Introduction
A brief burst of 500 Hz, bone-conducted vibration delivered to the midline of the forehead at the hairline (a location known as Fz) is known to cause linear acceleration of both mastoids. Furthermore, after a short latency period (10 milliseconds) following stimulus onset, there is also a change in electromyographic (EMG) activity recorded by surface electrodes beneath both eyes.Reference Iwasaki, McGarvie, Halmagyi, Burgess, Kim and Colebatch1, Reference Iwasaki, Smulders, Burgess, McGarvie, MacDougall and Halmagyi2
The latter myogenic potential is termed the ocular vestibular evoked myogenic potential (VEMP), and the first negative component of this potential, occurring after a latency period of approximately 10 milliseconds, is termed the ocular VEMP n10 component. This potential is probably due to activation of otolithic receptors by linear acceleration stimuli, since recordings of single primary vestibular afferent neurons in guinea pigs have shown that 500 Hz, bone-conducted vibration of the skull at comparable stimulus intensities causes comparable linear accelerations and selectively activates primary vestibular neurons sensitive to linear acceleration – the otolithic irregular neurons.Reference Curthoys, Kim, McPhedran and Camp3, Reference Curthoys and Vulovic4 These neurons have a very low response threshold for this stimulus, and a high sensitivity. At these low intensities, this same bone-conducted vibration stimulus has little effect on the firing rate of semicircular canal or regular otolith primary afferent responses.Reference Curthoys, Kim, McPhedran and Camp3, Reference Curthoys and Vulovic4
The ocular VEMP n10 component is negative (and so excitatory) and is probably due to the activation of a crossed otolith–ocular pathway from the otoliths to the inferior oblique and inferior rectus eye muscles. The evidence supporting these contentions is as follows. (1) Patients with unilateral vestibular nerve sectionReference Iwasaki, Smulders, Burgess, McGarvie, MacDougall and Halmagyi5, Reference Manzari, Burgess and Curthoys6 or vestibular schwannomaReference Iwasaki, Murofushi, Chihara, Ushio, Suzuki and Curthoys7 have a reduced or absent ocular VEMP n10 component beneath the contralesional eye, whereas the n10 component beneath the ipsilesional eye is of normal amplitude. (2) Suzuki et al. Reference Suzuki, Tokumasu and Goto8 showed that electrical stimulation of one utricular nerve in cats caused activation of the contralateral inferior oblique and inferior rectus muscles. (3) The amplitude of the n10 component in humans increases when the subject looks up, bringing the inferior oblique and inferior rectus muscles closer to the surface recording electrodes.Reference Iwasaki, McGarvie, Halmagyi, Burgess, Kim and Colebatch1, Reference Iwasaki, Smulders, Burgess, McGarvie, MacDougall and Halmagyi2, Reference Rosengren, Todd and Colebatch9, Reference Chihara, Iwasaki, Ushio and Murofushi10 (4) Patients without eye muscles do not show an n10 response.Reference Chihara, Iwasaki, Ushio, Fujimoto, Kashio and Kondo11 (5) Bone-conducted vibration activates the contralateral inferior oblique muscle in guinea pigs.Reference Yang, Liu, Wang and Young12
This same 500 Hz, bone-conducted vibration stimulus at the Fz site also causes a myogenic potential recordable by surface electrodes over tensed sternocleidomastoid muscles.Reference Manzari, Burgess and Curthoys6, Reference Halmagyi, Yavor and Colebatch13 This is the cervical VEMP. The first component of this potential, i.e. the positive–negative potential termed p13–n23, is an inhibitory potential probably caused by otolithic activation of descending, uncrossed, inhibitory, sacculo-collic projections (Colebatch et al.;Reference Colebatch, Halmagyi and Skuse14 see Rosengren et al. Reference Rosengren, Welgampola and Colebatch15 for a review). There is a wealth of evidence suggesting that the cervical VEMP is due to activation of the ipsilateral saccular macula, whose afferents course predominantly in the inferior vestibular nerve (for reviews of relevant anatomy, physiology and clinical evidence, see CurthoysReference Curthoys16 and Rosengren et al. Reference Rosengren, Welgampola and Colebatch15).
It appears that the cervical VEMP and the ocular VEMP reflect the function of different otolithic receptor regions, the ocular VEMP being mainly dependent on activation of utricular afferents and the cervical VEMP being mainly dependent on activation of saccular afferents.Reference Curthoys16 In humans (and guinea pigs), all afferents from the utricular macula travel in the superior division of the vestibular nerve,Reference de Burlet17 whereas in humans (and guinea pigs) most of the afferents from the saccular macula travel in the inferior division.Reference de Burlet17 This split is not perfect – a small contingent of afferents from the ‘hook’ region of the saccular macula travel in the superior vestibular nerve.Reference de Burlet17 Combining this anatomical evidence with the results of independent functional tests from patients with complete or partial dysfunction of the vestibular nerve (i.e. vestibular neuritis) provides a means of exploring the divisions of the vestibular nerve controlling the ocular VEMP and cervical VEMP (see Figure 1).
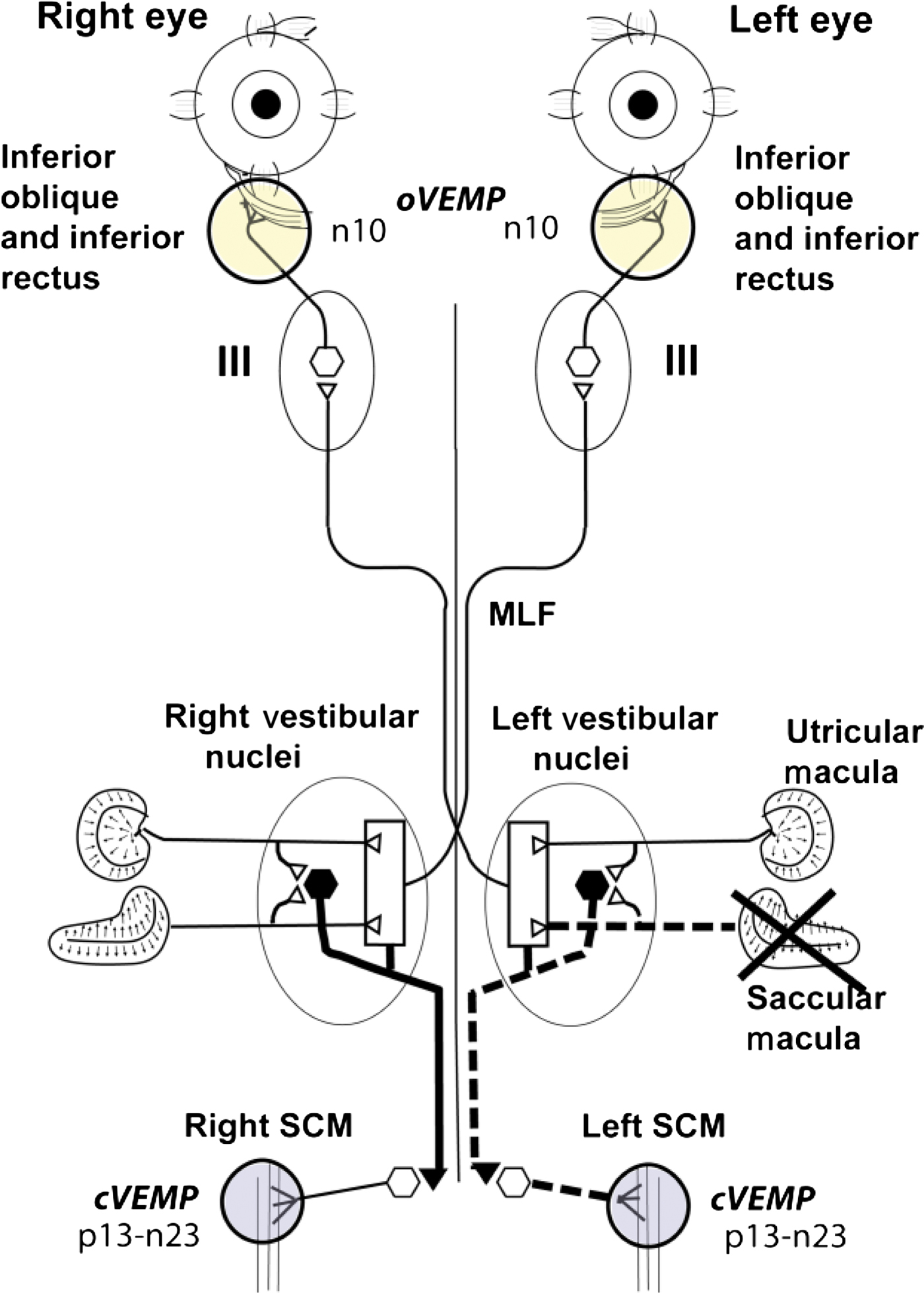
Fig. 1 Schematic diagram of some of the known vestibulo-ocular and vestibulo-collic projections which underlie the ocular and cervical vestibular evoked myogenic potential (oVEMP and cVEMP) responses to bone-conducted vibration delivered to the midline forehead at the hairline, based on known anatomical projections and physiological results from Suzuki et al. Reference Suzuki, Tokumasu and Goto8 and Uchino et al. Reference Uchino, Sasaki, Sato, Bai and Kawamoto30 Open symbols with thin lines indicate excitatory neurons, while closed symbols with thick lines indicate inhibitory neurons. Suzuki et al. Reference Suzuki, Tokumasu and Goto8 showed that high-frequency electrical stimulation of the utricular nerve results in activation of the contralateral inferior oblique and the ipsilateral superior oblique muscles, probably via some of the pathways shown here. Afferents from the saccular and utricular macula project to the vestibular nuclei; however, the exact termination of these afferents is not presently known, so the figure represents this uncertainty about the exact neural connections of these afferents by using an open box. The otolithic projections to other eye muscles are not shown. The afferents from the saccular macula course predominantly in the inferior vestibular nerve, and synapse on inhibitory neurons in the vestibular nucleus (black hexagons), which in turn project to spinal motoneurons controlling the sternocleidomastoid muscle (SCM). MLF = medial longitudinal fasciculus
Unilateral vestibular neuritis may affect the whole vestibular nerve or its branches.Reference Halmagyi, Aw, Karlberg, Curthoys and Todd18–Reference Strupp and Brandt25 Superior vestibular neuritis is distinguished by reduced or absent horizontal canal function, as shown by a canal paresis measure (via caloric testing) of greater than 22 per cent and/or the presence of a horizontal head impulse sign.Reference Halmagyi and Curthoys26 Superior vestibular neuritis is distinguished from neuritis affecting the entire vestibular nerve by the fact that superior vestibular neuritis patients have a normal p13 component of the ipsilateral cervical VEMP to either air-conducted soundReference Iwasaki, Chihara, Smulders, Burgess, Halmagyi and Curthoys27 or bone-conducted vibration.Reference Manzari, Tedesco, Burgess and Curthoys28 Based on this evidence, it is argued that if the ipsilateral cervical VEMP response to Fz-sited, bone-conducted vibration is present and symmetrical, then it is likely that the inferior vestibular nerve is still functional.Reference Manzari, Tedesco, Burgess and Curthoys28 Patients with superior vestibular neuritis have a reduced or absent ocular VEMP n10 component beneath the contralesional eye (in response to 500 Hz, Fz-sited, bone-conducted vibration), suggesting that the utricular afferents which have been affected by the superior vestibular neuritis are primarily responsible for the ocular VEMP n10 component.Reference Iwasaki, Chihara, Smulders, Burgess, Halmagyi and Curthoys27–Reference Curthoys and Manzari29
If the ocular VEMP n10 component is mainly due to utricular function, then unilateral dysfunction of the inferior vestibular nerve would be expected to have little or no effect on the amplitude of the ocular VEMP n10 component beneath the contralesional eye. On the other hand, if there is a saccular contribution to the contralateral ocular VEMP n10, then unilateral loss of saccular function should result in a reduced or absent contralateral ocular VEMP n10 (see Figure 1). In the present study, we tested this prediction by measuring the ocular VEMP n10 amplitude in patients with unilateral reduction or absence of cervical VEMPs.
Since the absolute value of VEMP amplitudes in response to Fz-sited, bone-conducted vibration stimulation varies between patients, we used a measure of the symmetry of the myogenic responses on the two sides in response to midline (Fz) stimulation, which stimulates both labyrinths approximately equally (see Iwasaki et al. Reference Iwasaki, Smulders, Burgess, McGarvie, MacDougall and Halmagyi2). In healthy people, the amplitude of the ocular VEMP n10 components beneath both eyes (in response to 500 Hz, Fz-sited, bone-conducted vibration) is approximately equal;Reference Iwasaki, Smulders, Burgess, McGarvie, MacDougall and Halmagyi2, Reference Manzari, Burgess and Curthoys6 similarly, the amplitude of the cervical VEMP p13–n23 components over the two sternocleidomastoid muscles (in response to the same vibration) is approximately equal.Reference Manzari, Burgess and Curthoys6 In Iwasaki and colleagues' study,Reference Iwasaki, Smulders, Burgess, McGarvie, MacDougall and Halmagyi2 the mean ± standard deviation (SD) ocular VEMP n10 asymmetry ratio (analogous to the Jongkees canal paresis score) for 67 healthy subjects was 11.73 ± 8.26 per cent. All healthy patients had asymmetry ratios of less than 40 per cent. Unilateral reduction or loss of utricular function causes asymmetrical ocular VEMP n10 responses, and so the asymmetry ratio in such patients is outside the normal range.Reference Iwasaki, Chihara, Smulders, Burgess, Halmagyi and Curthoys27
So, the question is: does unilateral reduction or loss of saccular function, as shown by the unilateral loss of cervical VEMPs, cause a corresponding asymmetry of the ocular VEMP n10? To test this question, we used data from 50 healthy subjects to establish the normal range for asymmetry ratios, for both ocular and cervical VEMPs. We then compared these normal results to those from 59 patients with probable inferior vestibular neuritis, who had unilaterally reduced or absent cervical VEMPs but in whom there was evidence that the superior vestibular nerve was functional (i.e. canal paresis index <22 per cent).
Patients and methods
Fifty-nine patients diagnosed as having probable inferior vestibular neuritis were enrolled in this study. In all these patients, there was independent evidence (from caloric and head-impulse responses) that the function of the superior vestibular nerve was within the normal range. The diagnosis of probable inferior vestibular neuritis derived from the fact that these patients had reduced or absent ipsilesional cervical VEMP responses, and that some cases also had a head impulse sign for excitatory head rotations in the plane of the posterior semicircular canal.Reference Cremer, Halmagyi, Aw, Curthoys, McGarvie and Todd31 Patients comprised 21 males (age range, 25–83 years; mean ± SD age, 55 ± 15 years) and 38 females (age range, 10–80 years; mean ± SD age, 54 ± 18 years).
All procedures were conducted in accordance with the Helsinki declaration, and were approved by the relevant institutional review board. All subjects and patients gave informed consent.
Table I summarises the relevant characteristics of the audiometric and vestibular test results of the 59 patients.
Table I Inferior Vestibular Neuritis Patients: Symptom Summary

*For 500, 1000, 2000 and 3000 Hz. Pt no = patient number; y = years; Migrn-reld = migraine-related; Full = fullness; Motn sick = motion sickness; CP = canal paresis; PTA = pure tone average; R = right ear; L = left ear; M = male; F = female; – = absent; += mild; ++= intense; +++= very intense
Patients with inferior vestibular neuritis reported symptoms of dizziness, postural unsteadiness and light-headedness, with vertigo and postural unsteadiness being the predominant symptoms. A detailed medical history was taken for every patient. In addition, all patients were assessed by: audiometric examination with air conduction and bone conduction threshold evaluation (even if the air conduction threshold was within normal limits); tympanometry with stapedial reflex testing; speech audiometry; auditory brainstem response testing; and caloric testing (modified Fitzgerald–Hallpike method).
This was the standard battery of audiological tests at the MSA Clinic in Cassino, Italy, and it was carried out to determine the site of the lesion by excluding other possibilities such as otosclerosis or retrolabyrinthine lesions.
None of our patients had a conductive hearing loss. In 13 patients, we identified a pure tone audiometry score asymmetry of 10 dB or more. We evaluated these asymmetric results firstly in light of the patient's history (looking for a lack of hearing level fluctuation and no concomitant increasing tinnitus with vestibular symptoms), and also in light of their auditory brainstem responses (ABRs) (looking for no asymmetry, in terms of latencies or amplitude, between the two sides, indicating integrity of the acoustic component of the VIIIth nerve).
On the basis of these tests, we concluded that the patients enrolled in the study did not have otosclerosis or vestibular schwannoma. However, to verify the latter point, we also referred all patients to a tertiary radiology centre for a magnetic resonance imaging scan of the posterior cranial fossa, using paramagnetic contrast enhancement. Radiological evaluation revealed normal and symmetrical VIIIth cranial nerves, and normal signal from the midbrain and posterior cranial fossa.
Horizontal canal function was tested in two ways. Every patient underwent caloric testing, and in order to be enrolled in the study the patient needed to have a canal paresis score of less than 22 per cent. Many patients were also tested by video recording of horizontal head impulses, to confirm that the dynamic function of the horizontal canal on the affected side was within the normal range.Reference MacDougall, Weber, McGarvie, Halmagyi and Curthoys32
On clinical testing, all these patients had normal or near-normal superior vestibular nerve function: the horizontal canal paresis index was less than 22 per cent in all cases, and there was no head impulse sign on horizontal head rotation in those patients thus tested. However, the inferior vestibular nerve on one side probably had reduced or absent function, since the p13 component of the cervical VEMP was reduced or absent following 500 Hz, Fz-sited, bone-conducted vibration. The net result was that the cervical VEMP asymmetry ratio was outside the normal range. In some cases, it was possible to establish the involvement of inferior vestibular neuritis more conclusively, by the presence of a corrective saccade following head rotations in the plane of the posterior semicircular canal.Reference Halmagyi and Curthoys26, Reference Cremer, Halmagyi, Aw, Curthoys, McGarvie and Todd31 Such confirmation was not possible in all patients.
Patients' results were compared to those from 50 healthy subjects without any vestibular disturbance (mean ± SD age, 38.3 ± 14.57 years; age range, 14–77 years), tested after informed consent had been obtained. These healthy subjects received exactly the same 500 Hz, Fz-sited, bone-conducted vibration stimulus as the patients, administered by the same operator, and their ocular and cervical VEMPs were measured in exactly the same way. None of the healthy subjects reported any auditory, vestibular, neurological or visual problems (apart from standard refractive errors).
Ocular vestibular evoked myogenic potential assessment
Full details of the procedures used are given in Iwasaki et al. Reference Iwasaki, Smulders, Burgess, McGarvie, MacDougall and Halmagyi2
In brief, subjects lay supine on a bed with their head supported on a pillow but positioned so that the head was horizontal or pitched slightly nose-down, with the chin on or close to the chest. The skin beneath both eyes was cleaned with alcohol wipes, and surface EMG electrodes were placed just beneath each eye to record the myogenic potentials. An active (i.e. positive), self-adhesive recording electrode was placed on the infra-orbital ridge just below the lower eyelid, and a reference (i.e. negative) electrode was placed about 2 cm below the active electrode. The electrodes were aligned with the centre of the pupil as the subject looked straight ahead. The self-adhesive pads around the electrode were trimmed to allow this very close placement, taking care that there was no electrical bridge formed between the two closely juxtaposed electrodes.
During testing, the subject looked up at a small fixation dot about 2 m from the eyes, positioned as high as was comfortable (the usual vertical visual angle was approximately 25–30° above the usual ‘straight ahead’ eye position, and exactly in the midline). The subject maintained visual fixation on the dot during testing.
The EMG surface potentials were amplified by alternating current coupled differential amplifiers (bandwidth 3–500 Hz (ocular VEMPs) or 3–2000 Hz (cervical VEMPs)), and the unrectified signals were averaged (n = 50 presentations for both ocular and cervical VEMPs) using a Medelec Amplaid Mk 12 averager (Milan, Italy) or an Otometrics Chartr system (Taastrup, Denmark); the sampling rate was 20 kHz in both cases. The electrical convention adopted was that negative potentials at the active electrode caused an upward trace deflection. A ground electrode was placed on the chin or sternum. Electrode impedance was maintained below 5 kΩ in all trials. Care was taken to ensure that the subject's jaw muscles were relaxed and that the person was looking straight up at the target point in their midline.
The ocular VEMP produced in response to 7 milliseconds of 500 Hz, Fz-sited, bone-conducted vibration stimulation is a series of negative and positive potentials. This study measured the amplitude of the first negative potential (i.e. the n10 component) from baseline to peak. The ocular VEMPs for both eyes were recorded simultaneously.
Cervical vestibular evoked myogenic potential assessment
Subjects lay supine on a bed. Alcohol wipes were used to clean the skin over the sternocleidomastoid muscles, and surface EMG electrodes were used to record the responses from both sternocleidomastoid muscles simultaneously. The subject was required to lift their head from the pillow while the operator stimulated the Fz site using 7 milliseconds of 500 Hz, bone-conducted vibration. The cervical VEMP in response to this stimulus is a series of positive and negative potentials; this study measured the peak-to-peak amplitude difference between the first positive and first negative potentials (i.e. the p13–n23 component).
Stimuli
The stimuli were delivered by a hand-held Bruel and Kjaer (Naerum, Denmark) 4810 Mini-Shaker instrument, fitted with a short bolt (2 cm long, M4) terminating in a bakelite cap, 1.5 cm in diameter, which was the contact point at the Fz point on the subject's forehead. Computer-generated 500 Hz tone bursts, lasting a total of 7 milliseconds and including a 2 millisecond rise and a 2 millisecond fall with a zero crossing start, were used to drive the Mini-Shaker instrument. The peak-to-peak linear acceleration at the mastoids was 0.4 g (Iwasaki et al. Reference Iwasaki, Smulders, Burgess, McGarvie, MacDougall and Halmagyi2) and the repetition rate was three per second, so the 50 stimuli took about 17 seconds to present. The Mini-Shaker instrument weighed approximately 1 kg, and the weight of the instrument was used to standardise the force applied in all subjects. The instrument was hand-held, but the operator simply maintained its near-vertical orientation at the Fz point, and did not apply any extra force to press the instrument against the subject's forehead.
Statistical analysis
The values in this paper are expressed as means ± SD. The significance level was set at 0.05. In this study, we calculated the absolute asymmetry ratio (AR) as follows:
Results
A typical example of cervical and ocular VEMPs from a patient with probable inferior vestibular neuritis is shown in Figure 2.

Fig. 2 Examples of averaged (a) contralesional cervical vestibular evoked myogenic potentials (VEMPs), (b) ipsilesional cervical VEMPs, (c) contralesional ocular VEMPs and (d) ipsilesional ocular VEMPs, in response to 500 Hz, bone-conducted vibration stimulation to the midline forehead at the hairline of a patient with unilaterally reduced cervical VEMP responses. The n10 component of the ocular VEMP response (V symbol) is the early negative component of this response, and is approximately equal beneath both eyes, as is the case in healthy subjects.
The asymmetry ratios for all healthy subjects were calculated for both ocular and cervical VEMPs, and are shown in Figure 3(a). For healthy subjects, the mean ocular VEMP asymmetry ratio was 7.09 ± 4.51 per cent, and the mean cervical VEMP asymmetry ratio was 8.23 ± 4.50 per cent.

Fig. 3 Plots of the asymmetry ratios of the ocular and cervical vestibular evoked myogenic potentials (oVEMPs and cVEMPs) for (a) healthy subjects and (b) patients with inferior vestibular neuritis. The patients show a significantly larger cVEMP asymmetry ratio compared with the healthy subjects, whereas there is no significant difference in oVEMP asymmetry ratios (comparing patients vs healthy subjects).
Similarly, the asymmetry ratios for all patients were calculated for both the ocular VEMP n10 component and the cervical VEMP p13–n23 component, and are shown in Figure 3(b). In a number of these patients, it was not possible to measure any meaningful, valid p13–n23 component from the electrode over the sternocleidomastoid muscle on the side of the affected ear, and so the value entered was 0 and the cervical VEMP asymmetry ratio became 100 per cent. The mean patient cervical VEMP asymmetry ratio was 70.71 ± 28.83 per cent, and the mean ocular VEMP asymmetry ratio was 12.30 ± 8.43 per cent.
A paired t-test was carried out to assess the significance of the difference between the patients' cervical and ocular VEMP asymmetry ratios; the mean difference was 58.40 ± 31.41, which was statistically significant (p < 0.001).
Patients who had asymmetrical cervical VEMPs had symmetrical ocular VEMPs. The reduced saccular function shown by the small cervical VEMP on the affected side did not detectably affect the contralateral ocular VEMP n10 component.
Discussion
The present study has shown that when the saccular macula and the inferior vestibular nerve have reduced function, there is no detectable effect on the ocular VEMP n10 component beneath the contralateral eye. This result implies that the afferents in the inferior vestibular nerve have little effect on the ocular VEMP n10 component beneath the contralateral eye in response to Fz-site, bone-conducted vibration.
Previous studies have shown the converse: that loss of superior vestibular nerve function, whilst the inferior vestibular nerve is still functional, leads to reduction or loss of the ocular VEMP n10 component in response to 500 Hz, Fz-sited, bone-conducted vibration beneath the contralateral eye.Reference Iwasaki, Chihara, Smulders, Burgess, Halmagyi and Curthoys27, Reference Manzari, Tedesco, Burgess and Curthoys28
These two results constitute a ‘double dissociation’: in patients with unilateral vestibular neuritis, if the superior vestibular nerve is affected then the ocular VEMP n10 beneath the contralesional eye is affected, whilst the cervical VEMP p13 remains; conversely, in patients with damage or loss of the inferior vestibular nerve, the ocular VEMP n10 remains but the cervical VEMP p13 is reduced or absent.Reference Manzari, Burgess and Curthoys33 This double dissociation supports the following conclusions: (1) that the ocular VEMP n10 component recorded by surface electrodes beneath the eye as the subject looks up reflects predominantly utricular activation; (2) that the cervical VEMP p13–n22 component recorded by surface electrodes on the tensed sternocleidomastoid muscles reflect predominantly saccular activation.
In our study, evidence of loss of inferior vestibular nerve function was complemented by evidence of normal function of the superior vestibular nerve (i.e. normal caloric responses, with a canal paresis of less than 22 per cent, and absence of head impulse sign following horizontal head rotations). This evidence implies that patients' ipsilesional horizontal canals were functioning normally; it is therefore likely that the superior vestibular nerve was not affected by the inferior vestibular neuritis.
• Vibration-induced cervical and ocular vestibular evoked myogenic potentials (VEMPs) probably reflect saccular and utricular function, respectively
• Inferior vestibular neuritis patients had asymmetrical cervical VEMPS but normal ocular VEMPs
• Thus, ocular and cervical VEMPs differentiate utricular from saccular function
Could the ocular VEMP n10 component have been due to preservation of the contingent of afferents from the hook region of the saccular macula, which travel in the superior vestibular nerve? The n10 component is a sharply defined potential at a very short latency, and appears to represent the EMG due to a synchronous volley of action potentials. It is unlikely to be caused by a volley arriving down a weak, polysynaptic pathway from the very small bundle of afferent fibres innervating the hook region of the saccular macula.
In the present study, exactly the same stimulus was used for both cervical VEMPs (just as was the case for the superior vestibular neuritis patients in the previous studyReference Manzari, Tedesco, Burgess and Curthoys28); thus, arguments about the effect of air-conducted sound versus bone-conducted vibration are not relevant.
Conclusion
Previous evidence indicates that the cervical VEMP response to bone-conducted vibration is probably due to ipsilateral saccular function, while the ocular VEMP response to bone-conducted vibration is probably due to contralateral utricular function.
The current study addressed the following questions: does saccular input contribute to the ocular VEMP, or are cervical and ocular VEMPs largely independent measures of otolithic function?
We found that 59 patients with asymmetrical cervical VEMPs had normal, symmetrical ocular VEMPs. This indicates that ocular and cervical VEMPs can be used to differentiate utricular from saccular function.
Acknowledgements
We are grateful for the support of the National Health and Medical Research Council of Australia (project grant numbers 457511 and 632746), and of the Garnett Passe and Rodney Williams Memorial Foundation.






