Introduction
Concha bullosa is an anatomical variation of the lateral nasal wall, characterised by the presence of an air pocket within the middle turbinate.Reference Ahmed, Hanci, Üstün, Aydogdu, Özdemir and Karaketir1 A large concha bullosa can narrow the middle meatus, and reduce the mucociliary clearance and ventilation, causing obstruction of the osteomeatal complex. Concha bullosa becomes pneumatised as a result of ethmoid cell extensions.Reference Lothrop2 The presence of concha bullosa can be identified using computed tomography (CT).
Concha bullosa, alone or in combination with other factors, may cause a variety of focal symptoms, ranging from pressure sensation or headache, to nasal obstruction.Reference La Mantia, Grillo and Andaloro3 Concha bullosa may lead to paranasal sinus infections, particularly after osteomeatal complex obstruction. However, this relationship is the subject of debate given the varying conclusions made in previous studies on the topic. Some authors suggest that concha bullosa promotes the development of sinusitis, whereas other studies have shown an inconsistent association between them.Reference Subramanian, Lekhraj Rampal, Wong, Mastura and Razi4–Reference Jones, Strobl and Holland6
Some studies have shown that odour neurofibrillaries are present in the middle concha mucosa.Reference Apuhan, Yildirim, Simsek, Yılmaz and Yılmaz7–Reference Nibu, Li, Zhang, Rawson, Restrepo and Kaga9 Hence, there is the question of whether surgical interventions cause damage to the odour epithelium in the middle concha, resulting in reduced odour function.
For symptomatic patients requiring surgical treatment, the concha bullosa should be approached endoscopically. During concha bullosa surgery, when accessing the inside of the concha, the medial or lateral mucosa of the concha can be removed together with the bone, submucoperiosteal resection can be performed, or concha size can be reduced by crushing without removing the mucosa.Reference Bhatt10–Reference Richtsmeier and Cannon12 This study compared odour test results and other nasal symptoms of patients with a symptomatic concha bullosa, before and after reduction surgery involving either lateral laminectomy or crushing.
Materials and methods
The study was approved by the Ethics Committee of the Santa Marta e Santa Venera Hospital in Acireale, Catania, Italy, and written consent was obtained from all patients prior to the procedure.
This study investigated 43 patients who underwent surgery because of a symptomatic concha bullosa between June 2017 and June 2018 at the Otolaryngology Unit of the Santa Marta e Santa Venera Hospital. All patients were aged over 18 years old. These patients had suffered nasal obstruction for more than three months, and the planned operation was a result of a symptomatic concha bullosa.
The exclusion criteria were: nasal septal deviation, previous nasal surgery, sinonasal polyposis, pre-existing sinus disease of any sort, positivity to a skin prick or radioallergosorbent test, previous subjective olfactory disturbance, intranasal drug addicts, diabetes mellitus, and rheumatological disease. Age, gender and patient complaints were recorded as medical data.
The patients were randomised to a lateral laminectomy group or a crushing group, for concha bullosa reduction treatment, according to a computerised random number generator performed by a statistician independent of the study.
Lateral laminectomy is performed as follows. Once the anaesthesia has been established, the concha is entered, with the help of a sickle knife, from the point where the pneumatisation is most prominent. This point can be identified using a sickle knife tip. After entering the concha, the incision continues along the free lower edge of the saw movement. Subsequently, the sickle knife tip is turned up and the incision is completed up to the concha sticking point. Care should be taken to avoid excessive pressure and breakage when entering into the concha. In cases where the concha lamellae are too thick, the incision can be completed with concha scissors. The separated lateral lamella of the concha is removed by performing a rotational motion with flat forceps.
The concha crushing is performed as follows. After anaesthesia, crushing with Bruening septum forceps is carried out, beginning from the point where the pneumatisation of middle concha is most prominent. Care should be taken when performing this technique while the concha is very movable.
Post-operatively, a NasoPore® nasal dressing was placed on the concha laterally. Patients were advised to lavage the site frequently. After 2 days post-operation, the scabs and clots were cleaned under endoscopy. The dressings were reapplied at weekly intervals.
All patients underwent ENT examination and nasal endoscopy pre-operatively. Coronal CT scans of the paranasal sinus were performed at the out-patient clinic examination. The nasal obstruction visual analogue scale (VAS), 22-item Sino-Nasal Outcome Test (SNOT-22), peak nasal inspiratory flow (PNIF) test and odour test were performed pre-operatively and at three months post-operatively. All assessments were performed by an otolaryngologist, who was blind to the treatment received.
Patients were asked to quantify their perceived nasal obstruction using the nasal obstruction VAS, as described previously.Reference Ciprandi, Mora, Cassano, Gallina and Mora13 All participants completed the SNOT-22 disease-specific questionnaire adapted and validated for Italian patients, which provides a quantitative measure of symptom severity and health-related quality of life for patients with sinonasal symptoms. The intensity of each symptom was scored on a Likert scale of 0–5, as previously described.Reference Mozzanica, Preti, Gera, Gallo, Bulgheroni and Bandi14
The PNIF measurement was performed using a nasal inspiratory flow meter (Clement Clark International, Harlow, UK). The patients were asked to expire forcefully while sitting, and then inspire forcefully through the nose, with an anaesthesia mask placed over the mouth. Of the three consecutive measurements with a maximum difference of 10 per cent, the highest measurement was recorded (in litres per minute) as the final value.
Olfactory function was evaluated using the Sniffin’ Sticks test (Burghart Messtechnik, Wedel, Germany) according to the aforementioned procedure.Reference La Mantia, Cupido, Castro and Andaloro15,Reference Haehner, Mayer, Landis, Pournaras, Lill and Gudziol16 It consists of three subtests; namely, the n-butanol odour threshold test, odour identification test and odour discrimination test. The results of the three subtests are presented as a composite ‘TDI’ score, which is the sum of the results obtained for threshold, discrimination and identification measures. Hyposmia was defined at a composite score of less than 30.Reference Oleszkiewicz, Schriver, Croy, Hähner and Hummel17
The software SPSS® version 15 was used for the statistical analysis. The student's t-test (or Mann–Whitney U test, as appropriate) was used for the continuous variables and in the dependent groups. The paired t-test (or Wilcoxon signed rank test, as appropriate) was used for the numerical variables. A p-value of less than 0.05 was considered statistically significant.
Results
Among the 43 participants selected, 53.5 per cent were male. The mean patient age was 29.6 years (range, 18–48 years). There was no statistical difference in age or gender (p > 0.05) between both groups. The results for nasal obstruction VAS, SNOT-22 and PNIF for the crushing and lateral laminectomy groups, before and after surgical treatment, are shown in Table 1.
Table 1. Comparison of treatment outcomes for crushing and lateral laminectomy groups in terms of VAS, SNOT-22 and PNIF

*n = 22; †n = 21. ‡Post-operative minus pre-operative value. †P < 0.05. VAS = visual analogue scale; SNOT-22 = 22-item Sino-Nasal Outcome Test; PNIF = peak nasal inspiratory flow; SD = standard deviation
In both groups, the pre- versus post-treatment changes in the mean values for the nasal obstruction VAS, SNOT-22 and PNIF test were statistically significant (intragroup comparison; all p < 0.05). However, there were no statistically significant changes when the scores were compared between the two treatment groups (intergroup comparison).
There were statistically significant pre- versus post-operative changes in the mean values for all the olfaction tests, and the composite threshold, discrimination and identification score, in both groups (p = 0.032 for crushing; p = 0.026 for lateral laminectomy). However, there were no statistically significant differences when these results were compared between the two groups (Table 2).
Table 2. Comparison of treatment outcomes for crushing and lateral laminectomy groups in terms of odour function
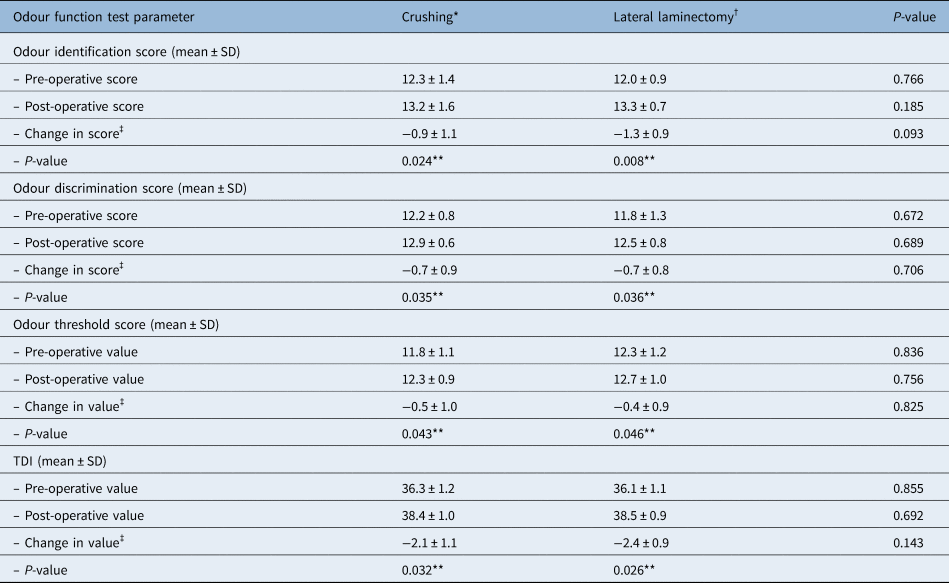
*n = 22; †n = 21. ‡Post-operative minus pre-operative value. †P < 0.05. SD = standard deviation; TDI = threshold, discrimination and identification sum score
No significant bleeding or major complications were detected among the participants after the procedure. However, light oedema and scabbing in the nose were observed.
Discussion
Concha bullosa is one of the most important factors providing resistance in the nose. It simultaneously affects respiration, air humidification, smell function and voice resonance. A middle concha often requires surgical intervention because of sinus diseases and nasal obstruction.
The distribution of the olfactory neuroepithelium in the nose is not clear.Reference Say, Leopold, Cochran, Smith and Greiner18 Olfactory neuroepithelium is thought to be located in the nasal olfactory cleft, septum, and upper concha in the dorsal region of the nasal cavity.Reference Ishimaru, Scheibe, Gudziol and Negoias19 Apuhan et al. observed that middle concha contained odour neuroepithelium.Reference Apuhan, Yildirim, Simsek, Yılmaz and Yılmaz7 According to their findings, there was more neuroepithelium on the lateral aspect of the concha, compared to medial and anterior surfaces. Indeed, recent studies indicate that the olfactory neuroepithelium is more prominent than previously thought. Several studies have revealed the presence of mature olfactory cells on electro-olfactogram measurements and biopsies.Reference Ishimaru, Scheibe, Gudziol and Negoias19–Reference Rawson, Gomez, Cowart and Restrepo21 Féron et al. showed the presence of olfactory epithelium in 50 per cent of healthy middle conchae biopsied.Reference Féron, Perry, McGrath and Mackay-Sim8 Rawson et al. identified olfactory neurons in the middle concha and septum corresponding to the upper part of the middle concha.Reference Rawson, Gomez, Cowart and Restrepo21 Additionally, Nibu et al. confirmed the presence of olfactory mucosa in the bottom of the medial surface epithelium of middle concha.Reference Nibu, Li, Zhang, Rawson, Restrepo and Kaga9
The excessive pneumatisation of middle concha can result in pathology of the nasal physiology. This variation can cause sinonasal complications by impairing nasal airflow and mucus drainage.Reference Cannon22 Indeed, concha bullosa is commonly seen in the middle concha, and rarely in the upper and lower conchae.Reference Christmas, Merrell, Mirante and Yanagisawa23 When large in dimension, it can cause nasal obstruction, without creating sinus problems.Reference Yasar, Verim and Özkul24 Moreover, concha bullosa might be the cause of nasal obstruction after primary septoplasty, and, if it remains unaddressed, could lead to the persistence of nasal obstruction even after the surgical nasal septum correction.Reference Derin, Sahan, Deveer, Erdogan, Tetiker and Koseoglu25,Reference Ozlugedik, Nakiboglu, Sert, Elhan, Tonuk and Akyar26 Bolger et al. classifies concha pneumatisations as lamellar, bullous or extensive.Reference Bolger, Parsons and Butzin27
The four most common methods used in concha surgery are concha crushing, lateral laminectomy, medial laminectomy and transverse excision.Reference Okuyucu, Akoğlu and Dağlı28 Partial concha surgery is a frequently performed procedure.Reference Dutton and Hinton29 Crushing the middle concha does not destroy the physiology or anatomy. However, a large concha bullosa requires partial resection. After concha crushing, medial adhesions are not an issue for surgeons, and sometimes this is utilised for the aperture of the osteomeatal complex.Reference Friedman, Tanyeri, Landsberg and Caldarelli30
The commonest complication after concha bullosa surgery is the development of synechia between the lateral nasal wall and middle concha.Reference Dutton and Hinton29 Some authors have stated that suturing the middle concha to septum, or medialisation by the creation of planned synechia, is beneficial, with no effect on smell function.Reference Dutton and Hinton29,Reference Friedman, Tanyeri, Landsberg and Caldarelli30 Complete resection of the middle concha leads to impaired nasal function, loss of a significant surgical marker, an increased risk of developing frontal sinusitis, and the development of large areas of scar tissue.Reference Ciprandi, Mora, Cassano, Gallina and Mora13 Hence, we provided concha medialisation by applying NasoPore to the middle concha laterally, at the end of the operation. Indeed, no synechia or complications were observed post-operatively among our patients.
The VAS is an easy method for evaluating nasal obstruction in a numerical manner. The results of rhinomanometry and VAS have been found to be correlated.Reference Ciprandi, Mora, Cassano, Gallina and Mora13 Although rhinomanometry is rarely available, it is possible to apply VAS everywhere. In this study, a VAS was used to compare the nasal obstruction scores of patients undergoing middle concha surgery. The VAS results showed a significant improvement post-operatively in both treatment groups compared to the pre-operative period. However, there was no statistical difference between the groups. These results are consistent with the literature review findings.Reference Kumral, Yıldırım, Çakır, Ataç, Berkiten and Saltürk31
The SNOT-22 is widely used to assess the effects of patient-based sinonasal problems on quality of life.Reference de Dorlodot, Horoi, Lefebvre, Collet, Bertrand and Eloy32 In our study, the SNOT-22 results showed a significant post-operative improvement in both groups compared to the pre-operative period. However, there was no significant difference found between the groups. These results are again consistent with the literature review findings.Reference Kumral, Yıldırım, Çakır, Ataç, Berkiten and Saltürk31
When measuring PNIF, the face mask is placed on the nose and mouth, and the mouth is kept closed. The patient breathes through the nose, and the peak current is recorded.Reference Youlten33 In our study, PNIF was used instead of a rhinomanometer to measure nasal resistance. The PNIF is easier to use in office settings than rhinomanometry, and only depends on the patient's exercise capacity.Reference Ozkul, Balikci, Gurdal, Celebi, Yasar and Karakas34 Both methods have shown similar results regarding obstructive nasal pathologies.Reference Bermüller, Kirsche, Rettinger and Riechelmann35 Few previous studies have investigated the decrease in nasal obstruction associated with the reduction of middle concha size.Reference Nease and Krempl36 In the current study, the post-operative PNIF findings showed improvement in both treatment groups. However, there was no difference between the two groups, in line with previous findings.Reference Kumral, Yıldırım, Çakır, Ataç, Berkiten and Saltürk31
The odour test, an electrophysiological test, is the only objective test. However, as it is not practical to use, physical tests have become more common for performing odour assessments. In our study, there was no significant pre- versus post-operative difference in n-butanol threshold values, in either treatment group. Kumral et al. showed an improvement in odour identification test findings post-operatively in both groups, for which treatment involved the use of medial or lateral laminectomy techniques.Reference Kumral, Yıldırım, Çakır, Ataç, Berkiten and Saltürk31 The current study showed post-operative improvements in odour function test results for both groups, but there were no significant differences between the groups. In some studies, a reduction of middle concha mass caused an increase in medial airflow, and improvement in smell function was observed.Reference Damm, Vent, Schmidt, Theissen, Eckel and Lötsch37,Reference Leopold38 There was no decrease in concha mass in the crushing group, and no change in smell function results was observed. Although previous studies have stated that there is odorous epithelium in the concha mucosa, a decrease in the odour function results was not observed, and the same level or better odour function results were obtained.Reference Kumral, Yıldırım, Çakır, Ataç, Berkiten and Saltürk31,Reference Nease and Krempl36
• Concha bullosa is a common variation of sinonasal anatomy, which may lead to headaches, nasal obstruction and impaired olfaction
• Olfactory neuroepithelium is thought to reside within the nasal cavity olfactory cleft, septum, upper concha and middle concha
• Olfactory neuroepithelium in middle concha mucosa may be damaged by surgical interventions, resulting in reduced odour function
• Lateral laminectomy and crushing are common surgical treatments for concha bullosa, and can improve nasal and olfactory functions
• The two techniques are similar in effectiveness, with no significant difference between them
Conclusion
Although the sample size was small, our study showed that both treatment techniques were comparably effective in improving patients’ quality of life, with reduced nasal obstruction, fewer nasal complaints, and improved olfactory function post-operatively. However, when the two techniques are compared, the effectiveness in terms of improved nasal and olfactory functions was similar, with no significant difference between them. Hence, lateral laminectomy and crushing are both effective methods for managing a symptomatic concha bullosa.
Competing interests
None declared




