INTRODUCTION
Human alveolar echinococcosis (AE), a highly pathogenic parasitic disease, is caused by the tumour-like growth of larval Echinococcus multilocularis in the liver (Brunetti et al. Reference Brunetti, Kern, Vuitton and Writing Panel2010). Human infection by E. multilocularis results from the accidental ingestion of tapeworm eggs that are passed into the environment through the faeces of definitive hosts (generally foxes or dogs). Until now, no curative treatment has been available for human AE, except for radical surgical resection of the entire lesion, which can only be performed at the early stages of the disease, and long-term treatment is needed for the majority of cases (Brunetti et al. Reference Brunetti, Kern, Vuitton and Writing Panel2010).
E. multilocularis is restricted to temperate cold regions of the northern hemisphere, including Eurasia and North America, where it appears to be the most serious parasitic zoonosis (Eckert et al. Reference Eckert, Conraths and Tackmann2000; Torgerson et al. Reference Torgerson, Keller, Magnotta and Ragland2010). An estimation of the worldwide median incidence indicates that AE occurs in more than 18 000 new human cases per year (Torgerson et al. Reference Torgerson, Keller, Magnotta and Ragland2010). The non-homogeneous geographical distribution of E. multilocularis is well known. Some of the highest historical incidences of human AE are recorded from St. Lawrence Island (SLI) and Western Alaska (Davidson et al. Reference Davidson, Romig, Jenkins, Tryland and Robertson2012); only two autochthonous cases of human AE have been reported in central Canada and the USA (Yamasaki et al. Reference Yamasaki, Nakao, Nakaya, Schantz and Ito2008). AE is widespread across the arctic, subarctic and temperate climate zones of Asia (Eckert et al. Reference Eckert, Gemmell, Meslin and Pawlowski2001). Human AE cases have been reported in Kazakhstan and central and eastern Anatolia, Turkey. In Russia and adjacent countries, there are few available recent data on the distribution and frequency of human AE (Jenkins et al. Reference Jenkins, Romig and Thompson2005). The Japanese island of Hokkaido remains an important endemic focus of E. multilocularis (Davidson et al. Reference Davidson, Romig, Jenkins, Tryland and Robertson2012). Human AE is highly endemic in nine provinces and autonomous regions of China (Xinjiang, Inner Mongolia, Heilongjiang, Qinghai, Gansu, Ningxia, Tibet, Sichuan and Shaanxi), which form three foci with the largest number of human cases in the world (Craig and Echinococcosis Working Group in China, Reference Craig2006; Torgerson et al. Reference Torgerson, Keller, Magnotta and Ragland2010). The prevalence of human AE ranged from 0·2% in northwestern Xinjiang to 4% in Gansu and northwestern Sichuan (Vuitton et al. Reference Vuitton, Zhou, Bresson-Hadni, Wang, Piarroux, Raoul and Giraudoux2003; Li et al. Reference Kulldorff and Nagarwalla2010; Giraudoux et al. Reference Giraudoux, Raoul, Pleydell, Li, Han, Qiu, Xie, Wang, Ito and Craig2013b), and presently there are inexplicably large differences in the incidence of human AE between neighbouring villages (Danson et al. Reference Danson, Graham, Pleydell, Campos-Ponce, Giraudoux and Craig2003, Reference Danson, Craig, Man, Shi and Giraudoux2004; Giraudoux et al. Reference Giraudoux, Raoul, Afonso, Ziadinov, Yang, Li, Quere, Feng, Wang, Wen, Ito and Craig2013a in press - this Special Issue of Parasitology).
In Switzerland, the annual incidence of human AE has more than doubled, increasing from a mean of 0·10 cases per 100 000 inhabitants/year during 1993–2000 to a mean of 0·26 cases per 100 000 inhabitants/year during 2001–2005, paralleling an increase in the fox population (Schweiger et al. Reference Schweiger, Ammann, Candinas, Clavien, Eckert, Gottstein, Halkic, Muellhaupt, Prinz, Reichen, Tarr, Torgerson and Deplazes2007). Identical increases likely occurred in other countries of the core endemic region (Austria, France and Germany) (Moro and Schantz, Reference Moro and Schantz2009). France represented 42% of human AE cases notified in Europe from 1982 to 2000 (Kern et al. Reference Kern, Bardonnet, Renner, Auer, Pawlowski, Ammann, Vuitton, Kern and European Echinococcsis2003; Vuitton et al. Reference Vuitton, Zhou, Bresson-Hadni, Wang, Piarroux, Raoul and Giraudoux2003). Combes et al. (Reference Combes, Comte, Raton, Raoul, Boué, Umhang, Favier, Dunoyer, Woronoff and Giraudoux2012) documented an increase of E. multilocularis infections in foxes in previously known endemic areas and its presence in 25 additional departments (French administrative divisions; median area of 5880 km2) where it had not been previously detected. Earlier studies on human AE distribution in France showed a significantly larger incidence of human AE in the eastern part of the country and the Massif Central (Kern et al. Reference Kern, Bardonnet, Renner, Auer, Pawlowski, Ammann, Vuitton, Kern and European Echinococcsis2003; Grenouillet et al. Reference Grenouillet, Knapp, Millon, Raton, Richou, Piarroux, Piarroux, Manition, Vuitton and Bresson-Hadni2010), with relative risks 52·8–117 times higher than in the rest of the country (Piarroux et al. Reference Piarroux, Piarroux, Knapp, Bardonnet, Dumortier, Watelet, Gerard, Beytout, Abergel, Bresson-Hadni and Gaudart2013). The majority of the analyses were conducted at the resolution level of the French arrondissements (French administrative division, median area of 640 km2) (Kern et al. Reference Kern, Bardonnet, Renner, Auer, Pawlowski, Ammann, Vuitton, Kern and European Echinococcsis2003) or departments (Grenouillet et al. Reference Grenouillet, Knapp, Millon, Raton, Richou, Piarroux, Piarroux, Manition, Vuitton and Bresson-Hadni2010; Piarroux et al. Reference Piarroux, Piarroux, Knapp, Bardonnet, Dumortier, Watelet, Gerard, Beytout, Abergel, Bresson-Hadni and Gaudart2013). In an earlier study conducted in the region of Franche-Comté (16 202 km2), at a finer resolution (French canton, average 140 km2), from 1971 to 1987, Vuitton et al. (Reference Vuitton, Bresson-Hadni, Liance, Meyer, Giraudoux and Lenys1990) reported clusters of human AE in the Doubs department and within the Doubs department on the Jura plateau (600–900 m of altitude), a location where human AE prevalence was shown to correlate with the population densities of the intermediate host Arvicola terrestris (Viel et al. Reference Viel, Giraudoux, Abrial and Bresson-Hadni1999). Elsewhere in the region of Franche-Comté (e.g. the departments of Jura, Haute-Saône and Territoire de Belfort), human AE cases were randomly distributed.
Giraudoux et al. (Reference Giraudoux, Delattre, Takahashi, Raoul, Quere, Craig, Vuitton, Craig and Pawlowski2002) highlighted the importance of clustering in E. multilocularis and human AE case distribution, which continues to pose unsolved epidemiological questions. Clustering may have consequences in the efficiency of national epidemiosurveillance systems. For instance, many local foci of clustered higher Em prevalence in foxes are likely to remain undetected until human cases are observed. Furthermore, in countries where human AE cases are under-reported (e.g. because of the lack of systematic collection of hospital records or where AE patients may not be systematically referred to hospitals), a number of human AE clusters are likely to be concealed. In those cases, the incidence rates that are averaged countrywide under the assumption of a random distribution do not represent the actual distribution and are barely relevant for the prevention and control of AE. Detecting spatial and temporal clusters has been facilitated by the Kulldorff's spatial scan statistics (Kulldorff and Nagarwalla, Reference Kulldorff and Nagarwalla1995) and its software implementation. However, using the method and interpreting the results are not trivial issues (Chen et al. Reference Chen, Roth, Naito, Lengerich and MacEachren2008), especially in cases when complex structures are at stake, such as nested hierarchies of clusters. A better understanding of the transmission processes and epidemiosurveillance efficiency should be facilitated by multi-scale approaches, which combine human and host animal epidemiology studies and landscape analysis (Giraudoux et al. Reference Giraudoux, Delattre, Takahashi, Raoul, Quere, Craig, Vuitton, Craig and Pawlowski2002).
A national population-based registry of human AE is an essential tool for understanding the spatio-temporal variation of the pattern of disease incidence on relevant temporal and spatial scales. In China, human case data are primarily derived from mass screenings in the local community, clinical case reports or hospital data that lack epidemiological details; thus, the prevalence of cases may be largely underestimated, and local foci of human AE may be missed (Zhou et al. Reference Zhou, Chai, Craig, Delattre, Quere, Raoul, Vuitton, Wen and Giraudoux2000; Torgerson et al. Reference Torgerson, Keller, Magnotta and Ragland2010). France, Germany and Switzerland have population-based data registries for human AE, but they have not been used to explore the detailed structure of human AE distribution on several spatial and temporal scales.
The present study is based on data from the French FrancEchino registry from 1982 to 2011. We explored the multi-scale space-time distribution of AE in France. We aimed to detect the spatial limits of large clusters in France and the presence of a nested hierarchy of clusters on the country scale. This study may pave the way toward a better analysis of the environmental and human factors that are responsible for the distribution of the disease and can also contribute to the development of predictive models that better target information and preventative action.
MATERIALS AND METHODS
Human AE register
The French population-based registry, FrancEchino, has recorded data on human AE cases since 1982. Created in 1997, this registry included AE cases retrospectively from 1982 to 1997 and prospectively since 1998, using a previously described methodology (Piarroux et al. Reference Piarroux, Piarroux, Giorgi, Knapp, Bardonnet, Sudre, Watelet, Dumortier, Gerard, Beytout, Abergel, Mantion, Vuitton, Bresson-Hadni and FrancEchino2011). The registry has been supported by the Institut de Veille Sanitaire (Institute of Public Health Surveillance) since 2003. The database includes only patients who present with the criteria of possible, probable or proven human AE (i.e. epidemiology, clinical history and a typical liver lesion morphologically identified by imaging techniques and positive serology, or lesions confirmed by positive histopathology and molecular techniques) (Brunetti et al. Reference Brunetti, Kern, Vuitton and Writing Panel2010; Piarroux et al. Reference Piarroux, Piarroux, Giorgi, Knapp, Bardonnet, Sudre, Watelet, Dumortier, Gerard, Beytout, Abergel, Mantion, Vuitton, Bresson-Hadni and FrancEchino2011). In the majority of cases, information about the commune (French administrative division of some tens of square kilometres) of the patient's residence at the time of diagnosis, previous residences, age, sex and occupation are obtained from epidemiological questionnaires. The data collection and recording for the FrancEchino registry received the ethical approval from the Protection of Human Subjects in Biomedical Research Committee (CCPPRB) and the National Commission on Informatics and Liberty (CNIL) for the use of nominative data. At the time of their diagnosis, all of the patients provided their informed consent regarding the use of their data for research purposes.
Reference population
Census population data were available at the National Institute for Statistics and Economic Studies (http://www.insee.fr) for 1982, 1990, 1999 and 2008. For each commune, we obtained the total population subdivided by sex and age-group in 5-year intervals.
To increase the sample size of each spatial unit, we used the canton as the statistical unit. The canton is an administrative unit that pools communes, with a mean area of 140 km2 and a mean population of approximately 16 000 inhabitants (Ozouf-Marignier and Verdier, Reference Ozouf-Marignier, Verdier, Lagadec, Le Bihan and Tanguy2009).
Statistics and graphical display
To visualize the spatial distribution of AE cases, we performed classical disease mapping of the number of cases per canton and of AE incidence rates using R 2.12.2 (R Development Core Team, 2012) and the package sp 1·0–5 (Bivand et al. Reference Bivand, Pebesma and Gomes-Rubio2008). We calculated the incidence rates as the number of cases per inhabitants/year for each canton. We estimated the number of inhabitants/year in the present study as the annual population count for each canton, from 1982 to 2011. The population data were available for census times only. For times between censuses, a linear interpolation was computed based on the population at the bracketing census times. We also studied the annual variation of AE cases.
Possible cluster location was analysed using the spatial scan statistic developed by Kulldorff and implemented in the SaTScan v9.1.1 software (Kulldorff and Nagarwalla, Reference Kulldorff and Nagarwalla1995). The scan statistic is a method for detecting non-random distributions in multidimensional point datasets. We tested the null hypothesis that the number of cases in each canton followed a Poisson distribution. Kulldorff's method imposes a circular window on the map and moves the circle centre over each point location (the centroid of each canton) so that the window includes different sets of neighbouring points at different positions. At each point location, the radius of the circle is increased continuously from 0 to a user-defined maximum radius. We adopted the default setting of a maximum containing at most 50% of the total population. SaTScan detects potential clusters by calculating a likelihood ratio for each circle comparing the relative risks in and outside the window. The circle with the maximum likelihood ratio among all radius circles at all possible point locations is considered to be the most likely cluster (called the primary cluster). SaTScan also identifies secondary clusters that have a significantly large likelihood ratio but are not the primary cluster. The number of expected cases was adjusted with age and sex as covariates, using indirect standardization (Naing, Reference Naing2000). For hypothesis testing, a Monte Carlo procedure was used to generate 999 random replications of the dataset under the null hypothesis. Chen et al. (Reference Chen, Roth, Naito, Lengerich and MacEachren2008) have noted and discussed a number of limitations of the Kulldorff's spatial scan statistic. The primary limitation of this type of statistic is that it is difficult to determine optimal settings for scaling parameters and that SaTScan may subsequently report statistically significant large clusters that contain a high proportion of low-risk areas. Chen et al. described those large areas as heterogeneous clusters. Smaller homogeneous subsets within the larger heterogeneous clusters may exhibit cumulative incidence values that are high enough to reject the null hypothesis on their own strength. Chen et al. (Reference Chen, Roth, Naito, Lengerich and MacEachren2008) described these subsets as core clusters. To account for the possible existence of a nested hierarchy of clusters, we adopted the following exploratory approach. First, we searched for statistically significant spatial clusters on the entire dataset using SaTScan; subsequently, we performed spatial scans within the subsets corresponding to each cluster detected. This search was repeated iteratively for each core cluster detected until we discerned no new clusters.
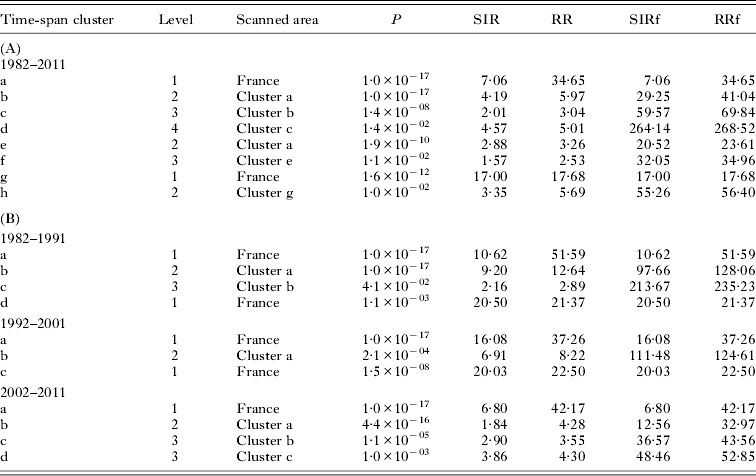
For each cluster, the relative risk (RR) and the standardized incidence rates (SIR) were generated using the SaTscan software. The RR corresponded to the ratio of the observed to expected cases inside the scanned area divided by the ratio of observed to expected cases outside the scanned area. The SIR was the ratio of observed to expected cases within the scanned area. We also calculated the relative risk (RRf) and the standardized incidence rates (SIRf) using the totality of the French population as the reference.
The low number of new AE cases each year did not permit us to perform the spatio-temporal scan analysis once with acceptable statistical robustness. The data were collapsed by 10-year time-spans for the spatial analysis, and temporal trends by year were analysed separately with no spatial component. We detected temporal clusters using SaTScan and a window moving over time. The maximum temporal window size was set to 50% of the total population.
RESULTS
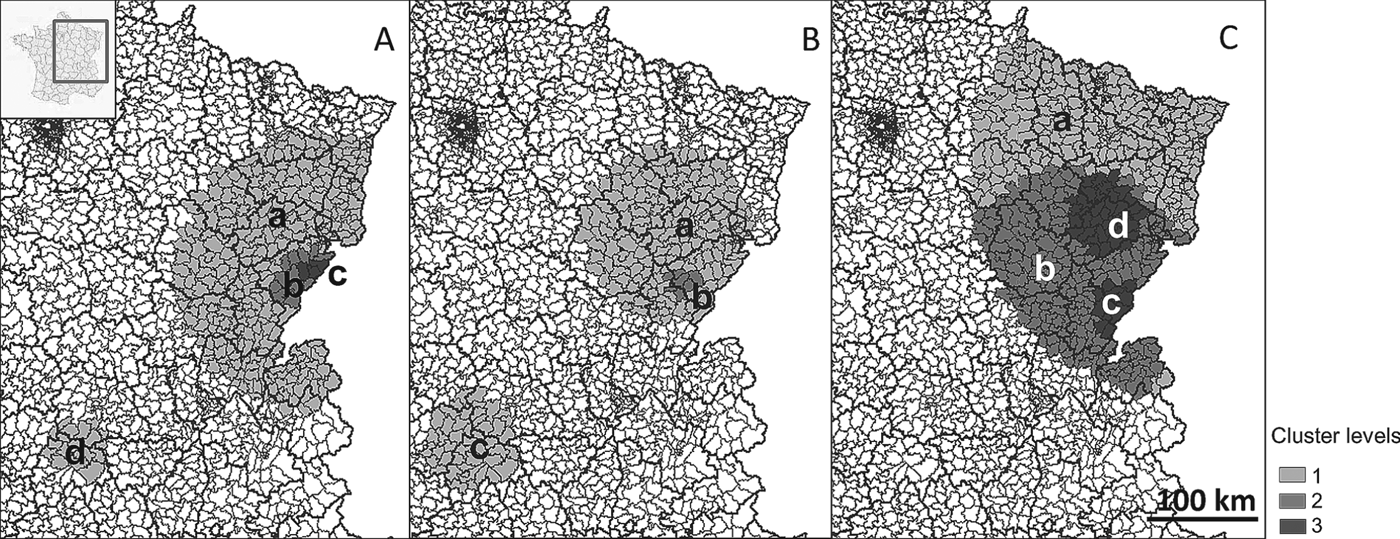
The July 2012 update of the FrancEchino registry reported a total of 509 diagnosed AE cases between 1982 and December 2011. This finding corresponded to an overall incidence rate of 0·027 cases per 100 000 inhabitants/year. Over the 30-year time-span of this study, the number of cases per canton varied from 0 to 10 cases (Fig. 1). This maximum number occurred in the canton of Pierrefontaine-les-Varans (located in the Doubs department) (Fig. 1A), which corresponds to an average incidence rate of 4·7 cases per 100 000 inhabitants/year (Fig. 1B). The maximum incidence rate was found in the canton of Amancey (also located in the Doubs department), with an average incidence rate of 8·1 cases per 100 000 inhabitants/year (Fig. 1A and B) with 8 cases diagnosed in 30 years.

Fig. 1. Human AE distribution in France, 1982–2011. Number of cases per canton (A); Incidence per 100 000 inhabitants/year (B); Cluster levels (C).
Information on the locality of diagnosis, age and gender was available for 489 cases only, which were included in the cluster analysis. The results of the spatial scan analysis for the entire study period are summarized in Fig. 1C and Table 1. On the country scale, two significant spatial clusters were detected, which corresponded to Eastern France (cluster a, P = 10−17, SIR = 7·06, RR = 34·7) and the Massif Central region (cluster g, P = 1·6×10−12, SIR = 17·0, RR = 17·7). Within the Eastern France cluster, 4 levels of nested clusters were detected. The core cluster (level 4) with the highest incidence was the canton of Amancey (P = 0·014, SIRf = 264·14, RRf = 268·5).
Table 1. Cluster characteristics, for 1982–2011 (A) and 1982–1991, 1992–2001, 2002–2011 (B)

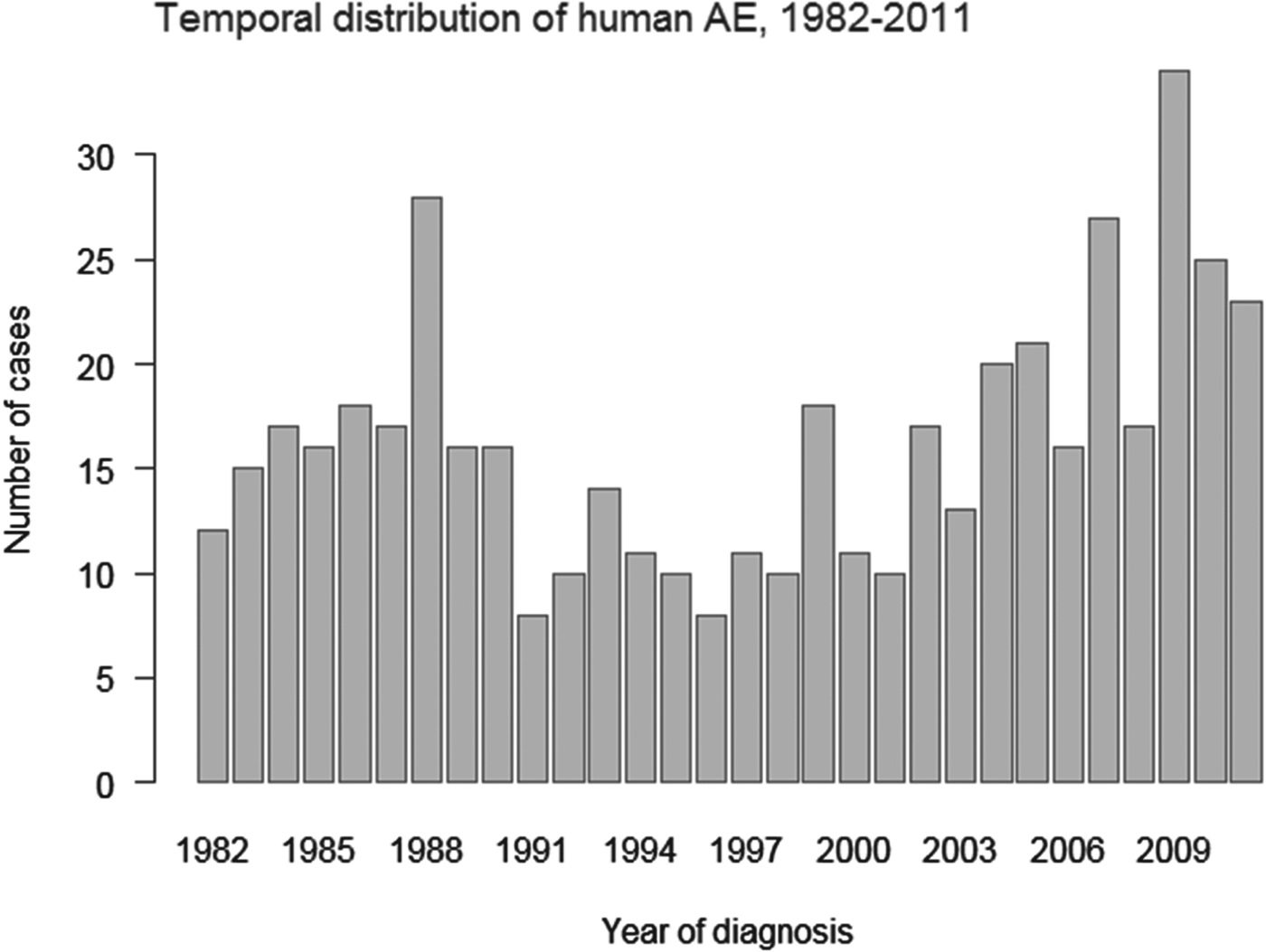
Fig. 2 shows that cluster locations varied over time, to some extent, with a similarly nested structure. Up to 3 cluster levels were detected in Eastern France for each 10-year time span. The Eastern cluster shrank during the 1991–2001 period and subsequently expanded during the 2002–2011 period. Furthermore, the Massif Central cluster faded during the last decade. The number of incident cases showed a temporal variation from 8 to 34 cases per year with a median of 16 cases per year (Fig. 3), with a minimum incidence rate of 0·014/100 000 and a maximum of 0·059/100 000. Purely temporal scan statistics detected a statistically significant lower incidence rate cluster from 1991 to 2003 (RR = 0·59, P = 0·001) and a higher incidence rate cluster from 2007 to 2011 (RR = 1·47, P = 0·011).

Fig. 2. Clusters for 1982–1991 (A), 1992–2001 (B) and 2001–2011 (C).

Fig. 3. Annual variation of AE distribution in France, 1982–2011.
DISCUSSION
The incidence of patterns of disease and the mortality rate over time and space can provide clues to detect the processes and the causes of diseases (Whittemore et al. Reference Whittemore, Friend, Brown and Holly1987). Those patterns can be complex, especially when the factors responsible for disease transmission differ at various spatial scales. Ecological systems are nested within one another, which also applies to disease transmission systems; this well-known fundamental hierarchical organization is easy to detect in nature (Allen and Star, Reference Allen and Star1982). Nevertheless, this hierarchical organization has been generally undervalued as a source of influence on the structure and development of pathogen transmission patterns and also as a means for understanding the crucial connections between the local processes and the large-scale distribution patterns (Giraudoux et al. Reference Giraudoux, Raoul, Pleydell, Li, Han, Qiu, Xie, Wang, Ito and Craig2013b).
Nested spatial and temporal structures are neither easily nor safely detected by simple examination of choropleth (value-by-area) maps. Epidemiological studies that investigate the nested spatial structure of disease distribution specifically are still rare. For instance, in earlier studies conducted in the United States, several researchers detected nested clustered distributions relative to cervical (Chen et al. Reference Chen, Roth, Naito, Lengerich and MacEachren2008) and prostate (Boscoe et al. Reference Boscoe, McLaughlin, Schymura and Kielb2003) cancer mortality. The authors identified well-defined core clusters that were included in less-certain periphery clusters on two hierarchical levels.
In the present study, on the scale of a 550 000 km2 country, we demonstrated that the distribution of human AE, a rare zoonotic parasitic disease, can be described as a nested hierarchy of clusters on 4 levels. Furthermore, although AE endemic areas were thought to be extremely stable spatially over time, our results indicated unexpected variations in cluster locations and limits over a 30-year time-span. This approach is one step closer to disclosing the processes that support such patterns. However, the source of variations may mix real variations in the transmission processes and exposure with epidemiological artefacts because of variations in recruitment biases over time and the choice of model parameters. Chen et al. (Reference Chen, Roth, Naito, Lengerich and MacEachren2008) have shown in their study on cervical cancer mortality how Kulldorff's statistics are sensitive to parameter choices related to cluster scaling (e.g. how SaTScan clusters tend to contain heterogeneous contents). In the present study, this difficulty has been overcome by iteratively scanning each cluster detected, top down, along a spatially nested hierarchy until the scan statistics could not detect a new cluster.
Temporal clusters were also found as follows: a significantly lower incidence from 1991 to 2003 and a higher incidence from 2007 to 2011. Those differences are attributable to selection biases. Those cases that were diagnosed before 1997 were recorded retrospectively. Since 1998, the official creation of the French registry permitted a prospective notification of cases. Since 2003, the support of national health authorities allowed for more active prospective surveys. They were and still are implemented by the FrancEchino coordination team and the development of the FrancEchino Network. This new combination of sources of information (microbiologists, pathologists and clinicians) presents a key point for more exhaustive surveillance (Jorgensen et al. Reference Jorgensen, an der Heiden, Kern, Schoneberg, Krause and Alpers2008). Moreover, a significant increase in the proportion of fortuitous cases diagnosed since 1982 has been reported. In 2011, more than 50% of the patients were asymptomatic at the time of diagnosis; for the majority of the cases, these patients were diagnosed using imaging techniques implemented for other reasons (Piarroux et al. Reference Piarroux, Piarroux, Giorgi, Knapp, Bardonnet, Sudre, Watelet, Dumortier, Gerard, Beytout, Abergel, Mantion, Vuitton, Bresson-Hadni and FrancEchino2011). This evolution may indicate that medical teams have enhanced awareness of this disease. From the beginning to the end of the study period, an increased efficacy in case detection has likely occurred. Another source of selection bias is that a large sero-epidemiologic screening was performed between 1987 and 1996 in the Doubs department, the most prevalent area for human AE in France at that time (Bresson-Hadni et al. Reference Bresson-Hadni, Laplante, Lenys, Rohmer, Gottstein, Jacquier, Mercet, Meyer, Miguet and Vuitton1994). This study led to the diagnosis of AE cases before 1991, which explains the high number of AE cases diagnosed early in 1988. This study may have led to a lower rate of AE diagnosis in the years after this study (although the number of cases detected during this screening does not totally compensate for the observed decrease in the following years).
Those biases alone can neither justify the patterns observed nor their variations. For instance, the shrinking of the eastern cluster during the period 1991–2003 does not parallel the putative increased detection efficiency of human AE over the study period. Furthermore, the spatial variations of the location of the low-level clusters over time in Franche-Comté cannot be attributed to the variations of public health awareness in this area where AE has been studied intensively from the 1980s (Vuitton et al. Reference Vuitton, Bresson-Hadni, Liance, Meyer, Giraudoux and Lenys1990). Schweiger et al. (Reference Schweiger, Ammann, Candinas, Clavien, Eckert, Gottstein, Halkic, Muellhaupt, Prinz, Reichen, Tarr, Torgerson and Deplazes2007) have shown in a 50-year survey that the increased prevalence of human AE paralleled the increased density of the fox population in Switzerland. Comparable studies are unavailable in France but, in a study conducted from 2005 to 2010, Combes et al. (Reference Combes, Comte, Raton, Raoul, Boué, Umhang, Favier, Dunoyer, Woronoff and Giraudoux2012) have shown an increase of E. multilocularis prevalence in foxes and the extension of the parasite's distribution range toward western France. The discovery of sporadic AE cases in these western areas from a decade ago may indicate that parasite transmission in wildlife and human exposure may have occurred and remained undetected long ago (Vuitton et al. Reference Vuitton, Wang, Zhou, Raoul, Knapp, Bresson-Hadni, Wen and Giraudoux2011).
In conclusion, our study demonstrates the intrinsically clustered distribution of AE and the fact that large clusters may hide core-clusters up to 4 levels, of which several vary in space and time. The processes that support those nested-clustered patterns are far from being understood, although the pattern itself may be general in Asia (see Giraudoux et al. Reference Giraudoux, Raoul, Afonso, Ziadinov, Yang, Li, Quere, Feng, Wang, Wen, Ito and Craig2013a, in press in this Special Issue of Parasitology). The validity of any epidemiosurveillance system depends on the data quality and the sampling strategy by which those data are acquired (Jorgensen et al. Reference Jorgensen, an der Heiden, Kern, Schoneberg, Krause and Alpers2008). Furthermore, human AE surveillance can hardly be considered an early warning system. Actually, the detection of human cases reveals the existence of intensive transmission years ago in animal hosts and subsequent human exposure, when the risk could have been disclosed by monitoring the definitive host (fox, dog) infection. The National Human AE registers are not exempted from epidemiological biases; contrary to the mass-screening of self-selected populations in areas generally known to be endemic, the registers are likely the best way to achieve a valid picture of human AE distribution on various scales. They can help to optimize the designs of epidemiological surveillance systems and cost-effective preventative strategies by considering the spatial and temporal structure of this helminthic zoonosis. The correlations between E. multilocularis or AE distribution and climate, land use, host population dynamics have been demonstrated for a long time (Giraudoux et al. Reference Giraudoux, Delattre, Takahashi, Raoul, Quere, Craig, Vuitton, Craig and Pawlowski2002, Reference Giraudoux, Craig, Delattre, Bartholomot, Bao, Barnish, Harraga, Quéré, Raoul, Wang, Shi and Vuitton2003, Reference Giraudoux, Raoul, Pleydell, Li, Han, Qiu, Xie, Wang, Ito and Craig2013b; Atkinson et al. Reference Atkinson, Gray, Clements, Barnes, McManus and Yang2013), but the details of the processes that explain the observed multi-scale patterns have not been established. The nested hierarchy of AE clusters disclosed in the present study has not been explored specifically from this perspective. Therefore, we anticipate that our study may be a reasonable starting point to pursue additional research in which environmental and social factors could be considered on several spatial and temporal scales to predict the risk of human disease and guide pre-emptive public health actions against human AE disease.
ACKNOWLEDGEMENTS
We thank all of the members of the FrancEchino Network and the clinicians, biologists and pharmacists who contributed to the reporting of cases and data collection in the present study. We appreciate the endeavours of everyone who worked diligently to conduct research and allow this manuscript to come to fruition.
FINANCIAL SUPPORT
The FrancEchino Registry is supported, in part, by the Institute of Public Health Surveillance (InVS). This research has been conducted within the context of the GDRI (International Research Network) ‘Ecosystem Health and Environmental Disease Ecology’.