Introduction
During the last three decades, accumulating clinical has evidenced THE superior canal dehiscence syndrome, which was first described by Lloyd B Minor in 1998.Reference Ward, Carey and Minor1 Superior canal dehiscence syndrome is caused by the presence of superior semicircular canal bone dehiscence, which leads to inner ear dysfunction, acting as a third mobile window. Specific manifestations include auditive hypersensitivity to body sounds, and sound- and pressure-induced vertigo. The diagnosis is based on specific physiological measures, including enhanced responses on vestibular evoked myogenic potential testing, an air–bone gap with normal ear immittance and stapedial reflexes, and sound-, vibration- and pressure-induced eye movements and nystagmus. Superior canal dehiscence should also be visualised on specific reconstructions of temporal bone computed tomography (CT) scans.
This report describes the case of a man who presented with audiovestibular symptoms consistent with unilateral Ménière´s disease, whose physiological testing and imaging findings were also compatible with superior canal dehiscence syndrome on the contralateral side where he did not have any specific symptoms. The study was approved by the Swedish Ethical Review Authority (application number: 2020-05237).
Case report
A 50-year-old man with a medical history of diabetes type II and prostate dysplasia was referred to our tertiary referral audiology and neurotology centre with a history of fluctuating hearing loss in the right ear and dizziness for four weeks.
The first audiometry findings showed unilateral sensorineural hearing loss (SNHL) in the right ear, with a pure tone average (PTA) of 52.5 dB HL (Figure 1a). On the left side, the hearing threshold was normal, with a PTA of 6.25 dB HL. The hearing loss was treated with high-dosage peroral cortisone. Control audiometry after one week showed hearing loss regression on the right side (PTA of 8.75 dB HL), with a remaining dip at 4 kHz at 50 dB HL (Figure 1b).
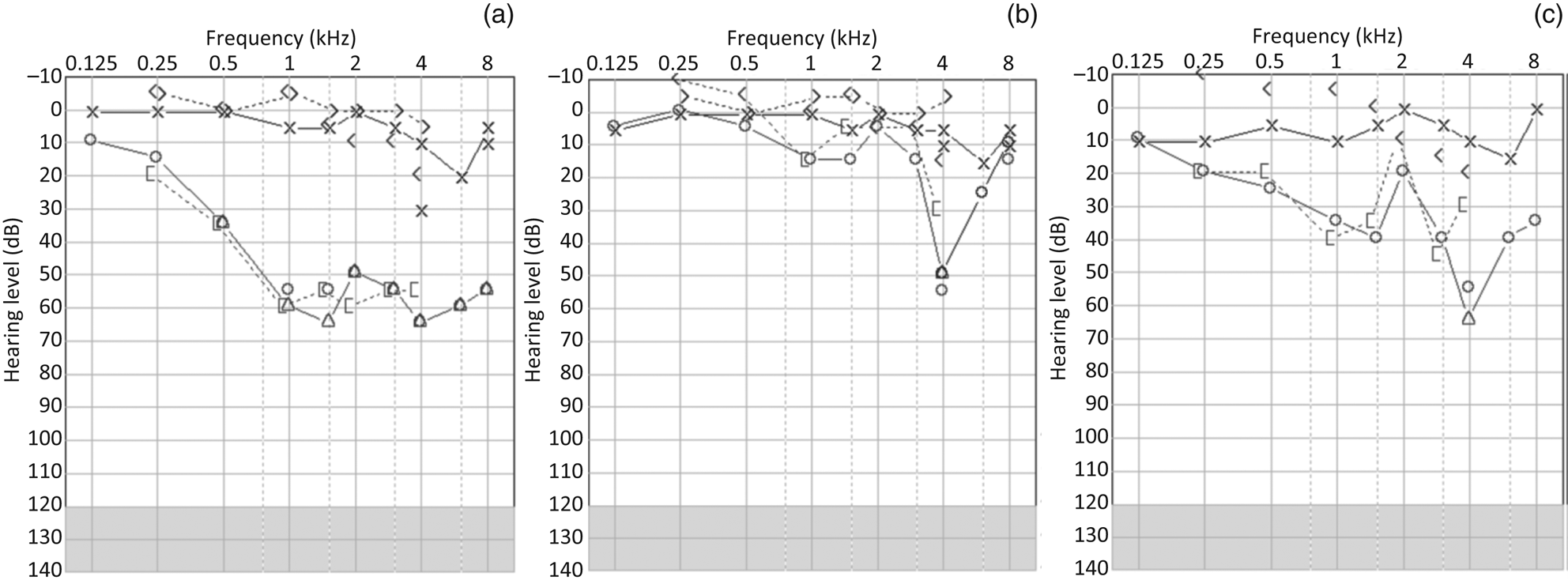
Fig. 1. Pure tone audiometry at: (a) initial presentation, (b) after the acute phase, and (c) at follow up. × = unmasked left air-conducted hearing threshold; ○ = unmasked right air-conducted hearing threshold; Δ = masked right air-conducted hearing threshold; < = unmasked right bone-conducted threshold; > = unmasked left bone-conducted hearing threshold; [ = masked left bone-conducted hearing threshold.
The patient was referred for a brain magnetic resonance imaging (MRI) scan. This showed normal structures at the cerebellopontine angles and temporal bones. An incidental small cavernoma of the right frontal lobe was also identified.
Six and a half months later, the patient sought treatment again because of worsened hearing on the right side, tinnitus, fullness, and a few vertigo spells lasting several minutes to hours. During the following five months, the right hearing threshold fluctuated between 16.25 and 50 dB HL in terms of PTA, and finally stabilised on a PTA of 30 dB HL (Figure 1c). Peroral cortisone did not appear to stabilise the hearing threshold in this period. Head impulse testing, videonystagmoscopy and positional testing showed normal findings during this follow-up period. Furthermore, vestibular testing revealed intact vestibular ocular and cervical reflexes on caloric irrigation, videonystagmography, and vestibular evoked myogenic potential testing. Surprisingly, vestibular evoked myogenic potential testing showed an enhanced vestibular response on the left side (i.e. the contralateral side to hearing fluctuation) (Figure 2).Reference Brantberg and Verrecchia2 The patient denied any auditive symptoms on the left side. Tullio's or Hennebert's phenomenon was not observed in successive visits. A CT scan of the temporal bone showed a lack of bone overlaying the left superior semicircular canal and normal findings on the right side (Figure 3). Eighteen months after the first episode of right SNHL, hearing function stabilised, as shown in Figure 1c.
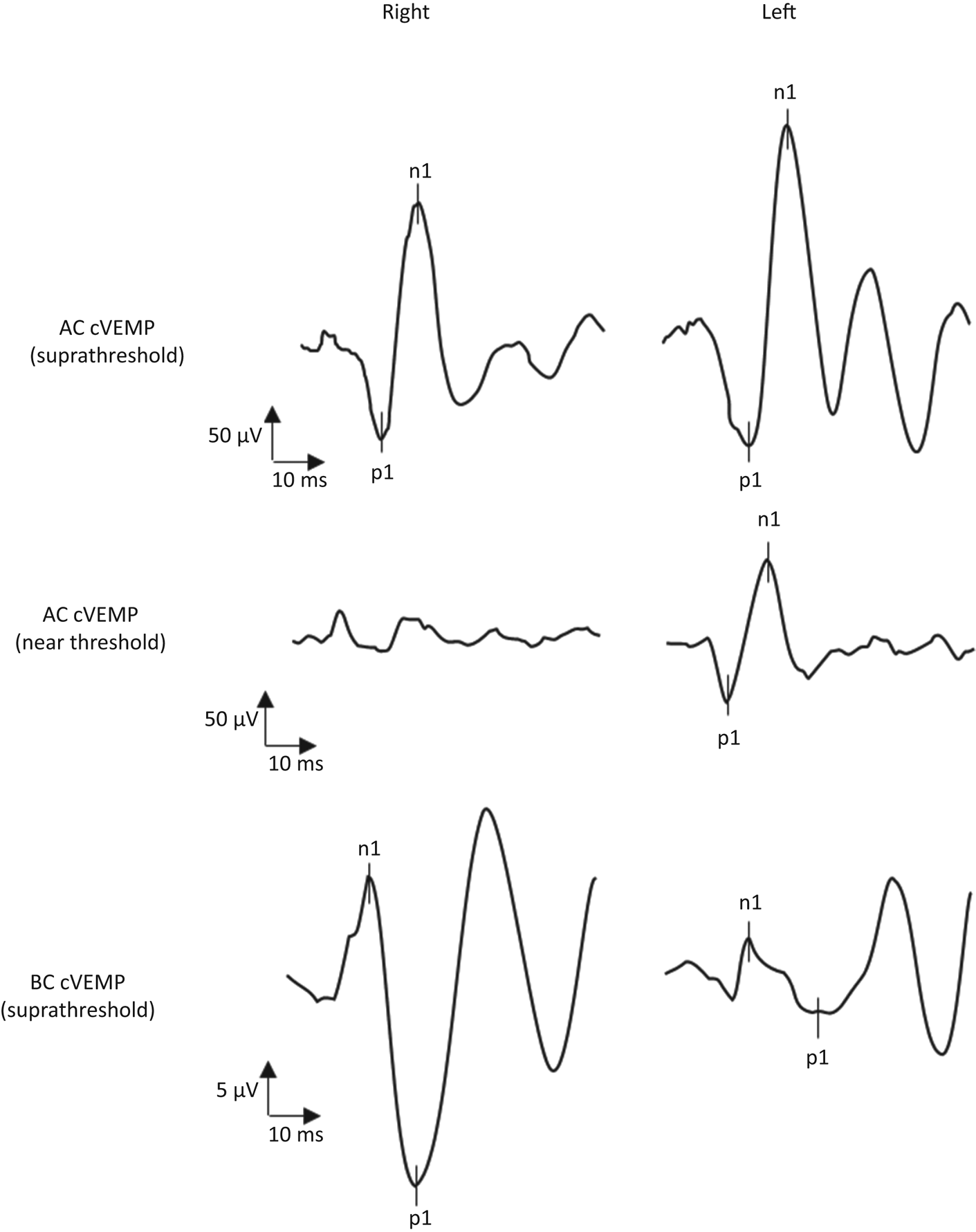
Fig. 2. Vestibular evoked myogenic potential recordings. The top row shows the cervical vestibular evoked myogenic potentials (cVEMP) evoked by ipsilateral air-conducted (AC) stimuli at suprathreshold levels (tone burst, 500 Hz; rise/plateau/fall = 1:2:1 ms; 130 dB SPL; 192 repetitions), and recorded at the right and left sternocleidomastoid muscles with surface electromyography (EMG). The middle row shows the cervical vestibular evoked myogenic potentials evoked by bilateral air-conducted near-threshold stimuli (click = 0.1 ms; 90 dB HL; 192 repetitions), and recorded simultaneously at the right and left sternocleidomastoid muscles with surface EMG. The bottom row shows the ocular vestibular evoked myogenic potentials (oVEMP) evoked by suprathreshold bone-conducted (BC) stimuli delivered at Fz (tone burst = 125 Hz; single cycle = 135 dB relative to 1 μN peak-to-peak equivalent force level; 192 repetitions), and recorded by surface EMG with an infra-orbital montage. The corrected amplitude of the suprathreshold air-conducted vestibular evoked myogenic potential recordings was 3.34 μV on the left and 4.22 μV on the right. When the stimulus was reduced to near threshold levels, a cervical vestibular evoked myogenic potential with a corrected amplitude of 1.86 μV was retrieved only at the left sternocleidomastoid muscle, with a weak inverted response on the right side. On ocular vestibular evoked myogenic potentials, a bilateral response with a larger amplitude on the right could be reproduced (right = 28.8 μV; left = 7.1 μV; asymmetry ratio = 0.60). This vestibular evoked myogenic potential pattern is indicative of left vestibular hypersensitivity to sound and vibration (according to Brantberg and VerrecchiaReference Brantberg and Verrecchia2, which is commonly found in superior canal dehiscence syndrome. Note the difference in amplitude scales for the cervical vestibular evoked myogenic potentials and ocular vestibular evoked myogenic potentials recordings.

Fig. 3. High-resolution computed tomography of the temporal bones. The reconstruction is on the plane of the superior semicircular canals of the (a) right and (b) left sides. The arrow indicates the extreme thinning or opening of the left superior semicircular bone canal.
Constant, non-pulsatile tinnitus and SNHL on the right side are the patient's only residual complaints. No hearing loss or auditive phenomena on the left side, and no autophony or hyperacusis for internal sounds, were reported by the patient at any time. Moreover, no sound- or pressure-induced vertigo, and no residual dizziness, were reported after a few vertigo spells occurred during right-sided SNHL fluctuation.
Discussion
This case report primarily describes a patient fulfilling the diagnostic criteria of unilateral definite Ménière´s disease.Reference Lopez-Escamez, Carey, Chung, Goebel, Magnusson and Mandalà3 Atypical sloping SNHL at lower frequencies, and a residual dip at higher frequencies, necessitated the exclusion of inner-ear or retrocochlear affections by MRI scanning. An enhanced vestibular response from the left ear was incidentally found on vestibular evoked myogenic potential testing, and was consistent with superior canal dehiscence syndrome. The presence of a left superior canal dehiscence was corroborated by CT scans, which also excluded the presence of superior canal dehiscence on the symptomatic right ear. To our knowledge, this is the first description of a clinical case in which an asymptomatic superior canal dehiscence could be identified by both physiological testing and imaging.
This case raises some concerns about vestibular evoked myogenic potentials in the diagnostic assessment of superior canal dehiscence syndrome. Asymptomatic superior canal dehiscence may represent a subclinical phase of the clinical progression of superior canal dehiscence syndrome. Superior canal dehiscence may already be present in childhood as a congenital thinning of canal bone,Reference Ward, Carey and Minor1 and evolve as progressive age-related osteopenia, often manifesting in middle age in relation to minor head trauma or barotrauma.
• An enhanced vestibular evoked myogenic potentials response is a common diagnostic marker of superior canal dehiscence syndrome
• In a unilateral Ménière´s disease patient, enhanced vestibular evoked myogenic potentials were associated with asymptomatic superior canal dehiscence on the contralateral side
• This finding was confirmed by computed tomography scans
• Superior semicircular canal dehiscence may alter the vestibular evoked myogenic potentials response before, or regardless of, specific superior canal dehiscence syndrome symptom development
A discrepancy between the symptoms and the physiological measurements in superior canal dehiscence syndromeReference Noij, Wong, Duarte, Masud, Dewyer and Herrmann4 is the rule, probably because the two manifestations have different dynamics. There is the possibility of an early phase, in which the physiological measures change, in the absence of symptomatic correlates. It follows that the number of superior canal dehiscence carriers may be greater than the number of superior canal dehiscence syndrome patients, with the difference represented by asymptomatic forms, as noted in the present case.
Currently, vestibular evoked myogenic potentials are considered the best clinical marker of superior canal dehiscence syndrome, followed by CT, which tends to overestimate the presence and dimension of superior canal dehiscence.Reference Mittmann, Ernst, Seidl, Skulj, Mutze and Windgassen5 The case described here reveals the potential risk of overestimating superior canal dehiscence syndrome based on vestibular evoked myogenic potential testing, given the possibility of detecting asymptomatic forms of superior canal dehiscence.
In our opinion, vestibular evoked myogenic potential testing remains a strong confirmatory marker of superior canal dehiscence syndrome, but it appears to be a weaker screening and exploratory test in the case of a less specific audiovestibular presentation. A hypersensitivity pattern on vestibular evoked myogenic potential testing acts as an independent diagnostic marker of superior canal dehiscence syndrome when specific symptoms are present and superior canal dehiscence is clearly visualised on CT. In cases of less specific symptoms (dizziness, ear lock, hyperacusis or non-pulsatile tinnitus) yet with clear superior canal dehiscence on CT, the vestibular evoked myogenic potential pattern should be accompanied by other congruent physiological measurements for a diagnosis of superior canal dehiscence syndrome.
Conclusion
This report describes a case of unilateral Ménière´s disease with a contralateral asymptomatic superior canal dehiscence, as revealed by vestibular evoked myogenic potential testing and CT scanning. The case seems to downplay the role of vestibular evoked myogenic potentials as independent markers of superior canal dehiscence syndrome given their ability to detect asymptomatic forms of superior canal dehiscence.
Acknowledgements
LV is thankful for the support of the Scientific Center for Advanced Pediatric Audiology at Karolinska Institutet, Stockholm, Sweden. LV was financially supported by the Tysta Skola Foundation, Stockholm, Sweden (grant number: FB20-0020), and by the foundation ‘Insamlingsstiftelsen för främjande och utveckling av forskning vid KI’ at Karolinska Institutet.
Competing interests
None declared





