Introduction
Bovine viral diarrhea virus (BVDV) was described in cattle in 1946 (Olafson et al., Reference Olafson, AD and FH1946) and is estimated to infect >80% of cattle populations worldwide (Ridpath, Reference Ridpath2010b). BVDV infections cause a loss of more than $400 million per year in the USA, leading to designation of BVDV as the most costly bovine viral disease in the country (Houe, Reference Houe1999; Fray et al., Reference Fray, Paton and Alenius2000; Gunn et al., Reference Gunn, Stott and Humphry2004). BVDV infections remain prevalent and unresolved because vaccination programs are not 100% effective and are not universally implemented. Also, it is impossible to distinguish vaccinated from infected pregnant cattle that carry a persistently infected (PI) fetus. Clarification of impaired or delayed fetal innate and adaptive immune mechanisms that lead to persistent BVDV infection, and diagnostics to detect cattle that are pregnant with PI fetuses, may greatly contribute to biocontainment and resolving infection.
The clinical indicators of acute infections are similar for both genotypes (1 & 2) and biotypes (cytopathic, cp; noncytopathic, ncp) of BVDV, and present as inapparent or subclinical infection to death of early embryos, abortion, congenital defects, and weak or stillborn calves. Acutely infected animals typically recover and, even though they eliminate the virus within 10–14 days post-infection, there are negative secondary consequences of these infections. A significant consequence is immunosuppression and pre-disposition to secondary infections (Dubovi, Reference Dubovi1994; Moerman et al., Reference Moerman, Straver, de Jong, Quak, Baanvinger and van Oirschot1994; Valle et al., Reference Valle, Skjerve, Martin, Larssen, Osteras and Nyberg2005). The impact of calves PI with BVDV on feedlot performance and secondary onset of bovine respiratory disease (BRD) can be significant (Loneragan et al., Reference Loneragan, Thomson, Montgomery, Mason and Larson2005; Hessman et al., Reference Hessman, Fulton, Sjeklocha, Murphy, Ridpath and Payton2009). Also, BVDV may provoke secondary infection such as BRD by altering pulmonary and systemic immune defenses and directly inducing lung damage, which, collectively, may facilitate secondary bacterial infection (Martin and Bohac, Reference Martin and Bohac1986; Ridpath, Reference Ridpath2010a). Interestingly, diarrhea is associated with mucosal disease, but is the least recognized clinical entity in BVDV-infected herds.
BVDV is in the genus Pestivirus (family Flaviviridae) and exists as a single-stranded RNA virus with ncp or cp biotypes (Bielefeldt-Ohmann, Reference Bielefeldt-Ohmann1995). Infection of naïve pregnant cows with ncpBVDV results in transplacental infection of the fetus (Harding et al., Reference Harding, Cao, Shams, Johnson, Vassilev, Gil, Wheeler, Haines, Sibert, Nelson, Campos and Donis2002). Infection with ncpBVDV after about day 150 of gestation results in transient infection (TI) wherein the cow and fetus clear the virus through innate and adaptive immune responses (Ridpath, Reference Ridpath2010b). If infection with ncpBVDV occurs before days 90–150, then virus is not recognized as foreign by the fetal immune system. Consequently, immune tolerance to the infecting BVDV strain occurs, which results in persistent infection (Stokstad and Loken, Reference Stokstad and Loken2002). Persistent infection with BVDV is currently thought to be caused by impaired innate immune response coupled with lack of adaptive immune responses. Because the virus is present prior to immune education, it is incorporated into the repertoire of host antigens (Potgieter, Reference Potgieter1995; Peterhans et al., Reference Peterhans, Jungi and Schweizer2003). Immune tolerance to BVDV results in failure to clear the infection by the adaptive immune system. Presented in this review is the work completed by our collaborative team to support the following hypothesis: fetal infection with ncpBVDV persists because of impaired fetal Type II interferon (IFN)-γ induction and responses in the face of robustly activated fetal Type I IFN responses. The central premise for this review is that establishment of fetal persistent infection with ncpBVDV occurs during fetal maturation of both branches of the immune system and is mostly caused by the inability of IFN-γ to facilitate acquired immune responses to ncpBVDV; although, an impaired type I response may also need to be considered.
Epitheliochorial placenta
Establishment of fetal persistent infection with BVDV prior to the development of fetal immune competency involves complex interactions between the dam, fetus, and placenta. The bovine epitheliochorial placenta contains placentomes, which are organs of nutrient exchange that are composed of fetal cotyledons and maternal caruncles (Hay, Reference Hay1991; Leiser and Kaufmann, Reference Leiser and Kaufmann1994; Leiser et al., Reference Leiser, Krebs, Klisch, Ebert, Dantzer, Schuler and Hoffmann1997; Igwebuike, Reference Igwebuike2006). Maternal viral infections, such as BVDV, can be transferred to the fetus. However, this placental organization precludes transplacental delivery of large maternal proteins such as immunoglobulins to the fetus and thus largely isolates the developing fetus from the maternal immune response (reviewed in (Chucri et al., Reference Chucri, Monteiro, Lima, Salvadori, Kfoury and Miglino2010)). Persistent infection with BVDV adversely affects fetal growth and development of multiple organs, including brain (Bielefeldt-Ohmann et al., Reference Bielefeldt-Ohmann, Tolnay, Reisenhauer, Hansen, Smirnova and Van Campen2008, Reference Bielefeldt-Ohmann, Smirnova, Tolnay, Webb, Antoniazzi, van Campen and Hansen2012) and bone (Webb et al., Reference Webb, Norrdin, Smirnova, Van Campen, Weiner, Antoniazzi, Bielefeldt-Ohmann and Hansen2012), and alters fetal antiviral immune responses (Shoemaker et al., Reference Shoemaker, Smirnova, Bielefeldt-Ohmann, Austin, van Olphen, Clapper and Hansen2009; Smirnova et al., Reference Smirnova, Webb, Bielefeldt-Ohmann, Van Campen, Antoniazzi, Morarie and Hansen2012).
Maternal and fetal infection with BVDV and induction of innate immune responses
To study why BVDV infection persists, we inoculated 46 pregnant BVDV-naïve heifers with 2 ml of media (controls) or 4.5 log TCID50 ncpBVDV2 strain 96B2222 intranasally on day 75 of gestation (Smirnova et al., Reference Smirnova, Webb, Bielefeldt-Ohmann, Van Campen, Antoniazzi, Morarie and Hansen2012) (Fig. 1). Maternal and fetal blood cell RNA was examined using real-time quantitative reverse transcriptase PCR (qRTPCR) for BVDV and markers of innate and adaptive immune responses. The greatest BVDV RNA concentration in fetal blood occurred on day 97 (22 days after infection), which significantly decreased by days 192 and 245 (P < 0.01). Thus, the temporal induction of BVDV in maternal blood is followed by appearance and replication of BVDV in fetal blood, with a reduction in amount of virus in PI fetuses over time to levels that are lower on days 192 and 245 compared with 97 (also see Fig. 8). Controls were BVDV-negative. The innate immune system responds to viral RNA and pathogen-associated molecular patterns (PAMPs) through pattern recognition receptors (PRRs) such as RNA Helicase; DEAD (ASP-Flu-ALA-ASP) box polypeptide 58 (DDX58, RIGI) and melanoma differentiation-associated protein 5 (MDA5) RNA helicases and Toll-like receptors that are induced shortly after viral infection (Saito and Gale, Reference Saito and Gale2007). This primary innate immune response activates downstream signal transduction, phosphorylation of transcription factors and upregulation of Type I IFN, which then induces antiviral and adaptive (response of antigen-specific lymphocytes to antigen and acquisition of immunological memory) immune responses (Alexopoulou et al., Reference Alexopoulou, Holt, Medzhitov and Flavell2001; Samuel, Reference Samuel2001).

Fig. 1. Experimental design to examine fetal response to persistent infection with ncpBVDV. Fetuses were collected by Cesarean section on days 82, 89, and 97 (n = 4 control and four PI fetuses), day 192 (n = 7 control and seven PI fetuses), and day 245 (n = 4 control and three PI fetuses). Fetal umbilical cord blood and fetal tissues were either preserved with Tri-reagent BD or snap-frozen in liquid nitrogen. Peak of maternal viremia was detected with qRTPCR for BVDV as described previously (Smirnova et al., Reference Smirnova, Bielefeldt-Ohmann, Van Campen, Austin, Han, Montgomery, Shoemaker, van Olphen and Hansen2008) on days 82–85, i.e. 7–10 days post-infection. Maternal seroconversion, through production of anti-BVDV antibodies, was confirmed at 28 days after infection using a standard serum neutralization assay. BVDV RNA was not detected in the blood of PI fetuses until day 89. Adapted from (Smirnova et al., Reference Smirnova, Webb, Bielefeldt-Ohmann, Van Campen, Antoniazzi, Morarie and Hansen2012).
Upregulation of RNA helicases and ISGs in response to fetal persistent infection
The viral recognition receptors, RIG-I and MDA5 helicases, are upregulated in response to persistent infection with BVDV in fetal blood cells with RIG-I being the earliest responder (Smirnova et al., Reference Smirnova, Bielefeldt-Ohmann, Van Campen, Austin, Han, Montgomery, Shoemaker, van Olphen and Hansen2008; Shoemaker et al., Reference Shoemaker, Smirnova, Bielefeldt-Ohmann, Austin, van Olphen, Clapper and Hansen2009) (Fig. 2). We also found significant upregulation of Type I IFN-stimulated genes (ISGs) in PI fetal blood and tissues (Shoemaker et al., Reference Shoemaker, Smirnova, Bielefeldt-Ohmann, Austin, van Olphen, Clapper and Hansen2009; Hansen et al., Reference Hansen, Smirnova, Van Campen, Shoemaker, Ptitsyn and Bielefeldt-Ohmann2010) suggestive of Type I IFN in fetal PI blood, but were not able to detect antiviral bioactivity in fetal blood on day 190 of gestation. Detection of IFN-α or IFN-β proteins in blood was not possible due to lack of specific and sensitive anti-bovine Type I IFN antibodies that have been standardized for enzyme-linked immunosorbent assay (ELISA) or radioimmunoassay. However, RIG-I, MDA5 and ISGs mRNA concentrations were increased either prior to (RIG-I, day 82) or by the time (MDA5 and ISG15 ubiquitin-like modifer (ISG15), day 97) of detection of fetal BVDV RNA peak. Based on these findings, we hypothesize that signaling of RNA helicases might be impaired in PI fetuses, which contributes to impaired secretion and action of Type I IFN. Alternatively, it is feasible that the Type I IFN production and response is localized to fetal tissues, rather than being detected as a peripheral response in blood leukocytes.

Fig. 2. Activation of innate immune response reflected in increase of RIG-I, MDA5, and ISG15 mRNA concentrations in fetal blood following infection with BVDV on day 75 of gestation (qRTPCR). Note: *P < 0.05; **P < 0.01; ***P < 0.001. Adapted from (Smirnova et al., Reference Smirnova, Webb, Bielefeldt-Ohmann, Van Campen, Antoniazzi, Morarie and Hansen2012).
Viral PAMPs induce caspase-associated recruitment domains (CARDs) of RIG-I and activate a CARD-containing adaptor called IPS-1 (IFN-β promoter simulator 1), which relays the signal to a complex of protein kinases. A critical regulatory kinase is TRAF family member-associated NFKB activator (TANK)-binding kinase 1 (TBK1), which phosphorylates IFN regulatory factors (IRFs) IRF3 and IRF7 and leads to heterodimerization and translocation of IRFs to the nucleus where they bind IFN-stimulated response elements (ISRE) within the promoters of Type I IFN genes and induce transcription (reviewed in (Tamura et al., Reference Tamura, Yanai, Savitsky and Taniguchi2008)). An impaired innate immune response in PI fetuses might occur through sub-optimal activation of the complete antiviral Type I IFN response (Schweizer and Peterhans, Reference Schweizer and Peterhans2001; Baigent et al., Reference Baigent, Zhang, Fray, Flick-Smith, Goodbourn and McCauley2002; Iqbal et al., Reference Iqbal, Poole, Goodbourn and McCauley2004; Gil et al., Reference Gil, Ansari, Vassilev, Liang, Lai, Zhong, Hong, Dubovi and Donis2006a, Reference Gil, van Olphen, Mittal and Donisb; Schweizer et al., Reference Schweizer, Matzener, Pfaffen, Stalder and Peterhans2006; Chen et al., Reference Chen, Rijnbrand, Jangra, Devaraj, Qu, Ma, Lemon and Li2007). For example, ncpBVDV might impair the induction of Type I IFN by interfering with function of IRF3 and IRF7 as described in a non-immune cell model of calf testes cells in vitro (Baigent et al., Reference Baigent, Zhang, Fray, Flick-Smith, Goodbourn and McCauley2002, Reference Baigent, Goodbourn and McCauley2004). This action of ncpBVDV is targeted for future study by our group using fetal tissues collected following in vivo infection. Furthermore, impaired Type I IFN response may also impact B-cell activation and production of antibody as well as induction of cytokines complimentary to antiviral action of Type I IFN through tumor necrosis factor receptor Type I-associated DEATH domain protein (TRADD)/Fas-Associated protein with Death Domain (FADD) and nuclear factor of kappa light polypeptide gene enhancer in B-cells (NF-κB) (Le Bon et al., Reference Le Bon, Thompson, Kamphuis, Durand, Rossmann, Kalinke and Tough2006) (Le Bon et al., Reference Le Bon, Thompson, Kamphuis, Durand, Rossmann, Kalinke and Tough2006).
Innate immune responses such as RIG-I were induced in fetal blood as early as day 82, and in addition to MDA5 and ISG15, continued to be upregulated on days 97 and 192 of gestation (Fig. 2). Detection of viral double-stranded RNA (dsRNA) by RIG-I and MDA5 normally leads to activation of the Type I IFN pathway through phosphorylation of IRF3 and IRF7 and, consequently, upregulation of ISGs. Upregulation of RIG-I is the earliest significant fetal response to BVDV infection and occurs prior to detection of virus by day 89. The upregulation of RIG-I is followed several days later by MDA5 as well as ISG15. Note that these ISGs are upregulated at a time immediately preceding decline in fetal viral RNA from day 97 to days 192–245. This decline in BVDV load in PI fetuses is of great interest in context of possible initiation of antigen presentation and recognition, followed by failure of adaptive immune responses.
RIG-I, MDA5, DEXH (Asp-Gu-X-His) box polypeptide 58 (LGP2), ISG15, myxovirus resistance factor 2 (MX2), and protein kinase R (PKR) mRNA concentrations increased in day 190 PI fetal blood (Shoemaker et al., Reference Shoemaker, Smirnova, Bielefeldt-Ohmann, Austin, van Olphen, Clapper and Hansen2009). These data confirm the long-term activation of RNA helicases and induction of ISGs in fetal persistent infection with BVDV, which may be caused through continued stimulation from Type I IFN as well as continuous presence of BVDV dsRNA and PAMPs. Exactly why these innate responses do not resolve persistent BVDV infection is not known and warrants further study. Also, the prolonged innate response in PI fetuses due to viral persistence is of great interest in the context of how it might impact fetal development and postnatal predisposition to secondary infections (Speer, Reference Speer2003; Toder et al., Reference Toder, Fein, Carp and Torchinsky2003).
IRF7, but not IRF3 mRNA is upregulated in response to fetal persistent infection
IRF3 and IRF7 are major transcriptional inducers of Type I IFN after RIG-I and MDA5-mediated activation through phosphorylation, heterodimerization and translocation to the nucleus to induce ISREs on Type I IFN genes. Microarray analysis comparing day 97 fetal spleen from control and PI fetuses (see Smirnova et al., Reference Smirnova, Webb, McGill, Schaut, Bielefeldt-Ohmann, Van Campen, Sacco and Hansen2014) revealed upregulation of IRF7, but not IRF3 mRNA in PI fetuses. IRF3 and IRF7 mRNAs were examined with qRTPCR in fetal spleen, liver and thymus to determine if downstream effectors of RIG-I and MDA5 were upregulated in fetal hematopoietic tissues in response to BVDV persistent infection (Fig. 3). IRF3 mRNA was abundant in these tissues with only a slight upregulation in concentration in PI thymus on day 89 of gestation. This is interpreted to mean that transcription of the IRF3 gene is not upregulated consistently in these tissues in response to fetal persistent infection. Whether this translates into lack of phosphorylation of IRF3 protein remains to be determined. In contrast, transcriptional upregulation of IRF7 mRNA was first noted by day 89 in thymus followed by day 97 in spleen and liver. Using an in vitro calf testes model, immunofluorescent staining and electrophoretic gel shift assay with an ISRE for ISG15, it was shown that both cpBVDV and ncpBVDV inhibit interaction of IRF3 with the ISRE. There was no effect on nuclear uptake of IRF7 in testes cells in vitro (Baigent et al., Reference Baigent, Zhang, Fray, Flick-Smith, Goodbourn and McCauley2002). Likewise, using in vitro approaches it was demonstrated that NPro from BVDV may block IRF-3 from binding to DNA and target IRF-3 to proteasomal degradation (Hilton et al., Reference Hilton, Moganeradj, Zhang, Chen, Randall, McCauley and Goodbourn2006). In contrast to the findings in these in vitro studies, IRF3 and IRF7 mRNA concentrations appear to be upregulated in fetal hematopoetic tissues, particularly IRF7, in response to ncpBVDV infection in vivo (Fig. 3).

Fig. 3. IRF3 and IRF7 mRNA concentrations in control and PI spleen, liver, and thymus. *P < 0.05; **P < 0.01; ***P < 0.001.
Type I IFN in fetal PI tissues
IFN-α and IFN-β were detected in very low concentrations that did not change in blood of PI compared with control fetuses on each stage of gestation examined. IFNα and IFNβ mRNAs were present in spleen and bone marrow, but concentrations did not differ in PI versus control fetuses. These data might reflect an impaired induction of Type I IFN transcription of genes by ncpBVDV at some point downstream of activation of RIG-I and MDA-5. The absence of upregulation of Type I IFN mRNA in response to persistent infection in these fetal tissues despite upregulation of IRF3 and IRF7 mRNAs might be explained through viral interference with IRF3/IRF7 mRNA, translation, phosphorylation, nuclear translocation of these proteins, or interaction of IRF3/IRF7 complexes with ISRE in the promoter region of Type I IFN gene (Peterhans and Schweizer, Reference Peterhans and Schweizer2013). Also, Type I IFN that is inducing ISGs might be from a different source, e.g. lymph nodes (Palomares et al., Reference Palomares, Walz and Brock2013). A lack of antibodies against these bovine IFNs impairs determination if these cytokines are circulating peripherally in the blood or are acting locally in tissues. Without validated ELISA or radioimmunoassay for bovine Type I IFN, indirect indicators through bioassays such as antiviral assays or upregulation of ISGs are commonly used when making inferences regarding release and actual circulating concentrations of Type I IFN. However, it is clear that any post-natal bovine that is infected with ncpBVDV (and intact Npro) is able to mount a Type I IFN response. Moreover, these animals do not become persistently infected.
Induction of ISGs in fetal PI tissues
The most compelling argument that the innate immune response through Type I IFN is active in fetuses PI with BVDV is the upregulation of ISGs encoding ISG15, MX2, PKR and the RNA helicases RIG-I and MDA5 (Shoemaker et al., Reference Shoemaker, Smirnova, Bielefeldt-Ohmann, Austin, van Olphen, Clapper and Hansen2009). Also, we have recently completed microarray screens of fetal spleens and found 14- to 29-fold increases in mRNA concentrations for many genes downstream of Type I IFN action (Fig. 4). Maternal blood cell ISG15 mRNA was upregulated on days 78 and 82, while fetal blood cell ISG15 mRNA was upregulated on days 89–192 of gestation (Fig. 2). Interestingly, ISG15 mRNA concentrations were similar in maternal and fetal blood, but upregulation was delayed 10–20 days in fetal compared with maternal blood. We propose to more extensively examine expression and function of these RNA helicase proteins in activation of kinases that phosphorylate downstream activators of Type I IFN transcription. Preliminary data to justify study include upregulation of IRF7 mRNA in response to fetal persistent infection without consistent upregulation of IRF3 mRNA. Because phosphorylation of IRFs and translocation to the nucleus is required for full transcriptional activation of Type I IFN genes (Yeow et al., Reference Yeow, Au, Juang, Fields, Dent, Gewert and Pitha2000; Smith et al., Reference Smith, Marie, Prakash, Garcia-Sastre and Levy2001; Taniguchi et al., Reference Taniguchi, Ogasawara, Takaoka and Tanaka2001), this process is targeted for study using our in vivo fetal infection model.

Fig. 4. Genes upregulated in response to persistent infection with BVDV in fetal spleen. Analysis of fetal spleen mRNA using microarray analysis revealed that IFN stimulated gene 15 (ISG15), guanlylate binding protein 1 (GBP1), interferon, alpha-inducible protein 6 (IFI6), interferon, alpha-inducible protein 27 (IFI27), and oligoadenylate synthetase 1 (OAS1) mRNA concentrations were upregulated (P < 0.001) 16-, 8-, 8-, 29-, and 15-fold; respectively. Data are described in more detail in (Smirnova et al., Reference Smirnova, Webb, McGill, Schaut, Bielefeldt-Ohmann, Van Campen, Sacco and Hansen2014).
Several ISGs have been described by our group to be upregulated in fetal PI blood and tissues with more extensive evidence demonstrating upregulation of ISG15 by immunohistochemical localization to ‘macrophage-like’ cells in PI fetal spleen (Shoemaker et al., Reference Shoemaker, Smirnova, Bielefeldt-Ohmann, Austin, van Olphen, Clapper and Hansen2009) and multiple cell types in the brain (Bielefeldt-Ohmann et al., Reference Bielefeldt-Ohmann, Smirnova, Tolnay, Webb, Antoniazzi, van Campen and Hansen2012). Even though these components of the innate response to ncpBVDV infection appear active, the virus persists. For this reason, we examined the Type II IFN-γ response to fetal persistent infection and are the first to report that the fetus mounts a significant IFN-γ response to infection with ncpBVDV (Smirnova et al., Reference Smirnova, Webb, McGill, Schaut, Bielefeldt-Ohmann, Van Campen, Sacco and Hansen2014).
Adaptive immune and IFN-γ response to persistent infection with BVDV
Antigen presenting cells (APC) and T cell recognition, education, clonal expansion, and induced apoptosis have been described to be impaired in cells infected with BVDV. Major histocompatibility complex (MHCI and MHCII) are upregulated in liver Kupffer cells isolated from day 89 PI fetuses (unpublished results with Morrarie and Chase; South Dakota State University). Because IFN-γ secretion is induced in fetal blood and IFN-γ mRNA concentrations are increased in fetal tissues on day 97 (Figs. 4 & 5), we hypothesize that antigen processing and presentation are activated to the point of inducing a greater Th1:Th2 T cell clonal expansion and action. IFN-γ activates macrophages, which leads to increased expression of CD40 (Kitagawa et al., Reference Kitagawa, Suzuki, Adachi, Nakamura, Yoshino and Sumida2001), tumor necrosis factor (TNF) receptors on T cells and release of TNF-α and Interleukin (IL)12 (Oriss et al., Reference Oriss, McCarthy, Morel, Campana and Morel1997; Kitagawa et al., Reference Kitagawa, Suzuki, Adachi, Nakamura, Yoshino and Sumida2001), and induces release of IL12 from dendritic cells (Schoenborn and Wilson, Reference Schoenborn and Wilson2007; Zhou, Reference Zhou2009). It is known that Th2 cytokines limit classical macrophage activation. IFN-γ inhibits release of Th2 cytokines such as transforming growth factor (TGF-β) and IL-10 that are produced by CD4+Th2 cells. This shift in Th1/Th2 favors Th1 clonal expansion and actions.
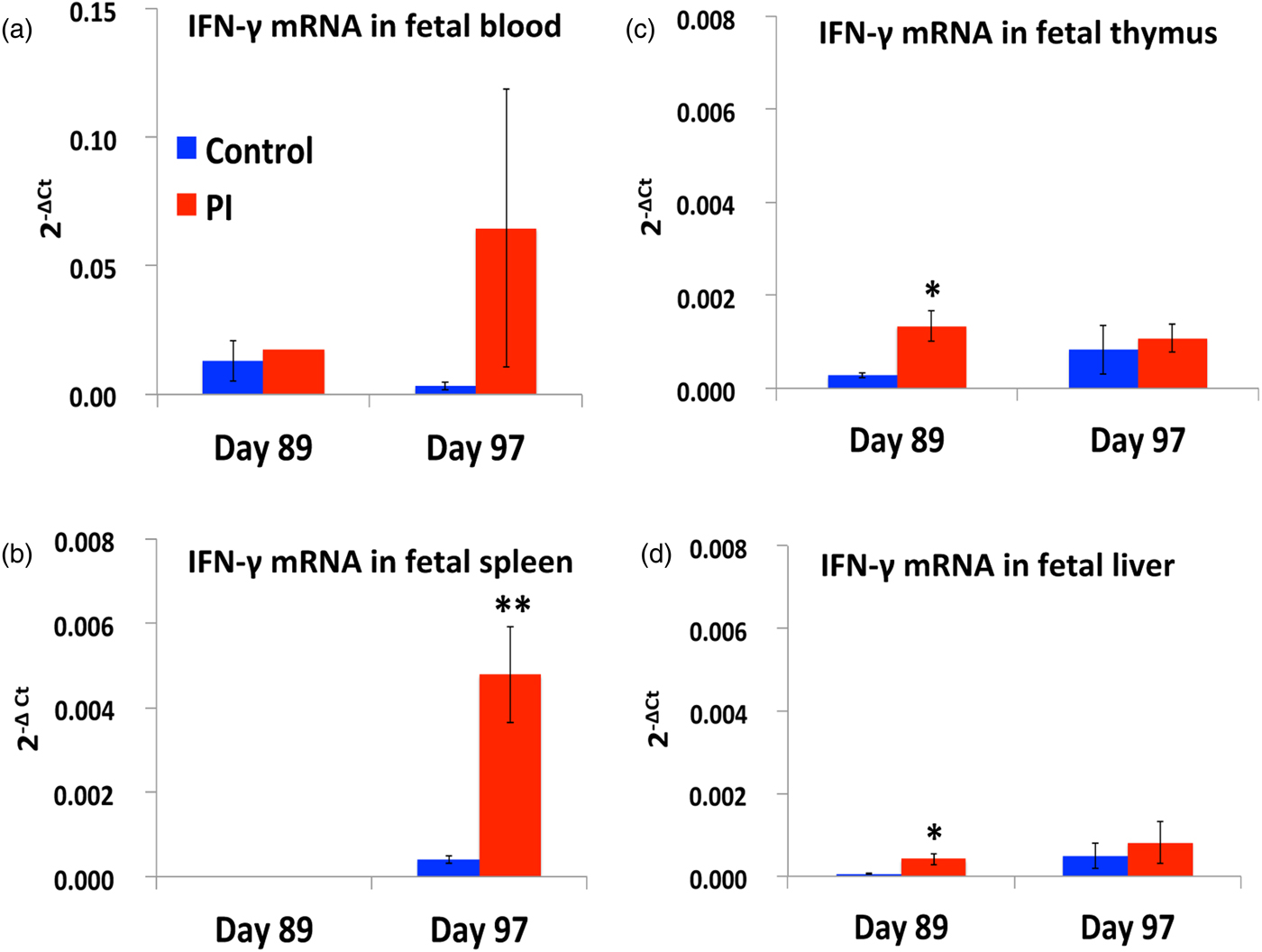
Fig. 5. IFN-γ mRNA concentrations in fetal lymphoid organs in response to persistent infection after ncpBVDV infection on day 75 of gestation. *P < 0.05; **P < 0.01.
MHCI/MHCII
ncpBVDV infects APCs (i.e. macrophages, dendritic cells, and B cells) (Bielefeldt Ohmann, Reference Bielefeldt Ohmann1988a, Reference Bielefeldt Ohmannb) and is recognized through the MHCI pathway by proteasome-mediated peptidases that clip viral envelope/capsid protein antigens into smaller peptides that can bind to MHCI. Also, BVDV proteins are synthesized in the rough endoplasmic reticulum (ER) in these cells during replication. Some viral proteins, however, will resist breakdown and will be incorporated into new viral particles. IFN-γ may regulate these processes by inducing ubiquitin-proteasome processing of peptides presented through MHCI (Glickman and Ciechanover, Reference Glickman and Ciechanover2002). It also activates an N-terminal peptidase, which shortens peptides so that they are essentially ‘tailored’ for binding to MHCI. Entry of antigenic peptides into the ER is mediated through the transporter TAP1/2 (Min et al., Reference Min, Pober and Johnson1996), which is also upregulated in response to IFN-γ. Peptide sorting, occurring in the ER, results in final binding to MHCI, association with β2 microglobulin (β2M), and export for expression on the cell membrane via Golgi and endosome-mediated vesicular trafficking. Once exposed to extracellular presentation, this MHCI/antigen complex activates T cells through binding to the T cell receptor (TCR) with co-activating CD8 and other antigen presenting facilitators (e.g., 41BBL) and Th1 interactions (CD28), which fully activate cytotoxic T cells for surveillance and killing of other cells that present virus antigen. The MHCII-mediated removal of inhibitory Class II-associated invariant chain peptide (CLIP), so that binding and recognition of BVDV peptides can lead to insertion into the cell membrane for extracellular presentation, could occur to some degree in fetal PI APCs.
MHCI/MHCII in PI fetal liver Kupffer cells
As liver Kupffer cells show high BVDV viral loads in PI fetuses, we examined these cells on day 89 of pregnancy. Kupffer cells are resident liver immune cells that mediate innate immune responses (Bilzer et al., Reference Bilzer, Roggel and Gerbes2006) and respond to antigen stimulation with release of IL-1, IL-6, and TNF-α as well as tolerogenic cytokines like IL-10 (Knolle et al., Reference Knolle, Lohr, Treichel, Dienes, Lohse, Schlaack and Gerken1995; Torres-Aguilar et al., Reference Torres-Aguilar, Aguilar-Ruiz, Gonzalez-Perez, Munguia, Bajana, Meraz-Rios and Sanchez-Torres2010; Risalde et al., Reference Risalde, Gomez-Villamandos, Pedrera, Molina, Ceron, Martinez-Subiela and Sanchez-Cordon2011). Kupffer cells were isolated from control and PI fetal liver using Percoll gradient centrifugation as described previously (Smedsrod and Pertoft, Reference Smedsrod and Pertoft1985) resulting in cell preparations of ~90% purity. After immunolabeling for MHCI and MHCII, flow cytometry analysis revealed that cells isolated from PI fetal liver had ~3 times more (P < 0.001) cells expressing MHCI compared with controls (50.47% PI cells versus 17.54% of control cells). The percentage of cells expressing MHCII also doubled (P < 0.05) in PI liver: 37.86% in PI versus 16.14% of cells in control samples. The ratio of MHCI:MHCII favored MHCI which is consistent with regulation by IFN-γ. Isolated PI fetal Kupffer cells also produced more IFN-γ and had greater phagocytic activity in vitro when compared with control Kupffer cells (unpublished data with S.E. Morrarie and C.C. Chase, South Dakota State University).
BVDV, IFN-γ, and adaptive immune response
The ratio of Th1:Th2 activation is hypothesized to favor MHCI:CD8 rather than MHCII:CD4 responses in fetuses PI with BVDV at least on day 97 of gestation, because of such dramatic increase in IFN-γ mRNA in fetal liver, spleen and thymus cells (Fig. 5) and circulating IFN-γ protein (Fig. 6) in fetal blood. IFN-γ mRNA concentrations did not change in PI fetal blood on days 89 and 97, although there was great variation noted in the day 97 PI fetuses. In contrast, IFN-γ mRNA concentrations were increased in fetal thymus and liver on day 89, and in spleen on day 97, suggesting that increased IFN-γ transcription may lead to secretion of IFN-γ protein into the circulation.

Fig. 6. IFN-γ protein concentrations in blood from control and PI fetuses. Maternal infection with ncpBVDV occurred on day 75 of gestation. Average concentration of IFN-γ protein in PI fetal blood reached 20 pg/ml by day 97 (a), which corresponded with the highest BVDV RNA concentrations (b), and subsequently declined by day 245 of gestation. Adapted from (Smirnova et al., Reference Smirnova, Webb, McGill, Schaut, Bielefeldt-Ohmann, Van Campen, Sacco and Hansen2014). A, a **P < 0.01 between groups.
Indeed, using a very sensitive and highly specific chemiluminescent ELISA-based multiplex assay (Aushon Biosystems, USA) for bovine IFN-γ, we detected significant increases of IFN-γ in blood of the day 97 PI fetuses (Smirnova et al., Reference Smirnova, Webb, McGill, Schaut, Bielefeldt-Ohmann, Van Campen, Sacco and Hansen2014). This increase of fetal blood IFN-γ temporally corresponded with the peak of fetal viremia, which was unexpectedly followed by a decline in viremia by day 192 and 245 of pregnancy (Fig. 6(B)). This serum IFN-γ profile is interpreted to reflect induction of IFN-γ followed by partial activation of a virus-specific adaptive immune response and associated decline in viral RNA concentrations from days 97 to days 192–245 of gestation. Although, we recognize that clonal selection or immunological memory would provide further evidence of adaptive immune response to persistent infection with BVDV.
Fetal spleen transcriptome
We have described precocious development of fetal spleen in PI fetuses (Shoemaker et al., Reference Shoemaker, Smirnova, Bielefeldt-Ohmann, Austin, van Olphen, Clapper and Hansen2009) and demonstrated upregulation of Type I IFN pathway genes, including ISGs, indicating strong involvement of this lymphoid organ in response to persistent BVDV infection. For this reason, fetal spleen was chosen for microarray analysis of differential gene expression. Because IFN-γ was detected in blood on day 97 and this coincided with a strong increase (12-fold) in IFN-γ mRNA concentration in fetal spleen – the highest increase in lymphoid tissues tested—we examined gene expression in fetal spleen cells prior to (on day 82) and during the peak of IFN-γ mRNA and protein expression on day 97.
There were subtle differences in gene expression on day 82 in control and PI fetal spleens in contrast to day 97, when 204 genes were differentially expressed (187 upregulated and 17 down-regulated at ≥ 1.5 fold; P < 0.0001) in day 97 PI compared with control fetal spleen. Many of these upregulated (up to 28-fold) genes represent innate (i.e. GBP5, GBP1, interferon-induced protein 44 (IFI44), IFI27, ISG15, GBP4, RIG-I, MDA5, tumor necrosis factor receptor associated factor 3 (TRAF3), IRF7, and IRF5), and adaptive (i.e. T cell receptor gamma, TCRG; lymphocyte-specific protein tyrosine kinase, LCK; zeta chain TCR associated protein kinase 70 kDa; ZAP70; natural killer cell receptor 2B4, CD244, delta CD3-TCR complex, CD3D; and TCRG6) immune responses to fetal PI with BVDV. Likewise, IFN-γ-induced genes were upregulated 2–4.8 fold (P < 0.001) and included signal transducer and activator of transcription 1 (STAT1) and TAP1 (downstream members of IFN-γ signaling pathway), as well as chemokine (C-X-C motif) ligand 10 (CXCL10), CXCL16, CXCR6 (promote monocyte, natural killer cell, and T cell migration), and IFI16 (viral restriction factor) (Smirnova et al., Reference Smirnova, Webb, McGill, Schaut, Bielefeldt-Ohmann, Van Campen, Sacco and Hansen2014). qRTPCR confirmed upregulation of STAT1, IFI16, CXCL10, CXCL16, and chemokine (C-X-C motif) receptor 6 (CXCR6), mRNA in day 97 fetal spleen and blood.
Induction of these genes diminished by days 192–245. Moreover, STAT1 became significantly downregulated by day 192 in PI fetal spleen. These data confirm that IFN-γ in fetal blood temporally induces downstream pathways, which might contribute to the development of an adaptive immune response and a significant decrease of BVDV replication in PI fetuses. The longer-term suppression of STAT1 mRNA concentrations in PI fetuses might be related to a failure of IFN-γ-induced adaptive immune response to be robust or complete enough to clear the BVDV infection. We have also discovered several genes associated with MHCI function that are upregulated in fetal spleen during persistent infection (Fig. 7).
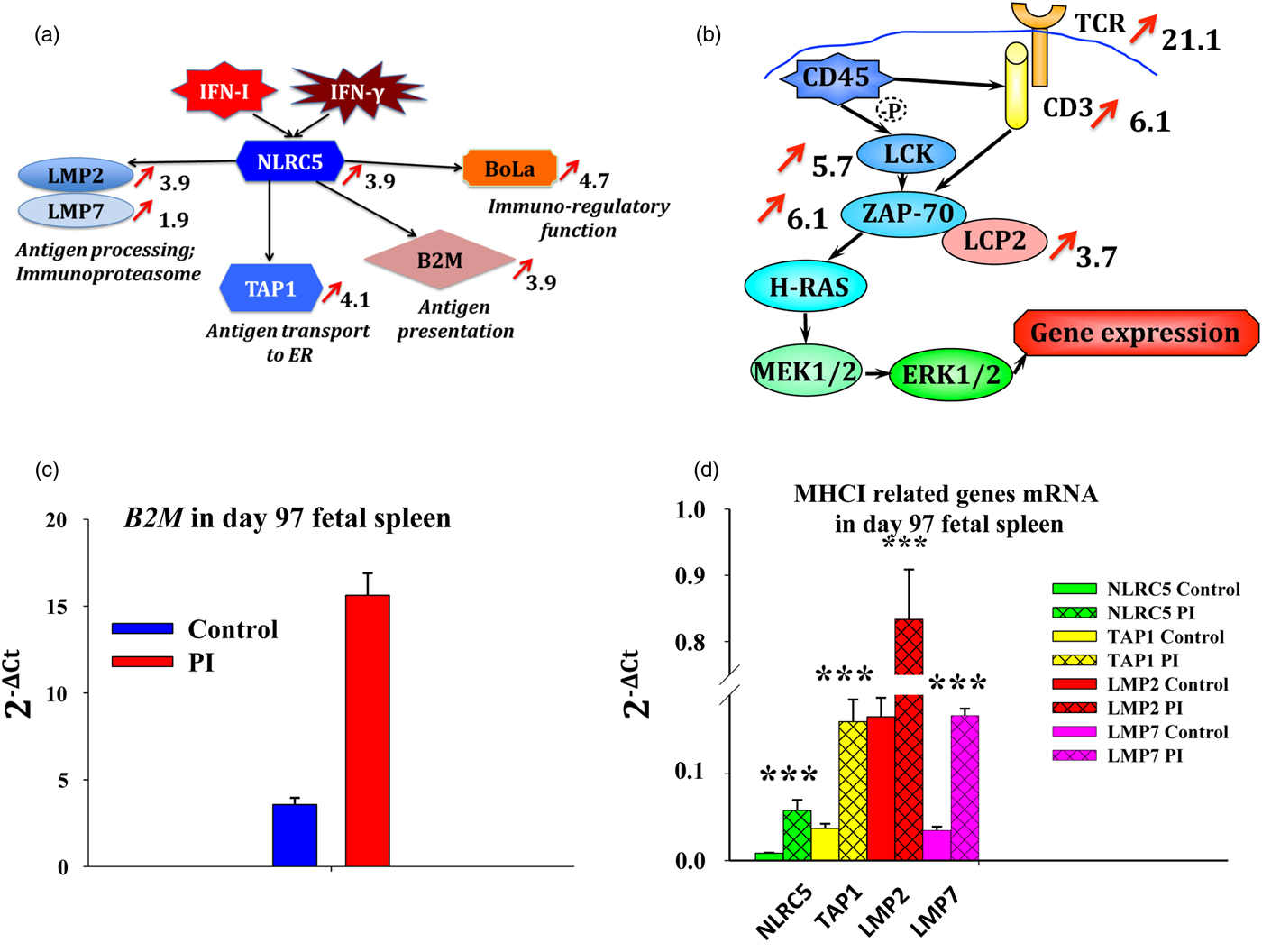
Fig. 7. Adaptive immune responses to persistent fetal infection with BVDV in spleen collected on day 97 of gestation. Panel (a) describes upregulation of genes downstream of IFN action that are associated with antigen processing, transport to ER, antigen presentation, and immunoregulatory function. Panel (b) describes upregulation (P < 0.05) of TCR and downstream genes in PI compared with control fetal spleen based on microarray analysis. Red arrows show fold change in Panels (a) and (b) (P < 0.001). Massive upregulation of TCR pathway genes indicates possibility of the developing cytotoxic T cell response in PI fetuses during establishment of persistent BVDV infection. Quantitative RTPCR revaled upregulation (P < 0.05) of beta-2-microglobulin (B2M) (c), and many MHCI-related genes (d) in fetal spleen PI with BVDV compared with controls. NLR family, CARD domain containing C5 (NLRC5) is predominantly expressed in hematopoietic cells, induced by Type I IFN and IFN-γ, and serves as a transactivator of MHCI-related genes (Meissner et al., Reference Meissner, Li, Biswas, Lee, Liu, Bayir, Iliopoulos, van den Elsen and Kobayashi2010, Reference Meissner, Li and Kobayashi2012; Kobayashi and van den Elsen, Reference Kobayashi and van den Elsen2012; Neerincx et al., Reference Neerincx, Castro, Guarda and Kufer2013). NLRC5 upregulates proteins low molecular weight mass protein (LMP) 2 and LMP7, forming immunoproteasome, which processes antigens into peptides through proteolysis for antigen presentation; TAP1, transporting antigen peptides to the ER for antigen processing; B2M, which forms complex with MHCI for antigen presentation; and non-classical MHCI antigen precursor BoLa, with possible immune-regulatory function. *P < 0.05; **P < 0.01; ***P < 0.001.
MHCI/antigen complex activates T cells through binding to TCR, which results in activation of cytotoxic T cells for surveillance and killing of virus-infected cells. Significant upregulation (5–21-fold, P < 0.001) of TCR pathway genes (CD3–TCR complex, including CD3, TCRγ, TCRζ, LCK, and ZAP70) and cytotoxic and regulatory T cell molecule (CRTAM), which is able to stimulate cytotoxic activity and promote IFN-γ secretion by CD8+ T cells, was detected in the microarray analysis. These data reflect upregulation of a cytotoxic T cell response to BVDV in fetal persistent infection and are interpreted to support our hypothesis that very discrete innate and adaptive immune responses become active in response to persistent infection with BVDV.
However, humoral immune responses are not completely developed and fail to initiate strong antibody responses, which, coupled with impaired cell-mediated responses, results in persistence of fetal viral infection.
Summary
Our interpretation (hypothesis) of the establishment of fetal persistent infection with ncpBVDV and development of innate (ISGs) and partial adaptive (IFN-γ) immune response in PI fetuses (Fig. 8), is different from established opinion and based on preliminary data presented herein (Smirnova et al., Reference Smirnova, Webb, McGill, Schaut, Bielefeldt-Ohmann, Van Campen, Sacco and Hansen2014) and previous publications from our group (Smirnova et al., Reference Smirnova, Bielefeldt-Ohmann, Van Campen, Austin, Han, Montgomery, Shoemaker, van Olphen and Hansen2008; Shoemaker et al., Reference Shoemaker, Smirnova, Bielefeldt-Ohmann, Austin, van Olphen, Clapper and Hansen2009; Hansen et al., Reference Hansen, Smirnova, Van Campen, Shoemaker, Ptitsyn and Bielefeldt-Ohmann2010; Webb et al., Reference Webb, Norrdin, Smirnova, Van Campen, Weiner, Antoniazzi, Bielefeldt-Ohmann and Hansen2012). Infection of the pregnant heifer with ncpBVDV induces rapid innate immune responses (Type I IFN and ISGs), which leads to maternal adaptive immune responses and clearance of the virus. During maternal acute infection, virus crosses the placenta, reaches the fetus and causes detectable viremia in the fetus 7–14 days after maternal infection. Fetal infection with BVDV activates innate immune responses (ISGs) and is accompanied by induction of the fetal adaptive immune response (IFN-γ and IFN-γ-induced genes) at the peak of fetal viremia (22 days after the maternal infection). Synergistic action of the fetal innate and components of the adaptive immune responses causes a reduction of the level of fetal viremia, but fails to produce neutralizing antibodies and T cells that are necessary to eliminate the virus, which consequently persists in the newborn calf (Collen et al., Reference Collen, Douglas, Paton, Zhang and Morrison2000).

Fig. 8. BVDV RNA concentrations and temporal activation of innate (Type I IFN) and adaptive (Type II IFN-γ) immune responses to ncpBVDV infection on day 75 of gestation in the mother and the PI fetus. Top panel describes BVDV RNA concentrations in maternal compared with fetal blood following infection on day 75. Bottom panel provides our interpretation of temporal activation of innate and adaptive immune response following infection with BVDV. Infection of pregnant heifers with ncpBVDV on day 75 of gestation activates the maternal innate immune response through upregulation of blood cell ISGs within 3 days (day 78) (Smirnova et al., Reference Smirnova, Bielefeldt-Ohmann, Van Campen, Austin, Han, Montgomery, Shoemaker, van Olphen and Hansen2008, Reference Smirnova, Ptitsyn, Austin, Bielefeldt-Ohmann, Van Campen, Han, van Olphen and Hansen2009; Hansen et al., Reference Hansen, Smirnova, Van Campen, Shoemaker, Ptitsyn and Bielefeldt-Ohmann2010). This is followed by peak of viremia within 10 days (days 82–85), a decline in viral titer by 14 days (day 89) and clearance of the maternal infection by 22 days (day 97). In maternal blood, IFN-γ, a mediator of the adaptive immune response, peaks between days 82 and 89 (~10–14 days after infection; Smirnova et al., Reference Smirnova, Webb, McGill, Schaut, Bielefeldt-Ohmann, Van Campen, Sacco and Hansen2014). The fetal innate immune response to persistent infection with BVDV includes induction of ISGs, which occurs as early as 10 days (days 82–89) following maternal infection (Shoemaker et al., Reference Shoemaker, Smirnova, Bielefeldt-Ohmann, Austin, van Olphen, Clapper and Hansen2009; Hansen et al., Reference Hansen, Smirnova, Van Campen, Shoemaker, Ptitsyn and Bielefeldt-Ohmann2010; Smirnova et al., Reference Smirnova, Webb, Bielefeldt-Ohmann, Van Campen, Antoniazzi, Morarie and Hansen2012). Type I IFN responses in PI fetuses are similar in scope, but are delayed for ~10 days, when compared with maternal responses to infection with ncpBVDV. Fetal viremia is not detected until 14 days after maternal infection (day 89) and peaks by 22 days (day 97), followed by a decline to lower levels of virus by days 192 and 245 of gestation. **P < 0.01 when compared to day 97 values. Reprinted with permission from (Smirnova et al., Reference Smirnova, Webb, Bielefeldt-Ohmann, Van Campen, Antoniazzi, Morarie and Hansen2012, Reference Smirnova, Webb, McGill, Schaut, Bielefeldt-Ohmann, Van Campen, Sacco and Hansen2014).
Recently, we described IFN-γ in PI fetal serum ~2 weeks following upregulation of a Type I IFN response (Smirnova et al., Reference Smirnova, Webb, McGill, Schaut, Bielefeldt-Ohmann, Van Campen, Sacco and Hansen2014). However, fetal IFN-γ levels are 10-fold less in PI compared with fetuses of heifers infected on day 175 of gestation, (i.e. TI; data not shown). Detection of IFN-γ in blood from PI fetuses may reflect activation of the adaptive immune response, which until now has never been described during fetal persistent infection with BVDV. Interestingly, this increase in PI fetal IFN-γ coincides with a decline in fetal blood BVDV RNA levels from day 97 to days 192 & 245 of gestation. Because IFN-γ and associated adaptive immune mechanisms necessary for antigen presentation and recognition are attenuated, PI fetal viremia is never cleared.
Significance and future direction
Currently, BVDV is thought to persistently infect cattle because of impaired innate defenses and evasion of adaptive immune response of the fetus by the virus at the time of in utero infection. Evasion of the innate immune system in persistent BVDV infection has been suggested to occur through inhibition of the antiviral Type I IFN response primarily through interaction with the viral Npro (Schweizer and Peterhans, Reference Schweizer and Peterhans2001; Baigent et al., Reference Baigent, Zhang, Fray, Flick-Smith, Goodbourn and McCauley2002; Iqbal et al., Reference Iqbal, Poole, Goodbourn and McCauley2004; Gil et al., Reference Gil, Ansari, Vassilev, Liang, Lai, Zhong, Hong, Dubovi and Donis2006a; Schweizer et al., Reference Schweizer, Matzener, Pfaffen, Stalder and Peterhans2006; Chen et al., Reference Chen, Rijnbrand, Jangra, Devaraj, Qu, Ma, Lemon and Li2007). Subsequently, the presence of BVDV in the fetus during thymic development is hypothesized to select T lymphocytes which view the viral antigens as part of the host antigen repertoire (Potgieter, Reference Potgieter1995; Peterhans et al., Reference Peterhans, Jungi and Schweizer2003). The failure of the adaptive immune system to recognize BVDV as other than ‘self’ results in a persistent viremia. Two novel findings related to these hypotheses have been discovered in our studies. The first is that the innate immune system is not completely impaired in fetuses and cattle PI with BVDV, which is supported by work from others (Yamane et al., Reference Yamane, Kato, Tohya and Akashi2008). The second is that BVDV does not completely evade the adaptive immune system, as suggested by increases in IFN-γ as well as IFN-γ-stimulated responses in PI fetuses (Smirnova et al., Reference Smirnova, Webb, McGill, Schaut, Bielefeldt-Ohmann, Van Campen, Sacco and Hansen2014).
The consequences of chronic persistent infection and chronic upregulation of ISGs in PI fetuses (Shoemaker et al., Reference Shoemaker, Smirnova, Bielefeldt-Ohmann, Austin, van Olphen, Clapper and Hansen2009) include impaired growth and predisposition to secondary infection (Munoz-Zanzi et al., Reference Munoz-Zanzi, Hietala, Thurmond and Johnson2003). After birth, some of the PI calves fail to thrive, have reduced fertility and show immunosuppression, resulting in death because of superimposed infections during the first year of life (Brackenbury et al., Reference Brackenbury, Carr and Charleston2003), or develop mucosal disease (Bielefeldt-Ohmann, Reference Bielefeldt-Ohmann1995). Surviving PI calves continually shed virus and infect other cattle, thus ensuring the survival of the virus (Fulton et al., Reference Fulton, Briggs, Ridpath, Saliki, Confer, Payton, Duff, Step and Walker2005). Infection of pen mates by PI calves can impair rate of gain, feed efficiency and carcass characteristics (Hessman et al., Reference Hessman, Fulton, Sjeklocha, Murphy, Ridpath and Payton2009; Burciaga-Robles et al., Reference Burciaga-Robles, Step, Krehbiel, Holland, Richards, Montelongo, Confer and Fulton2010). These effects of BVDV PI cattle impact the economics of cattle production worldwide.
After decades of study, the immunologic mechanisms involved in the creation of BVDV PI fetuses, including the mechanisms of virus-specific tolerance, escape of immune detection, and failure of the immune system to eliminate the virus, are incompletely understood (Peterhans and Schweizer, Reference Peterhans and Schweizer2013). Clarification of these mechanisms is best achieved by using in vivo bovine fetal models such as described here rather than in vitro models that do not accurately reflect the complex innate and adaptive immune responses of the fetus. This fundamental information can be used to develop new approaches to control viral infections. Genomic DNA from PI fetuses correlated with markers for innate and adaptive immune responses and levels of BVDV in fetal tissues can identify single nucleotide polymorphisms associated with resistance to infection with BVDV and, for that matter, viruses in general. This concept is supported by our findings of variation in amount of BVDV detected in PI fetuses. Perhaps some fetuses and their placentas are better able to control or eliminate viral infections compared with others that are less ‘immunocompetent’. Similarly, the genetic makeup of the cow may influence the likelihood of that she will control the BVDV infection and prevent the virus from reaching her fetus. These gene(s) could be selected for in breeding schemes to control BVDV. In addition, a better understanding of immunotolerance may be important in the ‘engineering’ of cattle that can produce foreign proteins (e.g. human insulin in milk) without complications of immune rejection. Key primary innate and adaptive immune responses have been described herein that may provide the information necessary to advance such new technologies.
Acknowledgments
This project was supported by Agriculture and Food Research Initiative Competitive (Grant no. 2008-35204-04652) from the USDA National Institute of Food and Agriculture.










