I. INTRODUCTION
Some of the reagents used to synthesize Sb-containing catalysts are variable in composition, hampering reproducible catalyst preparation. As part of a program to generate stoichiometric reagents, antimony oxalate hydroxide was prepared. Its crystal structure was solved by applying the charge flipping method (Oszlányi and Sütó, Reference Oszlányi and Sütó2008) and difference Fourier techniques to laboratory X-ray powder diffraction data and refined by the Rietveld (Reference Rietveld1969) method. Density functional calculations were used to understand the bonding.
II. EXPERIMENTAL
A. Sample preparation
Sb2O3 (25 g) and oxalic acid dihydrate (22 g) were slurried in 300 ml de-ionized water. The mixture was then refluxed for 3 h. After cooling, the resulting slurry was filtered to recover the Sb(C2O4)OH product. The white solid was washed several times with de-ionized water and dried at 110 °C for 3 h. A 96% yield was obtained. The compound has been prepared previously by reaction of oxalic acid dihydrate and SbCl3 (Korzun et al., Reference Korzun, Schorr, Schmitz, Fadzeyeva, Kommichau and Bente2005; Karlov et al., Reference Karlov, Butuzov and Dobrokhotova1983; Ambe, Reference Ambe1975).
B. Powder pattern
The white powder was examined as synthesized. The X-ray pattern was measured (Cu Kα radiation, 40 kV, 40 mA, 5° to 150° 2θ in 0.007 296 89° steps, 1 s/step) from a rotating specimen on a Bruker D8 Advance diffractometer equipped with a VÅNTEC-1 position-sensitive detector. Several weak Cu K(β) peaks were present in the pattern and were ignored in data processing. A search of the Powder Diffraction File (Faber and Fawcett, Reference Faber and Fawcett2002) using JADE 8.5 (Materials Data, Inc., 2008) indicated that the product was single-phase Sb(C2O4)OH (Karlov et al., Reference Karlov, Butuzov and Dobrokhotova1983) (Figure 1).
The pattern could be indexed using DICVOL06 (Louër and Boultif, Reference Louër and Boultif2007) on a high-quality [M(19)=123.3, F(19)=141.1] orthorhombic unit cell having a=5.827 13(3), b=11.294 48(10), c=6.313 77(3) Å, and V=415.537(5) Å3. Systematic absences were consistent with space groups Pnma or Pn21a. With Z=4, the calculated density was 3.625 g cm−3.
C. Structure solution
Attempts to solve the structure using Monte Carlo simulated annealing and direct method techniques were unsuccessful. Both methods yielded the Sb atom position but no other plausible atoms or fragments. Even Monte Carlo simulated annealing trials using Sb-OH and oxalate fragments were unsuccessful. We attribute these failures to the unrecognized preferred orientation, which tended to concentrate the electron density in the mirror plane.
Structure factors from a Le Bail (Reference Le Bail, Dinnebier and Billinge2008) extraction carried out using GSAS (Larson and Von Dreele, Reference Larson and Von Dreele2004) were used to create a SHELX HKLF 4 file, which was used as input to SUPERFLIP as incorporated into JANA2006 (Petříček et al., Reference Petříček, Dušek and Palatinus2006). The Sb and hydroxyl oxygen positions were identified easily. These two atoms were fixed in GSAS, and a difference Fourier map yielded the positions of the oxalate C and one of the O. Another O (the strongest peak in the difference map) was located only 1.06 Å from the Sb but in a direction expected for the remaining oxalate O. This atom was moved manually into approximately the correct position and refinement begun.
D. Refinement
The 15° to 150° portion of the pattern was used in the Rietveld refinement, with excluded regions 18.4° to 20.2°, 25.2° to 25.7°, and 40.5° to 40.9° to eliminate the strongest Kβ peaks. The background was described by a three-term shifted Chebyshev polynomial combined with a nine-term

Figure 1. (Color online) (Color online) Observed X-ray powder pattern of Sb(C2O4)OH. The vertical scale is (counts)1/2to emphasize the weaker peaks.
diffuse scattering function to describe the small concentration of amorphous material. The peak profiles were described using GSAS profile function No. 4, which includes the Stephens (Reference Stephens1999) anisotropic strain broadening model. The S(040)coefficient was fixed at 0, and X and shft were also refined. The significant preferred orientation was described by a sixth-order spherical harmonic expansion; the final texture index was 1.441.
The Sb was refined anisotropically, while a common Uiso was refined for the O and C atoms. The Uiso of the hydrogen atoms was fixed at 1.3 times that of the O/C. Restraints (soft constraints) were applied to the Sb-O bond distances: Sb1-O2=2.00(5) and 2.42(5); Sb1-O3/O4=2.25(5) Å. The bonded [C5-C5=1.55(4), C5-O3/O4=1.25(3) Å] and nonbonded [C5-O3/O4=2.32(5) Å] distances in the oxalate anion were also restrained. An angle restraint O3-C5-O4=126(5)° was also applied. The restraints contributed 0.9% to the final reduced χ2.
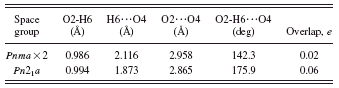
In space group Pnma, the hydroxyl group O2-H6 lies on the mirror plane at y=1/4. This results in rather weak and distorted O2-H6…O4 hydrogen bonds to two symmetry-equivalent O4 (Table I). Refinements in subgroups of Pnma such as Pn21a were unstable (they diverged). A quantum chemical geometry optimization in Pn21a led to a structure 93.8 kcal/mol lower in energy, with stronger and more-linear ordered hydrogen bonds (Table I). Refinement of ordered
TABLE I. Disordered and ordered hydrogen bonds in Sb(C2O4)OH.
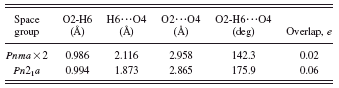
and disordered hydrogen models in Pnma yielded the same residuals. The final half-occupancy hydrogen position was therefore fixed off the mirror plane.
The final refinement of 49 variables using 18 139 observations yielded the residuals Rwp=0.0757, Rp=0.0604, χ2=5.651, R(F)=0.0837, and R(F 2)=0.0964. The largest peak in the difference Fourier map was +2.8e Å−3 (1.03 Å from Sb1) and the largest difference hole was −2.9e Å−3 (0.33 Å from Sb1). The largest errors in the final Rietveld plot (Figure 2) are in the shapes of the strong low-angle peaks and the

Figure 2. (Color online) (Color online) The final Rietveld plot for Sb(C2O4)OH. The red crosses represent the observed data points and the solid green curve; in magenta below is the calculated pattern. The difference pattern in magenta is plotted at the same scale as the other patterns. The row of tick marks indicates the calculated reflection positions. The vertical scale has been magnified by a factor of 20 in the 56° to 89° region and by a factor of 40 from 89° to 150°.
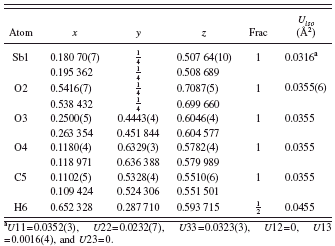
TABLE II. Refined (top) and optimized (bottom) structural parameters for Sb(C2O4)OH. Space group Pnma, a=5.827 13(3), b=11.294(10), and c=6.313 77(3) Å.
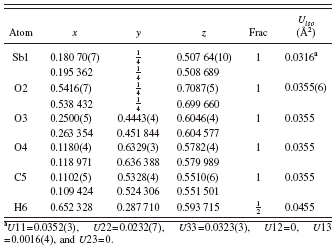
a U11=0.0352(3), U22=0.0232(7), U33=0.0323(3), U12=0, U13=0.0016(4), and U23=0.
high-angle background. The refined and optimized structural parameters are reported in Table II, and bond distances and angles are contained in Tables III and IV.
E. Quantum mechanics
Quantum chemical geometry optimizations were carried out using density functional plane wave pseudopotential techniques as implemented in CASTEP (Clark et al., Reference Clark, Segall, Pickard, Hasnip, Probert, Refson and Payne2005). The Perdew-Burke-Enzerhof functional with a 300 eV plane wave basis set cutoff was used with lattice parameters fixed at the experimental values. The Brillouin zone was sampled using four k points.
III. RESULTS AND DISCUSSION
The crystal structure contains pentagonal pyramidal Sb3+ cations, which are bridged by hydroxyl groups to form zigzag chains along the a axis (Figure 3). Each oxalate anion chelates to two Sb in approximately the ab plane, linking the chains into a three-dimensional framework (Figure 4).
The apical Sb-OH bond is relatively short [1.933(3) Å], while the other Sb-OH bond in the basal plane is relatively
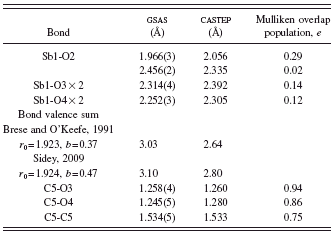
TABLE III. Bond distances in Sb(C2O4)OH.
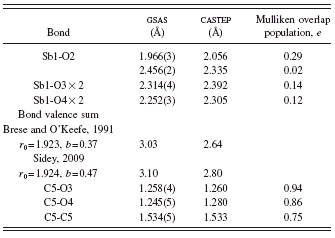
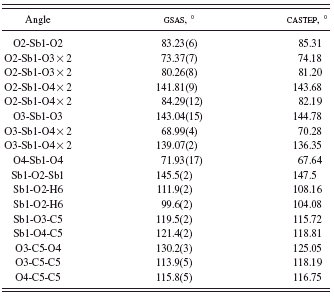
TABLE IV. Bond angles in Sb(C2O4)OH.

long [2.456(2) Å]. The Sb-oxalate bonds are slightly longer than expected from bond valence calculations (2.23 Å) (Brese and O’Keefe, Reference Brese and O’Keefe1991), but the Sb bond valence sum of 3.03 is very close to the expected value. The Sb coordination is “one sided;” the Sb lies 0.80 Å out of the pentagonal plane, as might be expected for a coordination sphere which includes a lone pair.
In the bond valence method (Brown, Reference Brown2002), the bond valence is most often calculated as s=exp[(r0−robs)/b], though other expressions have been used. In the most commonly used tabulation of bond valence parameters (Brese and O’Keefe, Reference Brese and O’Keefe1991), r 0 is tabulated for many cation-anion pairs and b is a universal constant equal to 0.37. For some cations which contain lone pairs, these default values might not be appropriate (Sidey, Reference Sidey2009), and alternate values have been derived. For this compound, both sets of parameters yield reasonable values for the Sb atomic valence (Table III).
The oxalate bond distances and angles fall within the normal ranges (Bruno et al., Reference Bruno, Cole, Kessler, Luo, Motherwell, Purkis, Smith, Taylor, Cooper, Harris and Orpen2004). The oxalate anion is planar to within 0.01 Å; the mean plane is approximately (5, 2, 11). The disordered hydroxyl group O2-H6 forms a normal-strength hydrogen bond to the oxalate oxygen O4, consistent with the shorter C5-O4 distance and higher calculated charge (O3=−0.56and O4=−0.62).
The Mulliken overlap populations (Table III) show that the short Sb-OH and the Sb-oxalate bonds have significant covalent character, while the long Sb-OH bond is nearly ionic. Although a ball-and-stick rendering of the structure

Figure 3. (Color online) The zigzag hydroxyl-bridged chains along the a axis in Sb(C2O4)OH. 50% probability ellipsoids/spheroids.

Figure 4. (Color online) The crystal structure of Sb(C2O4)OH, viewed approximately along the c axis.
(Figure 5) makes it appear that there are voids, no voids are detected by Mercury (Macrae et al., Reference Macrae, Bruno, Chisholm, Edgington, McCabe, Pidcock, Rodriguez-Monge, Taylor, van de Streek and Wood2008) and none are apparent in a space-filling rendering.
The density of states plot (Figure 6). The next lowest-energy filled orbitals are oxygen p orbitals.
The bischelating oxalate bridging of two Sb observed in this compound is the most common binding mode in antimony oxalates (Udovenko et al., Reference Udovenko, Sigula, Samarets and Davidovich1981b; Southerington et al., Reference Southerington, Begley and Sowerby1991; Schwarz et al., Reference Schwarz, Schmidt and Blösl1981; Coudreau-Ducourant et al., Reference Coudreau-Ducourant, Ducourant, Fourcade and Mascherpa1981; Millington and Sowerby, Reference Millington and Sowerby1992). Also observed are single chelation (Poore and Russell, Reference Poore and Russell1971; Millington and Sowerby, Reference Millington and Sowerby1992), chelation to one Sb and monodentate binding to another (Udovenko et al., Reference Udovenko, Sigula and Davidovich1981a; Davidovich et al., Reference Davidovich, Zemnukhova, Udovenko and Sigula1983), and trans-monodentate bridging of two Sb (Davidovich et al., Reference Davidovich, Zemnukhova, Udovenko and Sigula1983; Escande et al., Reference Escande, Tichit, Ducourant, Fourcade and Mascherpa1978a, Reference Escande, Tichit, Ducourant, Fourcade and Mascherpa1978b; Marsh, Reference Marsh1997).
Sb(C2O4)OH is isostructural to Bi(C2O4)OH, the structure of which was determined recently using single crystal techniques (Rivenet et al., Reference Rivenet, Roussel and Abraham2008). The analogy was not detected by a default reduced cell search in the Inorganic Crystal Structure Database (Hellenbrandt, Reference Hellenbrandt2004); the tolerances

Figure 5. (Color online) The crystal structure of Sb(C2O4)OH, viewed approximately along the a axis.

Figure 6. (Color online) The density of states plot for Sb(C2O4)OH. The heavy vertical line segment indicates the Fermi level.
on the unit cell edges had to be increased from 0.1 to 0.3 Å. The Bi coordination sphere is described as having six normal bonds and two long “secondary” bonds. Both bond valence considerations and Mulliken overlap populations suggest that the long (3.18 Å) Sb-O distances in this compound do not represent real bonds.
The Bravais-Friedel-Donnay-Harker morphology (Donnay and Harker, Reference Donnay and Harker1937) calculated from the crystal structures is blocky, but possibly consistent with elongated morphology along [100], or platy with {010} or {001} as prominent faces. Analysis of the calculated pole figure plots suggests a platy morphology with {001} as the large faces. The refined texture index is 1.441. Although not obvious from the pattern, preferred orientation is significant and made the structure solution very difficult. Undetected preferred orientation can be fatal to ab initio structure solution, resulting in collapsing of electron density into a plane.

Figure 7. (Color online) The HOMO, illustrating the Sb3+lone pairs.

Figure 8. (Color online) The powder pattern (blue) of Sb(C2O4)OH after refluxing in water, illustrating the conversion to nanocrystalline cervantite (Sb2O4, PDF entry 04-008-6495, red) and a trace of valentinite (Sb2O3, green). Only a small concentration of antimony oxalate hydroxide (black) remained.
Refluxing Sb(C2O4)OH in water converts it into nanocrystalline cervantite (Sb2O4) with a trace of Sb2O3 (Figure 8). The decomposition under relatively mild conditions shows that Sb(C2O4)OH can be a useful reagent.
IV. SUMMARY
The crystal structure of Sb(C2O4)OH has been solved by applying charge flipping and difference Fourier techniques to laboratory X-ray powder data exhibiting significant preferred orientation and refined by the Rietveld method. The structure is chemically reasonable compared to other antimony oxalates and to Bi(C2O4)OH . The experimental powder pattern has been submitted to ICDD for inclusion in future releases of the Powder Diffraction File.