfensberger et al. (Reference Wolfensberger, Veltman, Hoogendijk, Boomsma and de Geus2008) . Color photographs of angry, fearful, sad, happy and neutral facial expressions, in addition to a control condition consisting of scrambled faces, were presented to all participants. The photographs were selected from the Karolinska Directed Emotional Faces System (Lundqvist et al. Reference Lundqvist, Flykt and Ohmann1998), representing standardized facial expressions of emotions expressed by amateur actors. Twenty-four stimuli were selected for each of five facial expressions, comprising 12 female and 12 male faces. Each face was not presented more than four times. The control condition (scrambled faces) was presented 80 times. The experimental paradigm was presented using E-prime software (Psychological Software Tools, USA). To reduce anticipatory effects, an event-related design was used that involved a pseudo-random presentation of a total of 200 stimuli against a black background. Each photograph was shown on the screen for 2.5 s, with an interstimulus (black screen) interval varying between 0.5 and 1.5 s. The images were projected onto a translucent screen at the end of the scanner bed, visible by using a mirror above the participant's head. Participants were instructed to indicate each face's gender by pressing one of two buttons with the index finger of the left or right hand on two magnet-compatible button boxes. During the presentation of scrambled faces, participants had to press left or right buttons in conformity with the instruction presented on the screen (i.e. an arrow pointing to the left or to the right). The reaction time was recorded. The Faces paradigm was administered as part of a functional scanning session involving a planning task, a memory task and a resting state scan, the results of which will be reported elsewhere.
MRI data acquisition
Images were acquired on a Philips Intera 3-T MR scanner. SENSE-8 (UMCG and LUMC) and SENSE-6 (AMC) channel head coils were used for radio frequency transmission and reception. For each participant a series of 310 echo planar imaging (EPI) volumes, sensitive to the blood oxygenation-level dependent (BOLD) effect, were obtained, entailing a T2*-weighted gradient echo sequence [repetition time (TR)=2300 ms, echo time (TE)=28.0 ms at UMCG and TE=30.0 ms at AMC and LUMC, flip angle 90°] using axial whole-brain acquisition, with an interleaved slice acquisition order. The EPI volumes were acquired at 39 slices at UMCG and 35 slices at AMC and LUMC (0 mm gap, 3 mm thickness). The matrix sizes were 64×64 voxels at UMCG and 96×96 voxels at AMC and LUMC. The in-plane resolution was 3×3 mm at UMCG and 2.29×2.29 mm at AMC and LUMC. The images were acquired parallel to the anterior–posterior commissure plane. A T1-weighted anatomical MRI was also acquired for each subject (TR=9 ms, TE=3.5 ms, matrix size 256×256, voxel size 1×1×1 mm).
Data analysis
Performance on the faces task and psychometric data were analyzed in SPSS version 16.0 (SPSS Inc., USA) using the appropriate parametric (F) or non-parametric (χ2) test. Reaction times (averaged per subject) in the baseline condition were subtracted from those in the five emotional conditions, followed by a condition×group repeated-measures (RM) analysis of covariance (ANCOVA), with centers, age and years of education included as covariates. In case a significant effect was identified, post-hoc tests were conducted using Bonferroni correction for multiple comparisons.
Functional imaging data were pre-processed and analyzed using Statistical Parametric Mapping software (SPM5) implemented in Matlab version 7.1.0 (The MathWorks Inc., USA). Before pre-processing, manual origin setting was performed to the anterior commissure on the EPI volumes. Temporal and spatial correction of the data included slice timing correction, spatial realignment to the first image, co-registration between the anatomical and mean EPI images, spatial normalization to the standard Montreal Neurological Institute (MNI), resampling into a 3×3×3 mm grid, and spatial smoothing using a Gaussian kernel (8 mm full-width at half-maximum).
To remove low-frequency temporal noise, a high-pass filter was applied, with a cut-off of 128 s, to the fMRI time-series. A canonical hemodynamic response function (HRF), with the temporal derivative (TD) and the dispersion derivative (DD) (Friston et al. Reference Friston, Fletcher, Josephs, Holmes, Rugg and Turner1998), was used in a general linear model and parameter estimates were generated for each voxel, for each condition. For each subject, the following contrasts were computed: angry>scrambled, fearful>scrambled, sad>scrambled, happy>scrambled and neutral>scrambled.
A whole-brain analysis using a condition (angry, fearful, sad, happy, neutral)×group (HC, MDD, Anx, DAC) RM ANCOVA was conducted, with factor condition specified as the within-subject factor and with centers, age and education (years) added as nuisance factors. The main effect of condition is reported at a threshold of p<0.05 corrected for false discovery rate (FDR). The interaction effects (condition×group) were inspected at p<0.001 (uncorrected) and the clusters surviving p<0.05 corrected are reported.
To test for effects of medication on the neural response to facial expressions, further analysis was performed excluding medicated patients. A total of 176 participants were included in this analysis (56 HC, 45 MDD, 39 Anx, 36 DAC) using a 5×4 RM ANCOVA as described earlier. An additional RM ANCOVA was performed to test for differences between 62 medicated (14 MDD, 18 Anx, 30 DAC) and 120 unmedicated patients.
To identify brain regions associated with illness severity, regression analyses were performed within each group of patients using MADRS, BAI and FQ scores as regressors, and with centers, age and education (years) specified as nuisance factors. All three regressors were modeled simultaneously and the explained variance was then investigated for each one separately, thus correcting for the other two. The clusters surviving p<0.05 corrected value are reported.
The temporal profile of amygdala activation was investigated using a region of interest (ROI) approach, with an amygdala anatomical mask (Palmen et al. Reference Palmen, Durston, Nederveen and Van Engeland2006). For each subject, within each region, β values (HRF, TD and DD), were extracted using MarsBar (Brett et al. Reference Brett, Anton, Valabregue and Poline2002). The mean and standard deviation (s.d.) of the hemodynamic response shape within each group and for each condition were reconstructed and plotted for visual inspection. Furthermore, for each subject and for each response curve, the maximum amplitude and the corresponding time point of the peak amygdala response were calculated in the HRF and imported into SPSS version 16.0. Group effects on these parameters were investigated with non-parametric tests [Kruskal–Wallis (H) and Mann–Whitney (U) as post-hoc test].
Results
Characteristics of the groups
The results of the demographic and psychometric assessments of the participants are shown in Table 1. A significant group effect was present for MADRS [F(3, 231)=58.02, p<0.001], BAI [F(3, 209)=52.01, p<0.001] and FQ [F(3, 223)=37.01, p<0.001] scores. Post-hoc tests using Bonferroni correction (αcrit=0.0167) indicated that DAC patients scored significantly higher on MADRS compared to MDD [t(122)=5.47, p<0.001] and Anx [t(119)=5.54, p<0.001]. Depressed patients had mild-to-moderate depressive symptoms (MADRS score between 9 and 34; Müller et al. Reference Müller, Szegedi, Wetzel and Benkert2000). Anx patients showed greater anxiety severity compared with MDD [BAI: t(112)=3.93, p<0.001, FQ: t(107)=4.64, p<0.001]. Patients with DAC scored significantly higher on BAI and FQ compared with Anx [BAI: t(119)=2.54, p=0.012] and MDD [BAI: t(121)=7.58, p<0.001, FQ: t(118)=5.40, p<0.001].
No group [F(3, 221)=1.453, p=0.228] or condition effect [F(4, 884)=1.140, p=0.336] was found on reaction times to facial expressions versus baseline. The mean reaction times for each condition within each group are presented in Table 1.
Imaging data
Main effect of task within groups
Viewing facial expressions (>scrambled faces) elicited fusiform gyrus activation within each group of participants (p<0.05, FDR corrected; Supplementary online material S1). Amygdala activation to facial expressions (>scrambled) was found in MDD, DAC patients and HC (p<0.05, FDR corrected, Fig. 1, Supplementary material S1). In anxiety patients, amygdala activation to facial expressions (>scrambled) was not found at p<0.05 FDR corrected (but it was present at uncorrected p<0.005: right Z=2.59, left Z=2.64). Additionally, a conjunction analysis was performed to test whether the main effect of task within each group was consistently high and jointly significant. This analysis revealed significant (p FDR <0.05) activation in bilateral fusiform gyrus (right: x=39, y=−51, z=−21 and left: x=−39, y=−51, z=−21), right middle frontal gyrus (x=51, y=33, z=18) and bilateral amygdala (right: x=21, y=−6, z=−15 and left: x=−21, y=−12, z=−15).

Fig. 1. Main effect of viewing photographs of faces (>scrambled) within each group [False Discovery Rate (FDR) p<0.05]. Main activations were in the fusiform gyrus and amygdala. Color bar indicates t value. HC, healthy; MDD, major depression; Anx, anxiety disorder; DAC, depression-anxiety co-morbidity. (See also Supplementary Table S1, online.)
Condition×group interaction
A significant group×condition interaction effect was found in the right superior frontal gyrus (x=18, y=33, z=30, Z=6.28) and left cingulate gyrus (x=−12, y=−18, z=33, Z=4.29).
Between-group comparison
No significant differences in the neural response to negative (angry, fearful or sad) faces versus scrambled were found between MDD and HC. Patients with MDD showed right superior frontal gyrus extending into middle frontal gyrus hyperactivation in response to happy (>scrambled) faces, compared with HC (Fig. 2 a, Table 2).

Fig. 2. Group differences at p<0.05 corrected cluster-level. (a) Right frontal cortex activation to happy facial expressions (>scrambled) in MDD compared to HC (red)/Anx (blue)/DAC (green). White represents the overlapping of the clusters. (b) Increased right putamen activation to happy facial expressions (>scrambled) in HC compared to Anx out-patients. HC, healthy; MDD, major depressive disorder; Anx, anxiety disorder; DAC, depression-anxiety co-morbidity.
Table 2. Anatomical regions showing significant between-group differences in activation in response to facial expressions

MNI, Montreal Neurological Institute coordinates; R, right; L, left; k, cluster size in voxels; BA, Brodmann area; MDD, major depressive disorder; HC, healthy controls; Anx, anxiety disorder.
Anx patients showed right lentiform nucleus hypoactivation to happy>scrambled faces, compared to HC (Fig. 2 b, Table 2), whereas no significant differences in activation to angry, fearful, happy or neutral (>scrambled) faces were observed in Anx compared to HC. To further investigate differences between anxiety diagnoses, a separate analysis for patients with only social phobia (n=22) or only panic disorder (n=18) as compared to 20 randomly selected healthy control subjects was conducted. As this analysis concerned discrete diagnostic categories, patients with co-morbid panic disorder and social phobia (n=17) were left out. With regard to amygdala activation, the social phobia group did not differ from the panic disorder group or from the healthy controls. There was a trend for a reduced left amygdala response to happy faces in panic disorder patients compared to HC (x=−15, y=−6, z=−18; small volume correction, p FDR=0.063, Z value=3.55).
Like Anx, DAC patients did not show significant differences in the neural response to any facial expressions (>scrambled) compared to HC.
Medication effects
After excluding medicated patients, the analysis of demographic and clinical characteristics showed a significant group effect on age [F(3, 172)=2.85, p<0.05], years of education [F(3, 172)=8.97, p<0.05], MADRS [F(3, 170)=37.55, p<0.005], BAI [F(3, 172)=19.56, p<0.005] and FQ [F(3, 172)=16.97, p<0.005]. No significant group effect was found on gender [χ2(3)=3.13, p=0.37] or handedness [χ2(3)=0.87, p=0.83]. Psychometric measures of medicated and unmedicated patient groups are presented in the online Supplementary material S3.
Unmedicated MDD patients showed right middle frontal gyrus extending into cingulate cortex [Brodmann area (BA) 32] hyperactivation to happy>scrambled faces relative to HC (Table 3). In response to neutral>scrambled faces, greater right medial frontal gyrus activation was found in unmedicated MDD patients relative to HC. No significant differences in activation to negative (>scrambled) faces were found between MDD and HC. No significant differences in the neural response to any facial expression (>scrambled) were found between unmedicated Anx and HC. Unmedicated DAC patients showed right anterior cingulate cortex (ACC) hyperactivation in response to happy faces, compared to HC (Table 3). (As we found a significant group effect on age, we tested the effect of age on the neural response to facial expressions: we found no significant age effect.)
Table 3. Anatomical regions showing significant difference in activation, in response to facial expressions between unmedicated and medicated patients, and HC
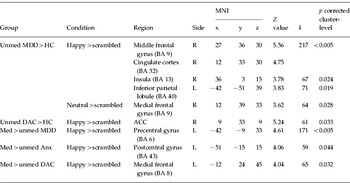
MNI, Montreal Neurological Institute coordinates; R, right; L, left; k, cluster size in voxels; BA, Brodmann area; ACC, anterior cingulate cortex; MDD, major depressive disorder; HC, healthy controls; Anx, anxiety disorder; DAC, depression-anxiety co-morbidity; Med, medicated; Unmed, unmedicated.
Unmedicated versus medicated out-patients
Medicated patients did not differ on depression and anxiety severity from unmedicated patients (p<0.05, S3). Significant differences in the neural response to facial expressions between medicated and unmedicated patients are presented in Table 3.
Correlations of activation with illness severity
No significant correlation was found between amygdala activation and illness severity in the patient groups. However, we did observe a significant correlation between left fusiform gyrus activation to angry and fearful (>scrambled) faces and depression severity in MDD patients. Supplementary online material S2 lists the regions showing significant correlation with illness severity.
Amygdala response shape
Supplementary online material S4 displays the response shape of the amygdala (mean and s.d.) during viewing facial expressions for each group. There was no group effect on left or right amygdala amplitude and on the time of the maximum peak (all p values >0.05).
Discussion
In the present study, we examined neural responses during implicit emotion processing in a large number of out-patients diagnosed with MDD, Anx and DAC disorders. Fusiform gyrus activation to facial expressions (>scrambled) was found within each group. Amygdala activation was found to all facial expressions (>scrambled) in MDD, DAC and HC. The conjunction analysis supported the presence of a common neural network implied in the perception of facial expressions across all groups. All of these regions have been previously reported to be involved in processing facial expressions of emotions (Haxby et al. Reference Haxby, Hoffman and Gobbini2000; Gorno-Tempini et al. Reference Gorno-Tempini, Pradelli, Serafini, Pagnoni, Baraldi, Porro, Nicoletti, Umit and Nichelli2001; Phan et al. Reference Phan, Wager, Taylor and Liberzon2002).
In contrast to our hypothesis and to some of the previous studies that reported amygdala hyperactivation to negative emotional stimuli in patients with major depression (Sheline et al. Reference Sheline, Barch, Donnelly, Ollinger, Snyder and Mintun2001; Anand et al. Reference Anand, Li, Wang, Wu, Gao, Bukhari, Mathews, Kalnin and Lowe2005; Fales et al. Reference Fales, Barch, Rundle, Mintun, Snyder, Cohen, Mathews and Sheline2008) and anxiety (Straube et al. Reference Straube, Kolassa, Glauer, Mentzel and Miltner2004; Bishop, Reference Bishop2007), in our study we failed to observe significant differences in amygdala response to facial expressions in out-patients with depression and/or anxiety relative to HC. This was the case for both medicated and unmedicated out-patients. Thus, although antidepressant medication has been shown to dampen the putatively excessive activation of the amygdala in depression (Sheline et al. Reference Sheline, Barch, Donnelly, Ollinger, Snyder and Mintun2001; Fu et al. Reference Fu, Williams, Cleare, Brammer, Walsh, Kim, Andrew, Merlo Pich, Williams, Reed, Mitterschiffthaler, Suckling and Bullmore2004; Fales et al. Reference Fales, Barch, Rundle, Mintun, Mathews, Snyder and Sheline2009) and anxiety disorders (Paulus, Reference Paulus2008; Engel et al. Reference Engel, Bandelow, Gruber and Wedekind2009), medication status could not explain the lack of group differences in amygdala activation in the present study.
However, our results are in agreement with previous findings in depression that suggest that there is no amygdala difference in depressed patients compared to HC (Lawrence et al. Reference Lawrence, Williams, Surguladze, Giampietro, Brammer, Andrew, Frangou, Ecker and Phillips2004; Gotlib et al. Reference Gotlib, Sivers, Gabrieli, Whitfield-Gabrieli, Goldin, Minor and Canli2005; Lee et al. Reference Lee, Seok, Lee, Cho, Yoon, Lee, Chae, Choi and Ham2008; Almeida et al. Reference Almeida, Versace, Hassel, Kupfer and Phillips2009; Norbury et al. Reference Norbury, Selvaraj, Taylor, Harmer and Cowen2009). A meta-analysis of functional brain activation to negative stimuli in depressed patients also failed to find amygdala hyperactivation (Diekhof et al. Reference Diekhof, Falkai and Gruber2008, table 4). In the studies that did not observe differences in amygdala activation between depressed patients and controls, the severity of the depression was diverse (recovered: Norbury et al. Reference Norbury, Selvaraj, Taylor, Harmer and Cowen2009; moderate: Gotlib et al. Reference Gotlib, Sivers, Gabrieli, Whitfield-Gabrieli, Goldin, Minor and Canli2005; Almeida et al. Reference Almeida, Versace, Hassel, Kupfer and Phillips2009; severe: Lawrence et al. Reference Lawrence, Williams, Surguladze, Giampietro, Brammer, Andrew, Frangou, Ecker and Phillips2004; Lee et al. Reference Lee, Seok, Lee, Cho, Yoon, Lee, Chae, Choi and Ham2008), as was medication use (all unmedicated: Norbury et al. Reference Norbury, Selvaraj, Taylor, Harmer and Cowen2009; some medicated: Gotlib et al. Reference Gotlib, Sivers, Gabrieli, Whitfield-Gabrieli, Goldin, Minor and Canli2005; Lee et al. Reference Lee, Seok, Lee, Cho, Yoon, Lee, Chae, Choi and Ham2008; Almeida et al. Reference Almeida, Versace, Hassel, Kupfer and Phillips2009; all medicated: Lawrence et al. Reference Lawrence, Williams, Surguladze, Giampietro, Brammer, Andrew, Frangou, Ecker and Phillips2004). Nonetheless, the findings presented here fit well and add to the aforementioned studies. For severe depression, amygdala response has shown abnormalities in previous studies (Sheline et al. Reference Sheline, Barch, Donnelly, Ollinger, Snyder and Mintun2001; Fu et al. Reference Fu, Williams, Cleare, Brammer, Walsh, Kim, Andrew, Merlo Pich, Williams, Reed, Mitterschiffthaler, Suckling and Bullmore2004; Anand et al. Reference Anand, Li, Wang, Wu, Gao, Bukhari, Mathews, Kalnin and Lowe2005) and medication may down-regulate the amygdala response (Sheline et al. Reference Sheline, Barch, Donnelly, Ollinger, Snyder and Mintun2001; Fu et al. Reference Fu, Williams, Cleare, Brammer, Walsh, Kim, Andrew, Merlo Pich, Williams, Reed, Mitterschiffthaler, Suckling and Bullmore2004; Lawrence et al. Reference Lawrence, Williams, Surguladze, Giampietro, Brammer, Andrew, Frangou, Ecker and Phillips2004). In our case, for mild-to-moderate depression, with and without medication, no aberrant amygdala activation was observed, whereas for a group of patients with severe or moderate depression and medication use, no aberrant amygdala response was found (Lawrence et al. Reference Lawrence, Williams, Surguladze, Giampietro, Brammer, Andrew, Frangou, Ecker and Phillips2004; Gotlib et al. Reference Gotlib, Sivers, Gabrieli, Whitfield-Gabrieli, Goldin, Minor and Canli2005; Lee et al. Reference Lee, Seok, Lee, Cho, Yoon, Lee, Chae, Choi and Ham2008; Almeida et al. Reference Almeida, Versace, Hassel, Kupfer and Phillips2009). Taken together, these studies suggest that amygdala response is influenced by both illness severity and medication and that there is an interaction effect between illness severity and medication. The antidepressant effect on downregulation of the amgydala may be primarily clear in severe depression. Caution is needed in interpreting these findings, however, as randomized designs with medication administration are needed to address this question adequately.
The fact that some studies do find amygdala hyperactivation and some do not may be due to methodological factors such as task design (subliminal presentation of emotional faces; Sheline et al. Reference Sheline, Barch, Donnelly, Ollinger, Snyder and Mintun2001) or task demands, for example unattended versus attended faces (Fales et al. Reference Fales, Barch, Rundle, Mintun, Snyder, Cohen, Mathews and Sheline2008), passive viewing (Anand et al. Reference Anand, Li, Wang, Wu, Gao, Bukhari, Mathews, Kalnin and Lowe2005), and implicit processing by asking for gender discrimination (Lawrence et al. Reference Lawrence, Williams, Surguladze, Giampietro, Brammer, Andrew, Frangou, Ecker and Phillips2004; Gotlib et al. Reference Gotlib, Sivers, Gabrieli, Whitfield-Gabrieli, Goldin, Minor and Canli2005). Straube et al. (Reference Straube, Kolassa, Glauer, Mentzel and Miltner2004) reported that implicit (photography versus schematic faces) processing of angry facial expression elicited significantly larger amygdala activation in social phobics relative to control participants, whereas explicit processing of angry faces elicited larger amygdala activation in both groups. Because we used an implicit or incidental facial affect-processing task, where participants were not explicitly instructed to pay attention to facial expressions, we cannot exclude the possibility that participants may have consciously processed facial expressions. Other task parameters that can contribute to the observed variability in amygdala activation concern the use of masked and/or block designs to overcome carry-over effects and to enhance sensitivity to brief automatic reactions.
Analysis of the amygdala response shape to facial expressions corroborated our findings that amygdala activation was not abnormal in patient groups. Although a comparatively early response of the left and the right amygdala activation to fearful faces was apparent in MDD patients (S4), this difference was not significant compared with HC or other patient groups. Two other studies did find a difference in amygdala response shape: sustained amygdala activation in response to negative emotions in seven depressed patients (Siegle et al. Reference Siegle, Steinhauer, Thase, Stenger and Carter2002) and a delayed amygdala response to angry, fearful and happy faces in 14 patients with generalized social phobia (Campbell et al. Reference Campbell, Sareen, Paulus, Goldin, Stein and Reiss2007). There are several factors that may contribute to this discrepancy, for example sample heterogeneity in terms of disease severity, the fact that patients in those two studies were more severely affected, but also differences in the experimental design (words versus pictures).
In the present study there was no significant correlation between illness severity and the magnitude of the amygdala response in out-patients. A positive correlation between fusiform gyrus activation to fearful and angry faces and depression severity was observed in MDD out-patients. We suggest that this pattern of activation to angry and fearful faces is symptom related in depression. In a previous study (Surguladze et al. Reference Surguladze, Brammer, Keedwell, Giampietro, Young, Travis, Williams and Phillips2005) larger fusiform gyrus activation to sad faces was reported in patients with severe depression compared to healthy volunteers, suggesting an attentional bias toward sad emotion in major depression.
Contrary to our expectation, ventral striatum hypoactivation to happy faces was not observed in MDD out-patients compared to HC. However, whereas Lawrence et al. (Reference Lawrence, Williams, Surguladze, Giampietro, Brammer, Andrew, Frangou, Ecker and Phillips2004) and Surguladze et al. (Reference Surguladze, Brammer, Keedwell, Giampietro, Young, Travis, Williams and Phillips2005) reported putamen hypoactivation in response to happy faces in 19 MDD patients relative to controls, other authors (Elliott et al. Reference Elliott, Rubinsztein, Sahakian and Dolan2002; Siegle et al. Reference Siegle, Steinhauer, Thase, Stenger and Carter2002; Davidson et al. Reference Davidson, Irwin, Anderle and Kalin2003a) reported no significant differences in ventral striatum activation to positive stimuli in depression, consistent with the present findings. A possible explanation for these inconsistencies may be the sample characteristics (severe versus mild depression, and sample size). Nevertheless, right putamen hypoactivation in response to happy faces was observed in Anx out-patients compared with HC. Activation of the basal ganglia, including the ventral striatum and putamen, in response to positive emotional stimuli has been reported previously (e.g. Phan et al. Reference Phan, Wager, Taylor and Liberzon2002), suggesting a role in reward-related processes (Elliott et al. Reference Elliott, Friston and Dolan2000). Decreased gray matter volume in the right putamen has also been reported to be associated with anxiety severity in panic disorders patients (Yoo et al. Reference Yoo, Kim, Kim, Sung, Sim, Lee, Song, Kee and Lyoo2005). Abnormal putamen function may be characteristic for anxiety disorders.
We did, however, observe right superior and middle frontal gyrus hyperactivation in response to happy (>scrambled) faces in MDD out-patients compared to HC. Larger middle frontal gyrus, cingulate cortex (BA 32) activation to happy faces was also found in unmedicated MDD out-patients relative to HC. We suggest that this stronger activation for happy faces may reflect increased attention to mood-incongruent stimuli. Frodl et al. (Reference Frodl, Scheuerecker, Albrecht, Kleemann, Müller-Schunk, Koutsouleris, Möller, Brückmann, Wiesmann and Meisenzahl2009) reported increased DLPFC activation during implicit emotional processing and failure of deactivation of this region during explicit emotional processing in patients with depression. They suggested that depressed patients needed stronger recruitment of this region to perform the task. Ochsner et al. (Reference Ochsner, Ray, Hughes, McRae, Cooper, Weber, Gabrieli and Gross2009) suggested that the DLPFC may be involved in mood-dependent attentional regulation of emotions. Although such a top-down component of emotion processing may be more apparent in explicit emotion evaluation tasks, they indicate that regulation can also be triggered by emotional stimuli presented in an implicit task.
Similar to MDD patients, unmedicated DAC patients compared to HC showed dorsal ACC hyperactivation to happy faces. The dorsal ACC activation has been suggested to mediate conflicts between emotion and cognition (Davidson et al. Reference Davidson, Pizzagalli, Nitschke and Kalin2003b) and is associated with attentional processing of emotional information (McRae et al. Reference McRae, Reiman, Fort, Chen and Lane2008). We can therefore conclude that dorsal PFC hyperactivation in depressed out-patients with or without anxiety suggests increased processing demands for mood-incongruent stimuli.
Limitations and strengths
As mentioned previously, the MDD patients included in the present study had mild-to-moderate depression severity [34 depressed out-patients were in remission (MADRS score <12; Zimmerman et al. Reference Zimmerman, Posternal and Chelminski2004), Supplementary material], which may have limited the sensitivity of our design. Although we analyzed the potential contribution of medication status, this might still be a confounder in the present study, as we did not study type or medication dosage. A major strength of the present study concerns the large sample size, relative to previous studies, which lends confidence to the non-trivial suggestion that altered DLPFC function is a key feature of the neurobiology of depression. In conjunction with this, the sample analyzed in this study is likely to represent a community out-patient population, the most prevalent community in mental health-care practice.
Conclusions
The present study demonstrates that perception of facial expressions elicits a common neural response in community out-patients with mild-to-moderate depression and/or anxiety, and healthy controls. The lack of group differences in amygdala activation to emotional facial expressions may be characteristic of a community-based sample of out-patients with depression and/or anxiety. However, we did observe diagnosis-specific activations for MDD, including dorsal PFC hyperactivation in response to happy facial expressions, which may reflect increased processing demands for mood-incongruent stimuli. Antidepressant and illness severity may influence amygdala response to emotional stimuli in major depression. Moreover, medication use seems to influence the neural response associated with cognitive control of emotion in MDD and DAC out-patients.
Note
Supplementary material accompanies this paper on the Journal's website (http://journals.cambridge.org/psm).
Acknowledgments
The infrastructure for the NESDA study (www.nesda.nl) is funded through the Geestkracht program of the Netherlands Organization for Health Research and Development (Zon-Mw, grant no. 10-000-1002) and is supported by the participating universities and mental health-care organizations: VU University Medical Center Amsterdam, University Medical Center Groningen, Leiden University Medical Center, GGZ inGeest, Arkin, GGZ Rivierduinen, Lentis, GGZ Friesland, GGZ Drenthe, Scientific Institute for Quality of Healthcare (IQ healthcare), Netherlands Institute for Health Services Research (NIVEL) and Netherlands Institute of Mental Health and Addiction (Trimbos Institute).
We thank A. Stan for his thoughtful and inspiring discussions, M. Messchendorp and J. Jansen for help with patient recruitment, E. Liemburg for patient management and A. Kuiper for operating the MRI scanner in Groningen.
Declaration of Interest
None.







