Introduction
Successful parasitism by parasitoids requires a sequence of hierarchical steps including host habitat location, host location, acceptance and suitability (van Alphen & Vet, Reference van Alphen, Vet, Waage and Greathead1986; Brodeur & Boivin, Reference Brodeur and Boivin2004). There are numerous works that relate the parasitism success for each of these hierarchical steps to external factors, such as host availability and host quality, and internal factors, such as egg load, life expectancy or energy reserves (Javoiš & Tammaru, Reference Javoiš and Tammaru2004; Mohamed et al., Reference Mohamed, Wharton, von Mérey and Schulthess2006; Wajnberg et al., Reference Wajnberg, Bernhard, Hamelin and Boivin2006; Canale & Benelli, Reference Canale and Benelli2012; Benelli et al., Reference Benelli, Gennari and Canale2013a ). Most of these studies were done on Hymenoptera, where the adult female carries out most of the behavioural steps of the parasitism process (Godfray, Reference Godfray1994). In a few Hymenoptera (Eucharitidae) and in other orders of parasitoids, such as Diptera (Tachinidae, Bomyiliidae), Lepidoptera (Epipyropidae), Neuroptera (Mantispidae) and most Coleoptera (Staphylinidae), location and selection of the host are performed by the first instar parasitoid larva (Eggleton & Belshaw, Reference Eggleton and Belshaw1992, Reference Eggleton and Belshaw1993; Yeates & Greathead, Reference Yeates and Greathead1997; Brodeur & Boivin, Reference Brodeur and Boivin2004; Stireman et al., Reference Stireman, O'Hara and Wood2006; Torréns, Reference Torréns2013). Nevertheless, relatively few studies have focused on the behavioural ecology of immature parasitoids (Royer et al., Reference Royer, Fournet and Brunel1999; Brodeur & Boivin, Reference Brodeur and Boivin2004; Crespo & Castelo, Reference Crespo and Castelo2009). This might be because the factors influencing host acceptance have been considered irrelevant since host-seeking larvae have a lower frequency of host encounter due to their limited dispersal ability (Feener Jr. & Brown, Reference Feener and Brown1997). However, for parasitoid species whose hosts are spatially aggregated, host discrimination might be advantageous for the larvae since they may encounter more than one host in their life (Royer et al., Reference Royer, Fournet and Brunel1999; Vet et al., Reference Vet, Hemerik, Visser, Wäckers, Lewis, Campbell and Sukhdeo2002; Brodeur & Boivin, Reference Brodeur and Boivin2004).
In parasitoids where the host-searching task is performed by the first instar larva, its entire fitness comes from a single host (Royer et al., Reference Royer, Fournet and Brunel1999; Brodeur & Boivin, Reference Brodeur and Boivin2004). Then, fitness can be directly related to host quality, which varies according to host species, age, sex, size, parasitism degree, host defences and nutritional state, and assessing host quality is of crucial importance for parasitoids (Godfray, Reference Godfray1994; Brodeur et al., Reference Brodeur, Geervliet and Vet1996; Brodeur & Boivin, Reference Brodeur and Boivin2004; Castelo & Crespo, Reference Castelo and Crespo2012). However, even when parasitoids can assess host quality efficiently, low-quality hosts are not always rejected (Heimpel et al., Reference Heimpel, Neuhauser and Hoogendoorn2003). Physiological state could influence the acceptance of low-quality hosts (Fletcher et al., Reference Fletcher, Hughes and Harvey1994; Sirot et al., Reference Sirot, Ploye and Bernstein1997; Javoiš & Tammaru, Reference Javoiš and Tammaru2006), and how the animal modifies its behavioural decisions throughout its life in response to its physiological and reproductive states is known as state-dependency (Mangel & Clark, Reference Mangel and Clark1988; Clark & Mangel, Reference Clark and Mangel2000; Roitberg & Bernard, Reference Roitberg, Bernard, Wajnberg, Bernstein and van Alphen2007; Bernstein & Jervis, Reference Bernstein, Jervis, Wajnberg, Bernstein and van Alphen2008).
Another important aspect for the successful parasitism is the capacity of parasitoids to attack one or more host species. The host range, i.e., the number and taxonomic diversity of species in which the parasitoid is able to develop, defines the host specificity (Futuyma & Moreno, Reference Futuyma and Moreno1988; Desneux et al., Reference Desneux, Barta, Hoelmer, Hopper and Heimpel2009). This specificity is affected by the physiological state and nutritional value of the host, behavioural host defences and presence of natural enemies (Slansky, Reference Slansky1986; Brodeur et al., Reference Brodeur, Geervliet and Vet1996). As host acceptance represents the last step of parasitoid host-searching, it might be used as a reliable indicator of parasitoid host-specificity (Brodeur et al., Reference Brodeur, Geervliet and Vet1996). However, the influence of internal factors such as parasitoid age, on the behavioural steps during parasitoid host-searching could influence the parasitism success due to the effects of the state-dependency on the process.
The intense selection pressure on parasitoids to locate hosts is well illustrated by the variety of cues and strategies used in host searching (Wang et al., Reference Wang, Yang, Gould, Wu and Ma2010; Joyce et al., Reference Joyce, Millar, Gill, Singh, Tanner and Paine2011; Benelli et al., Reference Benelli, Revadi, Carpita, Giunti, Raspi, Anfora and Canale2013b , Reference Benelli, Pacini, Conti and Canale c ; Colazza et al., Reference Colazza, Cusumano, Lo Giudice and Peri2013; Uefune et al., Reference Uefune, Kugimiya, Shimoda and Takabayashi2013). The stimuli sources used by parasitoids may be direct, as the host itself, or indirect, as the microhabitat of the host or a cue associated with its activity (Vet & Dicke, Reference Vet and Dicke1992; Steidle & van Loon, Reference Steidle and van Loon2003; Colazza et al., Reference Colazza, Cusumano, Lo Giudice and Peri2013; Uefune et al., Reference Uefune, Kugimiya, Shimoda and Takabayashi2013). In turn, these cues can be acoustic or visual but chemical cues seem to be the most frequent method of host location (Godfray, Reference Godfray1994; Rutledge, Reference Rutledge1996; Feener Jr. & Brown, Reference Feener and Brown1997; Cournoyer & Boivin, Reference Cournoyer and Boivin2004; Vet et al., Reference Vet, Hemerik, Visser, Wäckers, Lewis, Campbell and Sukhdeo2002; Gray et al., Reference Gray, Banuelos, Walker, Cade and Zuk2007). Since pheromones mediate the communication between conspecifics, they might be an important source of information for predators and parasitoids that can benefit from exploiting the host pheromones as kairomones (Dicke & Sabelis, Reference Dicke and Sabelis1988; Zuk & Kolluru, Reference Zuk and Kolluru1998; Ruther et al., Reference Ruther, Meiners and Steidle2002; Wertheim, Reference Wertheim2005; Wertheim et al., Reference Wertheim, van Baalen, Dicke and Vet2005). Kairomones are composed by a particular quali-quantitative blend of substances (Chapman, Reference Chapman1998; Greenfield, Reference Greenfield2002). The responses of parasitoids to host pheromones as kairomones appear to be host-specific, since they respond to pheromones of natural hosts, but not to those of a more distantly related non-target host species (Yong et al., Reference Yong, Pitcher, Gardner and Hoffmann2007). Thus, the quantitative blend composition of a cue, and the concentration in which it is released, could inform parasitoids about the identity of a host species among species belonging to the same genus.
Among dipteran parasitoids the most common strategy to locate hosts is the exploitation of the host's communication system, mainly through the detection of chemical cues (Feener Jr. & Brown, Reference Feener and Brown1997; Groba & Castelo, Reference Groba and Castelo2012). Most dipteran parasitoids, which have a split host location strategy with an active larval stage performing the final location and parasitism of the host, must use reliable cues, such as pheromones, to find them efficiently given their reduced mobility and the potential time-limitation (Eggleton & Belshaw, Reference Eggleton and Belshaw1992, Reference Eggleton and Belshaw1993; Stowe et al., Reference Stowe, Turlings, Loughrin, Lewis and Tumlinson1995; Feener Jr. & Brown, Reference Feener and Brown1997; Brodeur & Boivin, Reference Brodeur and Boivin2004; Stireman et al., Reference Stireman, O'Hara and Wood2006). The use of host-reliable cues enhances the efficiency in host finding and consequently increases the fitness on time-limited parasitoids (Vet et al., Reference Vet, Wäckers and Dicke1991; Wajnberg et al., Reference Wajnberg, Bernhard, Hamelin and Boivin2006).
Mallophora ruficauda Wiedemann (Diptera: Asilidae) is a robber fly endemic to the Pampas region of Argentina that inhabits open grasslands near apiaries (Rabinovich & Corley, Reference Rabinovich and Corley1997). This fly presents a biological duality; as an adult it is a predator that feeds mainly on honey bees and as a larva it is a solitary koinobiont ectoparasitoid of scarab beetle larvae (Coleoptera: Scarabaeidae), commonly known as white grubs. In this parasitoid, host searching is shared by adults and immature stages. During the summer, females M. ruficauda oviposit on tall grasses maximizing larvae dispersal by the wind (Castelo & Corley, Reference Castelo and Corley2004; Castelo et al., Reference Castelo, Ney-Nifle, Corley and Bernstein2006). After hatching, first instar larvae fall to the ground and rapidly bury themselves. Seven days later they moult into the second instar larva using their own reserves (Crespo & Castelo, Reference Crespo and Castelo2010). In this instar, the larvae acquire the ability to orientate towards the host through chemical cues originating in the hosts’ hindgut (Castelo & Lazzari, Reference Castelo and Lazzari2004; Crespo & Castelo, Reference Crespo and Castelo2008; Groba & Castelo, Reference Groba and Castelo2012). Crespo & Castelo (Reference Crespo and Castelo2010) estimated under laboratory conditions the median duration of the second instar larvae was 32 days in absence of the host and 109 days after they parasitized the host. Moreover, this larval instar is capable of discriminating the parasitism status of the host by means of chemical cues (Crespo & Castelo, Reference Crespo and Castelo2009). Once M. ruficauda larva locates the host and parasitism takes place, the larva remains attached to its host during the winter as a second instar. Then, at the end of the winter, when temperature slowly increases, the larva grows rapidly and one month later it completes its development, by consuming the host and pupating (Crespo & Castelo, Reference Crespo and Castelo2010). The successful parasitic relationship occurs between the second instar larva of M. ruficauda and the third instar scarab larva.
Scarabaeidae larvae are rhyzophagous and live in the soil during the winter (March to August) (Remedi de Gavotto, Reference Remedi de Gavotto1964; Alvarado, Reference Alvarado1983; Potter, Reference Potter1998). There are nine species of scarab beetles within the M. ruficauda distribution area (Alvarado, Reference Alvarado1980). Castelo & Corley (Reference Castelo and Corley2010) described the field specificity of M. ruficauda towards the species of white grubs and they found that M. ruficauda selects Cyclocephala signaticollis Burmeister among several scarab species because its relative frequency of parasitism towards this species is the highest (86.60%). In other species of the same genus, the relative frequencies of parasitism are smaller, being 6.70% for C. modesta Burmeister and 1.44% for C. putrida Burmeister. For Heterogeniates bonariensis Ohaus the frequency is 0%. The relative frequencies for the other species vary between 3.35 and 0%. However, this shows M. ruficauda to have a certain degrees of behavioural flexibility towards the acceptance of different host species and is not a strict specialist of C. signaticollis. However, it still remains poorly understood that if this parasitoid larva has the ability to orient itself towards different species of white grub in different scenarios according to its own physiological state.
This work seeks to determine the host orientation and host acceptance behaviours of the second instar larva of M. ruficauda for different white grub host species, as a measure of host's specificity. Furthermore, we test whether the larvae's host specificity is affected or modulated by larval life expectancy and concentration of host stimuli.
Materials and methods
Insects
Larvae of M. ruficauda were obtained from egg clutches collected from the grasslands in Moreno (34°46′S, 58°93′W), a locality associated with apiaries in Buenos Aires province, Argentina, between January and March 2009, 2010 and 2011. The egg clutches were individualized in Falcon type tubes and were observed daily to register hatching. When the eggs hatched, the larvae were separated individually in Eppendorf type tubes of 1.5 ml with a piece of filter paper as a substrate sowed with mineral water. Tubes were stored in complete darkness between 24 and 26 °C until they were used in the experiments. Before initiating any experiment every larva was checked to have moulted to the second instar (LII).
Hosts were collected in Moreno, Pilar (34°28′S, 58°55′W), General Rodríguez (34°27′S, 58°57′W), Escobar (34°20′S, 58°49′W) and Mercedes (34°65′S, 59°43′W), localities from Buenos Aires province and the Experimental Field of Ciudad Universitaria (Nuñez, Buenos Aires city, 34°32′S, 58°26′W) between March and August 2008–2011. White grubs were collected digging the soil to a depth of 0.3 m (López et al., Reference López, Alvarez Castillo, Carmona, Manetti and Vincini1994; Castelo & Corley, Reference Castelo and Corley2010). At the laboratory, each individual was identified up to species level using a taxonomic key (Alvarado, Reference Alvarado1980). The hosts, third instar larvae of white grubs, were maintained individually at room temperature in black tubes filled with soil and were fed weekly with fresh pieces of carrots until they were exposed alive in the experiments or used for preparing the stimuli extracts offered to the parasitoid larvae in the orientation experiments.
General considerations
M. ruficauda larvae used in all the experiments differed in their age and thus on their life expectancy. First, M. ruficauda larvae of 17–28 days old were considered ‘young larvae’. These larvae have recently moulted to the second instar and had a high life-expectancy, i.e., 86–94% (Crespo & Castelo, Reference Crespo and Castelo2010). Then, ‘old larvae’ were those with an age of more than 58 days old, where the risk of mortality was higher and the probability of survival decreased to 55% or less (Crespo & Castelo, Reference Crespo and Castelo2010).
Specificity experiments were performed to determine if host selection is determined by behavioural flexibility related to parasitoid age. We used as treatments chemical cues extracts and live white grubs of three species of Cyclocephala (C. signaticollis, Cs; C. modesta, Cm and C. putrida, Cp) tested on both young and old parasitoid larvae. The first species is the naturally most selected in the field and the other species have a relative low parasitism frequency. In all the experiments with live white grubs or with chemical cues extracts, individuals of H. bonariensis (Hb) (orientation and acceptance behaviour, tables 1 and 2, respectively) were used as a negative control due to their null parasitism frequency in the field (Castelo & Capurro, Reference Castelo and Capurro2000; Castelo & Corley, Reference Castelo and Corley2010). This species was used to evaluate the selectivity towards other species tested. The control species allowed us to compare the response of parasitoid larvae against the experimental groups and also rule out any age effect on the larvae's mobility, as indicator of larvae's health.
Table 1. Experimental design to determine the degree of specificity of the second instar larvae of M. ruficauda measured as orientation behaviour towards host chemical cues and live hosts, and if the specificity changes with the age of the parasitoid larva. This experimental series were performed to evaluate the responses of M. ruficauda larva to chemical cues and live hosts in olfactometer assays. N, number of replicates. Between brackets the total number of individuals that made a choice (left, stimulus; right, solvent/empty). The difference between the number of replicates and number of parasitoids that chose any side of the arena are called the ‘No decision’ larvae.

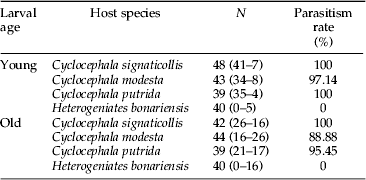
Table 2. Experimental design performed to evaluate the degree of specificity of the second instar larva of M. ruficauda regarding to age as the acceptance behaviour to different white grub species through no-choice tests (artificial parasitism). N, number of replicates. Between brackets the individuals found attached to the host (left) and number of missing larvae (right).
Specificity measured as orientation behaviour
In order to determine the degree of specificity of the LIIs, measured through the orientation behaviour towards host chemical cues extracts and live hosts, and if this specificity changes with the age of the parasitoid larva, we performed two laboratory dual-choice experiments in two rectangular static air two-way olfactometers. Since the natures of stimuli offered were different, the olfactometers used on each experiment differed in size (see details below). However, the principle was the same in both devices.
In both olfactometers, the arena was divided into three equal size zones (one middle and two laterals) along the long axis. On each lateral zone either the stimulus or control was placed. At the beginning of each trial, an individual M. ruficauda larva was released on the central area of the arena, and after 90 min, each device was examined to record the larva's position. Three possible responses could be obtained: choice for the stimulus (S), for the control (C) or no decision (ND) if the larvae remained in the middle zone. Replicates where M. ruficauda larva died during the assay were excluded from the statistical analysis. Because environmental factors can influence patch-time exploitation (Amat et al., Reference Amat, Castelo, Desouhant and Bernstein2006; Crespo & Castelo, Reference Crespo and Castelo2012), all the experiments of dual-choice were done between 10:00 and 17:00 h, under laboratory conditions (25.1±3.7 °C, 52±22% relative humidity) and in complete darkness. Additionally, olfactometry experiments were conducted only on sunny days with barometric pressure values between 1008 and 1021 hPa, since it has been shown that both a low and a sudden drop in barometric pressure has dramatic effects on patch-time exploitation and host-seeking behaviour in insects (Roitberg et al., Reference Roitberg, Mangel, Lalonde, Roitberg, van Alphen and Vet1993; Crespo & Castelo, Reference Crespo and Castelo2012).
Orientation to chemical cues
For assays with chemical cues extracts we used a 9 cm×6 cm×1 cm olfactometer, which is the same experimental arena used by Castelo & Lazzari (Reference Castelo and Lazzari2004) and Crespo & Castelo (Reference Crespo and Castelo2008). The stimuli were odour extracts from the four species of white grubs. They were offered a piece of filter paper (2 cm×1 cm) at each lateral zone containing either 10 μl of chemical cue or solvent. Experimental design and replicates for each treatment are detailed in table 1.
To obtain the stimuli extracts from the hosts with kairomones, the posterior body part of third instar larvae was homogenized using hexane as solvent (Castelo & Lazzari, Reference Castelo and Lazzari2004; Groba & Castelo, Reference Groba and Castelo2012). We used experimental solutions equivalent to 2.5 white grubs per ml, being more than double the attractive concentration used by Castelo & Lazzari (Reference Castelo and Lazzari2004), guaranteeing the occurrence of behavioural responses.
Orientation to live hosts
For live hosts we used an arena of 15 cm×4 cm×4 cm divided equally in three areas with a plastic mesh preventing the movement of the white grub outside the lateral zone but allowing the parasitoid larva to move freely. An individual host was placed in one of the lateral zones, whereas the other remained empty. Control series for assays with live hosts were performed without hosts in both sides. Experimental design and replicates for each treatment are detailed in table 1. These series allowed us to detect any possible asymmetry effect inherent to the experimental device.
Specificity measured as host acceptance behaviour
In order to evaluate the degree of specificity of the LII, measured through the acceptance behaviour towards different white grub species, and if the acceptance changes with the age of the parasitoid larva, we carried out no-choice tests consisting in artificial parasitism. For each assay, an individual host was placed in a 30 ml black tube filled with soil and food and with an LII. Each replicate, 39–49 per host species and parasitoid age treatment, was run for 1 week. After this period, the hosts were inspected to verify if the larva was attached to their cuticle, and had become a parasitized host. If the parasitism did not occur, we proceeded to register the soil to detect whether the larva was dead or remained free in the tube unattached to the host. Parasitism rate was calculated for each white grub species as the ratio between the number of parasitized hosts and the total number of hosts used in the experiment. Replicates where the white grub or the M. ruficauda larva died unattached to the host during the assay were not taken into account for calculating parasitism rate nor for the statistical analysis. Treatments and controls that were carried out for each white grub species and for each larval age are shown in table 2. Also, we registered when larvae got lost (i.e., not found neither attached to the host nor free in between the soil) to calculate the missing rate, as an indirect measure of the larvae physiological state (Crespo & Castelo, Reference Crespo and Castelo2008), and evaluate if there are any differences between treatments.
Orientation behaviour towards increasing concentrations of Cyclocephala chemical cues
To determine whether the specificity is influenced by host stimuli concentration in the Cyclocephala species we observed the orientation behaviour of the parasitoid larvae towards host chemical extracts with ascendant quantities of host cues. The aim of this experiment was to elucidate in a behavioural context whether the chemical cue that results attractive to M. ruficauda larvae is the same in all Cyclocephala species, if different host extracts promote the same orientation response, and if the parasitoid's interpretation of the host identity among species is due to differences in the cue concentration to which the parasitoid is exposed. We performed one experiment in the laboratory that is detailed in table 3. We used as the experimental arena a 9 cm×6 cm×1 cm static air two-way olfactometer, the same experimental arena and methodology as in ‘Specificity measured as orientation behaviour’ section. Three increasing concentrations of each host species chemical extracts were used: 5, 7.5 and 10 white grubs per m hexane. They were prepared using the same protocol as in ‘Orientation to chemical cues’ section. We performed experiments combining host species, extract concentration and parasitoid age (18 treatments). Experimental design and number of replicates for each treatment are shown in table 3.
Table 3. Experimental design series carried out in olfactometer assays to evaluate the degree of orientation (specificity) of the second instar larva of M. ruficauda to increasing concentrations of Cyclocephala hosts extracts according to the age of the parasitoid larva. N, number of replicates. Between brackets the total number of individuals that made a choice (left, stimulus; right, solvent).

Statistical analysis
In dual-choice experiments where the preference of M. ruficauda larvae for either side of the arena was tested against a random distribution, the data were analysed by means of chi-square (χ2) tests of goodness of fit (Zar, Reference Zar2010). To evaluate the influence of larval age, host species and nature of stimuli on M. ruficauda motivation to initiate movements related to host-searching behaviour, a three-way analysis of variance (ANOVA) was performed using M. ruficauda larval age, host species and cue nature as factors (Zar, Reference Zar2010). These data were checked for normality using a Shapiro–Wilk test. Previously, data from dual-choice experiments were randomized and the proportion of non-decision larvae was calculated every 16 observations. These proportions were used as response variable and were arcsin transformed and subjected to three-way ANOVA (Zar, Reference Zar2010). Post hoc comparisons for all treatments were performed using a Tukey–Kramer multiple comparison test (Zar, Reference Zar2010). When host specificity was measured through the host acceptance behaviour, the differences between parasitism rates for each treatment were analysed with a generalized lineal model analysis (GLM) with a logit link function, where the number of larvae successfully established to their respective host was defined as response variable (Hardy, Reference Hardy2002). Complementarily, data were transformed with a modification of the Freeman and Tukey transformation and a multiple comparison procedure analogous to the Tukey or Dunnett test was applied for non-parametric comparison (Zar, Reference Zar2010). Moreover, to assess any differences in the missing larvae along both the larval ages and host species, the ratio of missing larvae was tested with a GLM analysis, with a logit link function (GLM, GenStat 11.1) (Hardy, Reference Hardy2002).
Results
Specificity measured as orientation behaviour
Mallophora ruficauda young second instar larvae orientated significantly towards C. signaticollis chemical stimulus (fig. 1, table 1). This result is coincident with the one already found by Crespo & Castelo (Reference Crespo and Castelo2008). On the contrary, when young larvae were exposed to C. modesta and C. putrida stimuli, they distributed randomly in the experimental arena. The control series with H. bonariensis stimulus larvae also showed a random distribution (fig. 1, table 1).

Fig. 1. Orientation responses of M. ruficauda second instar larvae of different ages to extracts of Cyclocephala hosts species. Y, young larvae; O, old larvae; Hx, hexane (control) ; Cs, extract of C. signaticollis; Cm, extract of C. modesta; Cp, extract of C. putrida; Hb, extract of H. bonariensis; *, statistically significant differences (χ2 test, P<0.05).
Like the young larvae, the old larvae oriented towards C. signaticollis stimulus, but also larvae orientates significantly towards C. modesta stimulus (fig. 1, table 1). When larvae were exposed to C. putrida stimulus, they distributed randomly in the experimental arena. Similarly, larvae showed a random distribution when tested against to H. bonariensis extract (fig. 1, table 1). These results suggest that aged second instar larvae of M. ruficauda, with a high risk of mortality, are less selective and orientate towards other near potential host species as C. modesta.
When live white grubs were offered as stimuli the results were strikingly different (fig. 2, table 1). Both young and old larvae oriented not only towards C. signaticollis as we expected, but also oriented to C. putrida. When young larvae were exposed to C. modesta, they distributed randomly in the arena. Unlike the chemical stimulus experiments, where old M. ruficauda larvae were exposed to live C. modesta, they distribute randomly. When young and old larvae were exposed to live H. bonariensis, in both cases they distributed randomly in the lateral sides of the experimental arena (fig. 2, table 1).

Fig. 2. Orientation responses of M. ruficauda second instar larvae of different ages to live Cyclocephala hosts species. Y, young larvae; O, old larvae; E, empty (control) ; Cs, live C. signaticollis; Cm, live C. modesta; Cp, live C. putrida; Hb, live H. bonariensis; *, statistically significant differences (χ2 test, P<0.05).
Motivation of LII to initiate host-searching movements were analysed for young and old larvae stimulated with both chemical and live host stimuli from the different host species used in this work. Only the double interaction between larval age and cue nature was significant (ANOVA: F 1,116=10.78, P=0.0014), while other interactions were not statistically significant (ANOVA: M. ruficauda larval age * host species * cue nature: F 4,116=2.35, P=0.0587; host species * M. ruficauda larval age: F 4,116=2.14, P=0.0806; host species * cue nature F 4,116=1.76, P=0.1425). Furthermore, host species was not statistically significant (F 4,116=2.33, P=0.0601). Post hoc comparison showed that hosts’ chemical cues elicit a stronger response to initiate host-searching behaviour than live hosts, in both young and old larvae. Moreover, when experiments were performed with live host as stimuli, young larvae showed a higher motivation than old larvae. When young and old larvae exposed to chemical cues were compared, despite there is slightly higher values for young larvae than old ones, no significant differences were found (fig. 3).

Fig. 3. Motivation of young and old second instar M. ruficauda larvae to initiate movements related to host-searching behaviour, when they are exposed to host's chemical cues and live hosts as stimuli. Chem, chemical host stimuli; Live, live host; Y, young larvae; O, old larvae. Different letters indicate significant differences (ANOVA, Tukey–Kramer test, P<0.05).
Specificity measured as host acceptance behaviour
When the specificity of M. ruficauda was analysed through the acceptance behaviour between the larval ages and among host species, it was found that both young and old second instar larvae were attached, in high proportion, to the three species of Cyclocephala. For H. bonariensis the proportion of attached larvae drastically decreased to zero (fig. 4, table 2). In particular, we found that 100% of young larvae were attached both to C. signaticollis and C. putrida, 95.6% to C. modesta and 0% to H. bonariensis. Similar values were found in the experiments with old larvae, being 100% to C. signaticollis and C. putrida, 93.7% to C. modesta and 0% to H. bonariensis. Responses were significantly different according to the host, being higher to species belonging to genus Cyclocephala (GLM: deviance=207.8, P<0.001; Hb-Cs q0.05,∞,4=23.523; Hb-Cm q0.05,∞,4=19.153; Hb-Cp q0.05,∞,4=21.073; Cs-Cm q0.05,∞,4=3.027; Cs-Cp q0.05,∞,4=1.525; Cm-Cp q0.05,∞,4=1.476). The effect of the interaction between larval age and host species was not significant (GLM: deviance=3.17, P=0.075), neither larval age (GLM: deviance=1.09, P=0.297).

Fig. 4. Specificity of young and old second instar larvae of M. ruficauda measured as acceptance behaviour towards different Cyclocephala hosts species. Y, young larvae; O, old larvae; Cs, live C. signaticollis; Cm, live C. modesta; Cp, live C. putrida; Hb, live H. bonariensis. Different letters indicate significant differences (χ2 test, P<0.05).
When the percentage of missing larvae in the arena was analysed for different hosts and M. ruficauda larval ages, no host species effect was found (GLM: deviance=0.049, P=0.824), neither the interaction between host species and parasitoid larval age (GLM: deviance=0.321, P=0.571). For larval age, old larvae showed significantly more ‘disappearance’ rate than young larvae (GLM: deviance=43.75, P<0.001). The number of dead larvae in acceptance experiments was four in a total of four replicates and the mortality rate was insignificant among the host species assays, being zero for C. signaticollis, three for C. putrida, and one for C. modesta, and they were not considered in the analysis.
Orientation behaviour towards increasing concentrations of Cyclocephala chemical cues
When orientation to increasing chemical cues was evaluated, young larvae oriented to C. signaticollis concentrations of 5 and 7.5 white grubs per ml extract, but the distribution in the arena was at random in the 10 white grubs per ml concentration experimental series (fig. 4, table 3). When young larvae were exposed to each of these concentrations of C. modesta and C. putrida extracts, they distributed randomly in the experimental arena in all the experimental series (fig. 5, table 3).

Fig. 5. Orientation responses of M. ruficauda second instar larvae of different ages to extracts of Cyclocephala hosts species of increasing concentrations. Y, young larvae; O, old larvae; Hx, hexane (control); Cs, extract of C. signaticollis; Cm, extract of C. modesta; Cp, extract of C. putrida; Hb, extract of H. bonariensis; 5, 7.5 and 10 correspond to concentrations of white grubs per ml; *, statistically significant differences (χ2 test, P<0.05).
When host orientation was tested on old larvae with C. signaticollis increasing concentrations, the larvae only oriented significantly to the host extract of 5 white grubs per ml, and distributed randomly in the experimental arena with 7.5 and 10 white grubs per ml concentrations. Similar results were observed with C. modesta. Old larvae oriented to host extract of 5 white grubs per ml, but not to 7.5 and 10 white grubs per ml concentrations. No preference for either side of the arena was registered to any concentration of C. putrida extract, and the larvae distributed randomly in both sides of the arena (fig. 5, table 3). In every case, i.e., each combination of host species and larval age, M. ruficauda did not orientate to extracts of 10 white grubs per ml.
Discussion
Our results indicate that host orientation changes with the host species, type of cues and parasitoid age; however, host acceptance is only influenced by the host species. Moreover, we found that increasing concentrations of the host stimuli might impair the parasitoid capability to distinguish the host identity.
As in previous works, M. ruficauda shows a high preference for parasitizing C. signaticollis and detects its chemical cues originating in the posterior half of the host's body (Castelo & Lazzari, Reference Castelo and Lazzari2004; Groba & Castelo, Reference Groba and Castelo2012). However, our work suggests that orientation to potential hosts of the genus Cyclocephala can show some flexibility depending on the nature of the cue detected by the parasitoid and its age, and this behaviour is modulated by the different chemical cues released by the potentials hosts. Particularly, besides the expected response to C. signaticollis chemical cues, only old M. ruficauda larvae orientated to C. modesta chemical cues, suggesting that C. modesta odours are attractive to larvae with low life expectancy. The fact that life expectancy can influence and decreases parasitoids selectivity thresholds has been suggested many times for female adult parasitoids and, sometimes, for host-seeking larvae (Mangel, Reference Mangel1987; Royer et al., Reference Royer, Fournet and Brunel1999; Javoiš & Tammaru, Reference Javoiš and Tammaru2004). In Aleochara bilineata Gyllenhal (Coleoptera: Staphylinidae), a solitary parasitoid of puparia of some species of Diptera, host acceptance is influenced by their life expectancy and the host condition, and was observed that the degree of acceptance of suboptimal hosts (parasitized hosts) increases significantly with the age of the larvae (Royer et al., Reference Royer, Fournet and Brunel1999).
The positive attraction of the aged parasitoid to C. modesta host odours is irrespective of the concentration used. Differences observed in young larvae orientation pattern towards C. modesta and C. signaticollis could be due to the quantities of attractive cue contained in the extract since they differ in size (one C. signaticollis equals 2.13 C. modesta in weight, Castelo & Crespo, Reference Castelo and Crespo2012). In this scenario, we expect the response changes when young larvae are exposed to a higher concentration of C. modesta extract. Given that orientation patterns in both larval age groups are similar when we increased the extract concentration, quantity of cue contained in extracts cannot explain the observed differences. The behavioural responses of M. ruficauda larva suggest that C. signaticollis and C. modesta might have similar cues because the larvae orientate to both hosts but in different conditions. These cues may share chemical components but vary in their proportions, or differ in their components, while close structural similarities; these similarities are likely a result of the phylogenetic relationship between them (Tillman et al., Reference Tillman, Seybold, Jurenka and Blomquist1999; Guerrieri et al., Reference Guerrieri, Schubert, Sandoz and Giurfa2005; Yong et al., Reference Yong, Pitcher, Gardner and Hoffmann2007; Félix et al., Reference Félix, Calatayud, Le Ru, Silvain and Frérot2011).
Given that when high concentration stimuli were used no orientation was observed, it might suggest that there was no odour plume because the olfactometer was odour saturated. Another possible explanation involves saturation of chemoreceptor structures. Crespo et al. (Reference Crespo, Lazzari and Castelo2011) suggested that M. ruficauda larva orientates by means of klinotaxis by the successive comparison of stimulus concentration during the insect movement. If the parasitoid's chemoreceptors were saturated, there is an impossibility to detect and to orient through the odour gradient. At intermediate concentration, only young larvae show a similar orientation pattern to the two lower concentrations, but the older larvae do not choose any side of the experimental arena, even when C. signaticollis extract was used as stimulus. The parasitoid age might be influencing the sensitivity of chemoreceptors and old larvae chemoreceptors might become saturated at lower odour concentrations than younger ones, and cannot detect changes in stimulus strength (Blaney et al., Reference Blaney, Schoonhoven and Simmonds1986). So, we found that old larvae fail to discriminate the odours of alternative hosts when they are concentrated. On the contrary, young larvae only failed to discriminate them at the highest concentration.
Some works show that parasitoids can exploit a multiplicity of cues during the host orientation process (Fischer et al., Reference Fischer, Samietz, Wäckers and Dorn2001; Wang et al., Reference Wang, Yang, Gould, Wu and Ma2010). In this work, when orientation to live white grubs was tested in young and old parasitoid larvae, we found that they orientated not only towards C. signaticollis but also towards C. putrida, and in no case towards C. modesta, as it happened when stimulated with chemical cues. This fact suggests that M. ruficauda may be using another type of cue to detect and orientate towards the hosts, such as mechanical cues, due to the vibrations caused by hosts on the substrate, as observed in other insects (Laumann et al., Reference Laumann, Blassioli Moraes, Čokl and Borges2007; Wang et al., Reference Wang, Yang, Gould, Wu and Ma2010). If this were the case, differences between the species tested could be explained given the differences and similarities between sizes of host species. C. signaticollis and C. putrida are similar in size as C. modesta and H. bonariensis, but the first pair is considerably larger than the second one (Castelo & Crespo, Reference Castelo and Crespo2012). So, it is possible that M. ruficauda cannot discriminate among different species of live hosts with similar size, because mechanical cues originated by the movement of the host could be similar between C. signaticollis and C. putrida. Moreover, according to C. modesta, it is possible that the parasitoid larva detects a chemical cue similar to the other Cyclocephala species, but may recognize this species as not the preferred one by detecting other cues such as movement pattern, size, stridulation pattern, other odours, as was observed in other parasitoids. In addition, Alvarado (Reference Alvarado1980) has made an exhaustive morphological description of the four species tested in this work and found that these scarab larvae have structures for stridulation in their mandibles and maxillae. These structures might generate a detectable and recognizable cue for M. ruficauda larva as other parasitoids do (Schmidt & Smith, Reference Schmidt and Smith1987a ,Reference Schmidt and Smith b ; Laumann et al., Reference Laumann, Blassioli Moraes, Čokl and Borges2007; Flores-Prado & Niemeyer, Reference Flores-Prado and Niemeyer2012).
Regarding the orientation experiments, we observed that motivation to initiate exploratory movements depended on the stimulus nature and the larva's age used in the experiments and not on the host species. Chemical stimulus might imply a more reliable indicator of host presence as opposed to mechanical stimulus. However, when we offered live hosts chemical stimuli are present. The difference in larvae's motivation might be due to a higher concentration of the attractive odours in the chemical stimulus than in the live host. Also, the olfactometer used with live white grubs was higher in size than that used with chemical solutions. Thereby, differences in olfactometers size could reinforce the difference in attractive odour concentrations. Young larvae showed greater motivation than older larvae, but only when live hosts are offered. As observed in previous works, second instar of young and old ages have a similar motivation to initiate locomotive movements to chemical stimulus (Crespo & Castelo, Reference Crespo and Castelo2008).
Unlike the field studies, no-choice experiments allow us to find out whether the parasitoid attempts to attack unsuitable hosts or conversely, whether potentially suitable hosts are not attacked at all (Morehead & Feener Jr., Reference Morehead and Feener2000; Desneux et al., Reference Desneux, Barta, Hoelmer, Hopper and Heimpel2009). In this case, our laboratory experiments showed that H. bonariensis is not attacked by M. ruficauda, but C. modesta and C. putrida are attacked even though the parasitoid cannot develop successfully, i.e., parasitoid cannot complete its development until adulthood or the emerged adult was of lower weight or malformed in relation to parasitoids emerged from optimal host (unpublished data). These aspects have been observed also in other parasitoid species (Godfray, Reference Godfray1994; Desneux et al., Reference Desneux, Barta, Hoelmer, Hopper and Heimpel2009). Differences between results from orientation and acceptance behaviours may be understood as a response of M. ruficauda, in absence of optimal hosts, accepting other Cyclocephala species available as hosts, like occur in other insect species (Stephens & Krebs, Reference Stephens and Krebs1986; Janssen, Reference Janssen1989; Ellers et al., Reference Ellers, van Alphen and Sevenster1998).
Finally, the physiological condition of parasitoids is crucial on the host location process and on the subsequent parasitism success. In the experiments where we measured the acceptance of hosts, we also registered when larvae were not found. We found that the proportion of missing larvae was significantly higher with increasing M. ruficauda age. Only in some cases, dead larvae were found in the soil, but in almost all cases they were not found. Dead larvae were probably degraded quickly due its minute size. The death of larvae may be because of natural causes (i.e., the mortality rate proper of each parasitoid age) or larvae might have suffered some kind of injury derived from mechanical host defences when the parasitoid larva was trying to parasitize the white grubs (Castelo & Crespo, Reference Castelo and Crespo2012). Old larvae could have probably been attacked more due to their low mobility and their poor physiological condition. However, the difference in the death proportion between young and old larvae could be due to the specific mortality rate of each age, being higher in older parasitoids.
Throughout this work we attempted to show the complexity of the chemical ecology in the Cyclocephala species host-M. ruficauda parasitoid model. Although M. ruficauda could be considered a specialist host, it has been demonstrated that the host selection is not completely rigid, showing certain flexibility to choose other potential hosts. Under particular conditions, hosts can be considered as suboptimal for the parasitoid, such as a limited availability in the environment or decreased larval survival probability. The time-limited survival probability, as an example of state-dependency, has been largely addressed with adult parasitoid models. In the models where the larvae perform host location, this topic was addressed from host-discrimination, where aged larvae select a previously parasitized host, and this mechanism also seems to occur in M. ruficauda, suggesting an age-dependency host selection in this dipteran host-seeking larva.
Acknowledgements
We thank local beekeepers from Moreno (Quinta Zanotti), SERPAJ (General Rodríguez and Pilar), Escobar and Mercedes, province of Argentina, for allowing us to work on their farms. We specially thank D. Steve Dennis for his very useful comments and suggestions on the manuscript. This work has been funded through grants UBACyT 2011–2012 no. 1031 and 2012-2015 no. 0125, and CONICET PIP 2009–2013 no. 1597, to M. Castelo.










