Introduction
Gastrointestinal nematodes are common parasites of herbivores. Usually, a single host is infected by several nematode species distributed along the abomasum, small intestine and large intestine. Additionally, it is quite frequent to find different host species sharing the same parasite species. This is due to nematode egg dispersal through faeces and, consequently, to the presence of infective larvae in areas where several susceptible host species share the same trophic resources. Thus, parasite transmission and infection rates involve highly stochastic dynamics (Cornell, Reference Cornell2005) including intrinsic and extrinsic factors such as host immune status, host behaviour or climate conditions (Agosta & Klemens, Reference Agosta and Klemens2008; Hoberg & Brooks, Reference Hoberg and Brooks2008; Agosta et al., Reference Agosta, Janz and Brooks2010). These interactions have increased interest on how parasite exchanges between host species may affect closely related hosts (Morgan et al., Reference Morgan, Milner-Gulland, Torgerson and Medley2004; Obanda et al., Reference Obanda, Maingi, Muchemi, Ng'ang’a, Angelone and Archie2019), a process that becomes most notorious in natural ecosystems where different host species share the same habitat.
The multi-host parasite paradigm has proven itself particularly useful to study the role of pathogens influencing wildlife population dynamics (Smith et al., Reference Smith, Sax and Lafferty2006; Delogu et al., Reference Delogu, Ghetti, Gugiatti, Cotti, Piredda, Frasnelli and De Marco2013; Sinclair et al., Reference Sinclair, Melville, Sargison, Kenyon, Nussey, Watt and Sargison2016; Leivesley et al., Reference Leivesley, Bussière, Pemberton, Pilkington, Wilson and Hayward2019). Examples have been documented in literature for closely related ungulates as showcased by a recent study dealing with sarcoptic mange by Iacopelli et al. (Reference Iacopelli, Fanelli, Tizzani, Berriatua, Prieto, Martínez-Carrasco, León, Rossi and Candela2020); this study describes a spatio-temporal pattern of sarcoptic mange infection data in two wild ruminants in south-east Spain, showing that the disease is directly responsible for the decline of Iberian Ibex (Capra pyrenaica) populations. Although gastrointestinal nematode infections usually cause no clinical signs in wild ruminants (Gunn & Irvine, Reference Gunn and Irvine2003), nematode burden has shown to exert a negative impact on wild ungulate population dynamics (Gulland, Reference Gulland1992; Albon et al., Reference Albon, Stien, Irvine, Langvatn, Ropstad and Halvorsen2002). However, the gastrointestinal multi-host/multi-parasite system parasite richness occurring in sympatric wild ruminants in south-east Spain still remains to be studied (Parker et al., Reference Parker, Chubb, Ball and Roberts2003).
Wild ruminants have been shown to be highly exposed to pathogens due to the variety of their grazing resources, often shared with domestic ruminants as well (Ocaido et al., Reference Ocaido, Siefert and Baranga2004). Sierras de Cazorla, Segura y Las Villas Natural Park (SCSV) is the largest protected area in Spain, harbouring a wide array of habitats and trophic resources to host four different wild ungulates: mouflon (Ovis aries musimon), red deer (Cervus elaphus), fallow deer (Dama dama) and Iberian ibex. Grazing by livestock (mainly small ruminants) is allowed in peripheral areas of SCSV, with clearly defined park boundaries within which the presence of any livestock species is forbidden (BOJA, 2017; PORN, 2017). Given this background, we aim to compare patterns of parasite abundance and the degree of parasite sharing within the naturally occurring multi-host parasitism affecting these four wild ungulates. The SCSV is a paradigmatic area to study parasite transmission among ruminants in a multi-host/multi-parasite system. In view of the prevalence and impact that nematodes might have on wild ruminants, a better understanding of their dynamics could shed further light on the epidemiology of polyparasitism and, consequently, favour the management and conservation of their populations.
Material and methods
Area of study and collection of the samples
The Sierras de Cazorla, Segura y Las Villas Natural Park (SCSV) is the second largest protected area in Europe. It is located on the eastern side of the Baetic Mountains (Andalusia, Spain), spanning an area of 2140 km2 (Fandos, Reference Fandos1991). The area has a Mediterranean mountain climate with mild winters and hot summers. The average annual precipitation is less than 448 mm and the average annual temperature of 15°C.
We collected 252 digestive tracts from February to April throughout the years 2003 to 2005 from a set of wild ruminants including two cervid species, fallow deer (n = 109) and red deer (n = 64);and two bovids, mouflon (n = 59) and Iberian ibex (n = 20). Samples originated from free-ranging animals hunted at SCSV an integral part of wildlife population control, the estimated annual census for each ruminant species in the park is listed in table 1. Mouflon and fallow deer were introduced in the 1950s of the last century for hunting purposes, while Iberian ibex and red deer are native species in the study area (De Leyva, Reference De Leyva2002; Herrera, Reference Herrera2008; Masseti & Mertzanidou, Reference Masseti and Mertzanidou2008; Cassinello & Salvador Milla, Reference Cassinello and Salvador Milla2017).
Table 1. Total animal census during the sampling period.

ND, not determined.
All animal manipulations were performed according to the Animal Care Committee guidelines and the Bioethical Committee of Murcia University (Murcia, Spain), the local Committees for animal research (REGA ES300305440012), and in accordance to the current European Animal Welfare Legislation (ART13TFEU).
Collection, storage and identification of parasites
Field necropsy was performed immediately after animals were shot by park rangers, and the whole digestive tract was removed. In order to record the exact location of the nematodes along the tract, each section was clamped, double ligated and preserved in identified plastic bags until further laboratory processing. Then, gastrointestinal nematodes were collected by separate processing of abomasum, small and large intestines. Following a longitudinal cut, mucosae of each digestive section were examined by scraping. Digestive tract content was washed and sieved through a series of mesh screens (mesh pore sizes: 1 cm, 0.6 mm and 0.3 mm). The resulting sediment was preserved in 10% formalin samples.
Samples from abomasum and small intestine were diluted with water up to 2 l, and thoroughly mixed. One aliquot, representing 10% of the volume was examined in small portions under a stereoscopic microscope to collect the nematodes. When there were not sufficient nematodes for identification purposes (up to 100 individuals), one or two more aliquots (up to a total of 30% of the volume) were analysed. Male and all female nematodes were collected but only male specimens were identified. The number of male nematodes was expanded to the whole sample volume in order to calculate the abundance of each parasite species. In the specific case of the large intestine, all nematodes present were counted.
Morphometric characteristics were used to categorize the adult male parasites by species following Durette-Desset (Reference Durette-Desset1989) for the sub-family Ostertagiinae, and Skrjabin et al. (Reference Skrjabin, Shikhobalova, Schulz, Popova, Boev and Delyamure1961) and Yamaguti (Reference Yamaguti1961) for the Strongylida suborder. All nematode specimens were analysed as described by Ortiz et al. (Reference Ortiz, Ybáñez, Garijo, Goyena, Espeso, Abáigar and Cano2001).
Epidemiological parameters and statistical analysis
Prevalence, intensity and abundance of infection for each parasitic species were defined according to Margolis et al. (Reference Margolis, Esch, Holmes, Kuris and Schad1982) and Bush et al. (Reference Bush, Lafferty, Lotz and Shostak1997). Briefly, prevalence is defined as the number of hosts infected with a particular parasite species (or taxonomic group) within the number of hosts examined, expressed as a percentage; intensity is defined as the number of individuals of a particular parasite species in a single infected host, expressed as the number of specimens per infected animal; and abundance is defined as the number of individuals of a particular parasite species per host examined. At a population level, the intensity refers to the mean number of parasites within infected members of that host population (hence excluding uninfected hosts). In contrast, abundance refers to the mean number of parasites within the entire studied host community. Fisher's exact test was used to analyse the prevalence at different host population levels. A model-based analysis of multivariate abundance data was used to evaluate frequency distribution of the abundance for the parasites shared by the four host species. Multivariate analysis of parasite abundance was carried out using the mvabund package (Wang et al., Reference Wang, Naumann, Wright and Warton2012). Subsequently, Kruskal–Wallis analysis was performed to statistically test the outcome. Analyses were carried out using R software (RStudio Team, 2015).
Results
General descriptive patterns of the gastrointestinal nematodes
Twenty-nine nematode species were found and 81.52% of the analysed ruminants were parasitized with nematodes at least in one gastrointestinal section. Spiculopteragia asymmetrica (55.9%), Oesophagostomum venulosum (58.7%) and Spiculopteragia quadrispiculata (45.3%) were the most prevalent species overall. All identified nematode species, as well as their respective prevalence, abundance and intensity are listed in table 2.
Table 2. List of identified nematode species and their prevalence, abundance and intensity per host.

P: Prevalence; A: Abundance; I.R.: Intensity Range.
Prevalence values differed among the host species. Fallow deer presented the highest nematode prevalence (n = 109; 91.5%), followed by the wild bovids (mouflon n = 59, 85.5%; Iberian ibex n = 20, 83.3%) and the red deer (n = 64, 61.4%). For more details see supplementary material (S1).
Richness in nematode species was different between host species: mouflon and fallow deer were found to host up to 12 different species, followed by Iberian ibex and red deer, where up to ten and seven different nematode species were isolated, respectively (fig. 1 and table 2). Our results showed a significantly positive correlation between nematode species richness and intensity (fig. 2c, d). This correlation was significant for all analysed hosts, with the mouflon and the red deer presenting the highest correlation (see supplementary material, S2).
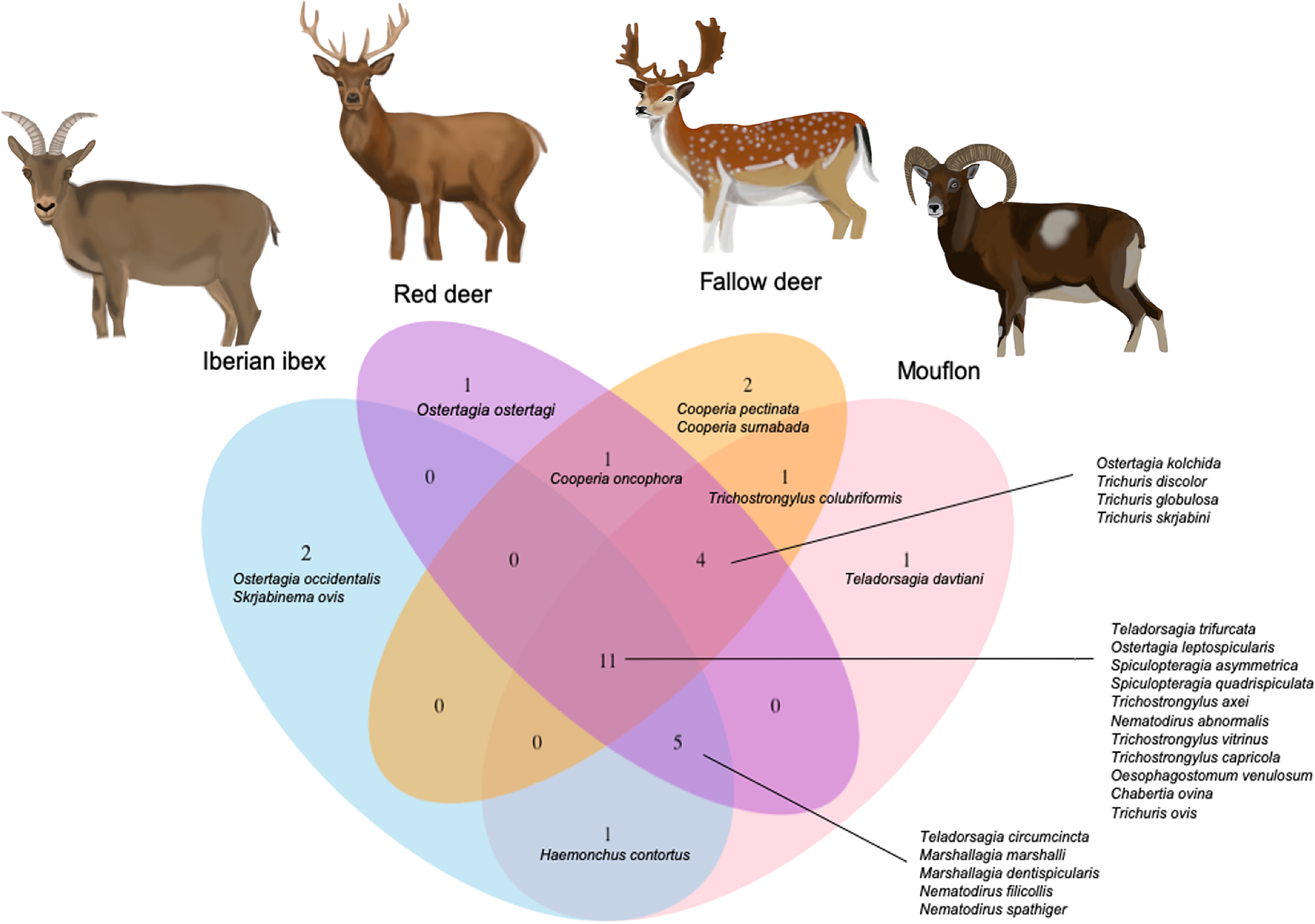
Fig. 1. Venn diagram representing the nematode species found in each species of wild ruminant host.

Fig. 2. Richness and intensity was evidenced in this study as shown by the bubble plot (a) and significantly represented in the correlogram (b). P values lower than 0.05 were considered statistically significant: *P < 0.05, **P < 0.01, ***P < 0.0001.
Multi-host nematode distribution
Eleven nematode species were commonly found parasitizing all host species: Teladorsagia trifurcata, Ostertagia leptospicularis, S. asymmetrica, S. quadrispiculata and Trichostrongylus axei, Nematodirus abnormalis, Trichostrongylus vitrinus, Trichostrongylus capricola O. venulosum Chabertia ovina and Trichuris ovis.
Except for T. ovis, prevalence for the above mentioned species differed significantly between cervids and bovids, as represented in fig. 3. Mean abundance differed significantly between host species as shown in fig. 4a. Additionally, the abundance of these 11 species showed significant differences among hosts (fig. 4b). In particular, we identified different parasite communities on different host species (likelihood ratio test – LRT = 613.6; P < 0.001). The differences among host species are maintained even at the parasite species level (adjusted for multiple testing), with the exception of T. ovis (LRT = 0.431; P > 0.05) and T. axei (LRT = 0.498; P > 0.05). The differences at the parasite community level (adjusted for multiple testing) were significant even between bovids and cervids (LRT = 472.5; P < 0.001) (fig. 4b). In this case the univariate analysis adjusted for multiple testing confirmed the significant differences at the single species level for all parasite species with the exception of T. axei (LRT = 1.886; P > 0.05), O. venulosum (LRT = 2.685; P > 0.05) and T. ovis (LRT = 0.078; P > 0.05) (supplementary fig. S1).

Fig. 3. The mean nematode prevalence of the eleven commonly found species showed significant differences between hosts. Different letters indicate significant effect.

Fig. 4. Abundance of total identified nematodes (a) and the 11 commonly found parasite species (b) described in all studied wild ruminants (b). Different letters indicate significant effect.
Single-host nematode distribution
The Iberian ibex was the only ungulate to be infected by Marshallagia occidentalis and Skrjabinema ovis with 7.1% and 29.4% prevalence, respectively. Likewise, 2.2% of the red deer were also infected with Ostertagia ostertagi in the abomasum, with an average intensity of 13 nematodes per host (table 2). Similarly, Cooperia pectinata and C. surnabada were only reported in the small intestine of fallow deer, showing 1.0% prevalence and intensities of 21 and ten nematodes per host, respectively. Finally, mouflon was the only host species in which Teladorsagia davtiani was isolated from the abomasum, with a prevalence of 8.9% and an intensity range of 2–30 nematodes (table 2).
Discussion
Our study highlights the occurrence of a very rich and diverse parasite community shared among wild ungulates in SCSV. It represents a clear example of a multi-parasite/multi-host scenario in which the vast majority of nematodes are shared between at least two sympatric host species and only a few parasites are exclusive to a single host species. The structure of the parasite community was significantly host influenced, with the highest parasite prevalence and richness consistently appearing in mouflon and fallow deer hosts. These results were confirmed through an innovative model-based approach to the analysis of multivariate abundance data (Yee, Reference Yee2010; Ives & Helmus, Reference Ives and Helmus2011; Ovaskainen & Soininen, Reference Ovaskainen and Soininen2011), that provides more accurate results than traditional distance-based methods (Warwick et al., Reference Warwick, Clarke and Gee1990).
Wild animals are more likely to host a larger parasite richness in comparison with livestock because of increased exposure to infective stages in natural areas (Bordes et al., Reference Bordes, Morand, Kelt and Van Vuren2009; Walker & Morgan, Reference Walker and Morgan2014). Macroparasites are important components of ecological communities (Pedersen & Fenton, Reference Pedersen and Fenton2007), with single-host polyparasitism being a common state for many wild animals (Cox, Reference Cox2001; Polley & Thompson, Reference Polley and Thompson2015). Ecological interactions and their implications in polyparasitism and multi-host co-infection must be understood to better explain parasite patterns of richness and diversity (Craig et al., Reference Craig, Tempest, Pilkington and Pemberton2008; Morand, Reference Morand2015). From this integrative perspective, SCSV offers a perfect scenario for the study of a natural multi-host/multi-parasite system, since it is a large natural area with four sympatric wild ruminant species that interact with domestic livestock.
We have recorded up to twenty-nine nematode species belonging to the superfamilies Strongyloidea and Trichostrongyloidea, the latter being the most dominant in our study because of its higher species diversity (Hoberg & Lichtenfels, Reference Hoberg and Lichtenfels1994). Potentially pathogenic genera, such as Ostertagia and Haemonchus, as well as individual species such as C. pectinata, O. venulosum, C. ovina and T. ovis were found in our study. Previous studies have reported that these nematodes can have a negative impact on both domestic and wild ruminants (Herlich, Reference Herlich1965; Jackson et al., Reference Jackson, Jackson and Williams1988; Parkins & Holmes, Reference Parkins and Holmes1989; Lavín et al., Reference Lavín, Marco, Rossi, Meneguz and Viñas1997). The repercussion of gastrointestinal nematodes on wildlife is also well known, and previous studies have already shown that gastrointestinal nematodes reduce food intake in parasitized cervids and/or cause severe lesions that can lead to reduced weight gain (Gulland, Reference Gulland1992; Arneberg et al., Reference Arneberg, Folstad and Karter1996; Lavín et al., Reference Lavín, Marco, Rossi, Meneguz and Viñas1997; Coltman et al., Reference Coltman, Pilkington, Smith and Pemberton1999; Albon et al., Reference Albon, Stien, Irvine, Langvatn, Ropstad and Halvorsen2002; Fanelli et al., Reference Fanelli, Menardi and Chiodo2020).
Interestingly, parasite richness varied greatly among hosts, ranging from one to up to twelve different nematode species in a single host. The parasite richness found in the mouflon population was particularly high. This wild ruminant was introduced in Spain for hunting purposes in 1953 (Cassinello & Salvador Milla, Reference Cassinello and Salvador Milla2017). When allochthonous species are introduced in a new ecosystem, their successful reproduction, colonization of new habitat and eventual occupation of existing ecologic niches, can result in a threat to local biodiversity through the displacement of native species (Hulme et al., Reference Hulme, Roy, Cunha and Larsson2009; Kelly et al., Reference Kelly, Paterson, Townsend, Poulin and Tompkins2009). The mouflon has adapted extremely well to its new habitat in the Iberian Peninsula (Cassinello & Salvador Milla, Reference Cassinello and Salvador Milla2017) and, as demonstrated in our study, to the parasite fauna of its sympatric ruminant community. The mouflon may act as an efficient spreader of parasites, as it shares the same pastures with other wild bovids such as the Iberian Ibex in winter and in spring (Martínez & Fandos, Reference Martínez and Fandos1989), and in summer with cervids, such as red deer (Miranda et al., Reference Miranda, Sicilia, Bartolomé, Molina-Alcaide, Gálvez-Bravo and Cassinello2012). Moreover, this allochthonous species shares the majority of its gastrointestinal parasites with domestic sheep (Pisanu et al., Reference Pisanu, Chapuis, Et and Durette-Desset1996; Balicka-Ramisz et al., Reference Balicka-Ramisz, Laurans, Jurczyk and Ramisz2017). In fact, cross-infection has been described by Bartczak & Okulewicz (Reference Bartczak and Okulewicz2014), whose work evidenced that the mouflon may play a significant epidemiological role in the exchange of parasites between cervids and domestic ruminants (sheep and goats). Under this perspective, the mouflon might act as an epidemiological link of nematode transmission between wild ruminants and small ruminant farms located in the peripheral area of the SCSV, where livestock is allowed to graze (Walker & Morgan, Reference Walker and Morgan2014).
Furthermore, parasite richness was linked to higher parasite intensity for all wild ruminant species. This may be due to a combination of several factors including high host population density (Ezenwa, Reference Ezenwa2004), the direct life cycle of some macroparasites that may facilitate transmission rate (Arneberg, Reference Arneberg2001), and environmental changes that affect host/parasite-mediated speciation (Brunner & Eizaguirre, Reference Brunner and Eizaguirre2016). The abundance and intensity patterns of some parasites, such as T. circumcincta, T. trifurcata, T. vitrinus and T. capricola, among others, have been directly related to rainfall, which tend to increase in intensity during spring (Valcárcel & Romero, Reference Valcárcel and Romero1999). Nonetheless, further studies should be carried out to understand the influence of external and/or internal factors on the gastrointestinal nematode intensity and richness in its wild ruminant community (Ortiz et al., Reference Ortiz, Ybáñez, Garijo, Goyena, Espeso, Abáigar and Cano2001).
Finally, differences in the abundance pattern of some nematodes were also observed. The presence of specific patterns was highlighted by the multivariate analysis of abundance, which demonstrated significant differences among the 11 nematode species shared by all host species. Clear patterns were observed at both the host species (fallow deer, Iberian ibex, mouflon and red deer) and the host group levels (bovids vs. cervids). These findings highlight the ability of parasites to adapt to the community of hosts and shape their ecological distribution according to the most suitable host species available (Winter et al., Reference Winter, Rehbein and Joachim2018).
The clearest example under this perspective is represented by the nematode species in the abomasum, which were found in all host species but with significantly higher intensity in cervids than in bovids. This dichotomous pattern has already been reported by Zaffaroni et al. (Reference Zaffaroni, Teresa Manfredi, Citterio, Sala, Piccolo and Lanfranchi2000). In particular, S. asymmetrica is usually found parasitizing the gastrointestinal tract of cervids (Dróżdż, Reference Dróżdż1966; Santín-Durán et al., Reference Santín-Durán, Alunda, Hoberg and Fuente2004) and is rare in sylvatic and domestic bovids (Suarez & Cabaret, Reference Suarez and Cabaret1991). A positive association has already been described between strongyle co-infection prevalence and level of habitat overlap across taxa. This is because sharing of nematodes is more likely in closely related hosts because of their similar ecology, physiology and behaviour (Ezenwa, Reference Ezenwa2003; Gruijter et al., Reference Gruijter, Ziem, Verweij, Polderman and Gasser2004; Ocaido et al., Reference Ocaido, Siefert and Baranga2004; Archie & Ezenwa, Reference Archie and Ezenwa2011).
Conclusion
The results of our study may represent a baseline to be considered for the planning and implementation of wild ruminant management projects. We consider that evaluating nematode richness, prevalence and intensity is helpful to better understand the health situation of free-ranging ruminants, in particular when multiple hosts share the same area in high population densities. An additional but non-negligible benefit in understanding and eventually reducing the impact of diseases linked with overabundance is represented by the fact that they can affect not only fitness and trophy quality, but also human and livestock health, as well as the success of conservation measures for endangered species.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0022149X21000183
Acknowledgements
The authors thank the staff and guards of SCSV for the facilities, sample collection and overall help to carry out this study.
Financial support
This study has been funded by the Spanish Ministry of Science and Technology projects AGL2002-02916.
Ethical standards
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Conflicts of interest
The authors declare that they have no conflict of interest.








