Introduction
The yak (Bos grunniens) is the principal meat and dairy animal in the Qinghai-Tibet plateau, as few other animals survive in this area. In vitro production (IVP) of embryos is a well established embryonic biotechnology with a variety of applications in basic and applied sciences (Sirard, Reference Sirard2017). IVP has made great progress in cattle and is becoming one of the most exciting and progressive procedures available for today’s producers, however the efficiency of yak IVP is still low (Marinho et al., Reference Marinho, Sanches, Rosa, Tannura, Rigo, Basso, Pontes and Seneda2015).
Although many advances have been made in cell culture techniques, the proportion of embryos that develop to the blastocyst stage is variable. Oxidative stress, a cellular condition caused by the accumulation of reactive oxygen species (ROS), is thought to contribute significantly to defective embryo development (Loren et al., Reference Loren, Sanchez, Arias, Felmer, Risopatron and Cheuqueman2017; Tian et al., Reference Tian, Wang, Zhang, Ji, Wang, Lv, Li, Chai, Lian and Liu2017). Despite the fact that low concentrations of ROS have been shown to reduce DNA fragmentation and improve the developmental potential of porcine embryos, excessive ROS levels are one of the main determinants of embryo quality produced in vivo and in vitro, and are caused by exposure to elevated oxygen concentrations, light, and elevated concentrations of metabolites and substrates (Mata-Campuzano et al., Reference Mata-Campuzano, Alvarez-Rodriguez, del Olmo, Fernandez-Santos, Garde and Martinez-Pastor2012).
One method that may help to overcome this problem is the supplementation of antioxidant compounds. The addition of antioxidants has been reported to decrease ROS levels and increase blastocyst production rates (Nikseresht et al., Reference Nikseresht, Toori, Rahimi, Fallahzadeh, Kahshani, Hashemi, Bahrami and Mahmoudi2017). Melatonin is a potent free radical scavenger and antioxidant. The beneficial effects of melatonin are evident, particularly with regard to its antioxidant properties, as ROS production is decreased compared with untreated controls (Mehrzadi et al., Reference Mehrzadi, Safa, Kamrava, Darabi, Hayat and Motevalian2016). Furthermore, as melatonin is amphiphilic, it can easily reach any cellular compartment, including the membrane, cytosol, and mitochondria (Asghari et al., Reference Asghari, Abdollahi, de Oliveira and Nabavi2017). Importantly, it inhibits peroxidation, which is a common feature of other antioxidants. With regard to its free radical scavenging capacity, melatonin is five-fold more potent than glutathione (GSH) (Liu et al., Reference Liu, Qin, Qian, Shi, Deng, Zhu, Zhang, Tao and Liu2014). Although the antioxidant effect of melatonin has been reported in some mammalian cell models, there is currently no research regarding the effect of melatonin on yak preimplantation embryo development (Song et al., Reference Song, Peng, Yin, Zhao, Fu, Zhang, Mao, Wu and Zhang2016).
The aim of the present study was to determine whether melatonin has the ability to protect preimplantation yak embryos against oxidative injury and to determine the underlying mechanisms. We show, as far as we can ascertain for the first time, the embryo-protective effect of melatonin against oxidative injury. Furthermore, the improvement in embryo viability was associated with the decreased ROS levels, stable mitochondrial function, and modulation of the activity of enzymatic antioxidants.
Materials and methods
Animals and reagents
All animal experiments and treatments were approved and supervised by the Animal Care Commission of the College of Veterinary Medicine, Northwest A&F University. The ovaries of slaughtered cattle were obtained from Datong Abattoir in Qinghai, China.
Dulbecco’s phosphate-buffered saline (DPBS) was purchased from HyClone Laboratories Inc. (Logan, UT, USA), follicle-stimulating hormone (FSH) from Bioniche Inc. (Belleville, Ontario, Canada) and fetal calf serum (FCS) from Gibco (Grand Island, NY, USA). All other chemicals and reagents were cell culture tested and were obtained from Sigma-Aldrich (St. Louis, MO, USA). Synthetic oviductal fluid (SOF) medium was prepared according to a previous study.
Oocyte collection and in vitro maturation (IVM)
Ovaries were collected from yaks at the abattoir from October to December, and transported to the laboratory in DPBS maintained at 28–34°C. Cumulus–oocyte complexes (COCs) were aspirated from follicles (2–8 mm diameter), and collected in DPBS supplemented with 6 mg/ml bull serum albumin (BSA) under a low-power (×20 magnification) stereomicroscope. Unselected COCs were rinsed three times in DPBS containing 5% (v/v) fetal calf serum (FCS) and twice in TCM-199 supplemented with 20% (v/v) fetal calf serum (FCS), 1 μg/ml estradiol-17β, 100 U/ml penicillin, and 100 μg/ml streptomycin. Only COCs with one or more layers of cumulus cells and an evenly granulated ooplasm were selected. Up to 30 COCs were placed in each culture well (Nunc Inc., Naperville IL, USA) containing 600 μl of maturation medium and covered with 300 μl mineral oil. The COCs were allowed to mature for approximately 24 h at 38.5°C in an atmosphere of 5% CO2 in humidified air.
In vitro fertilization and embryo culture
Frozen semen was thawed and washed by centrifugation through a Percoll gradient (30%/45%) containing HEPES-buffered SOF medium supplemented with 5 mg/ml BSA, 50 μg/ml caffeine, 30 μg/ml glutathione and 20 μg/ml heparin (sperm medium) and centrifuged at 500 g for 10 min to separate motile sperm. After centrifugation, 120 μl of the concentrated sperm fraction was removed and placed into 200 μl of sperm medium and incubated at 38.5°C for 15 min.
Oocytes and sperm were then co-incubated for 24–26 h at 38.5°C in an atmosphere of 5% CO2 in humidified air. The putative zygotes were then washed three times in SOF medium. The number of cleaved zygotes was recorded 48 h post-insemination. The culture medium was changed at 96 h and blastocyst development was determined on days 7–9 post-insemination (Day 0 = insemination).
Melatonin and hydrogen peroxide (H 2 O 2 ) treatment
Zygotes were selected for the experiments. To determine oxidative damage, the zygotes were exposed to different concentrations of H2O2 (0–120 μM) for 15 min, thoroughly washed and then cultured in SOF until the day of blastocyst stage. For the melatonin dose–response study, zygotes were cultured with different concentrations of melatonin (0–100 μM) in the blastocyst stage throughout the development period, and the SOF medium containing melatonin at the specified concentrations was replenished after 72 h. To determine the protective effect of melatonin against oxidative stress, zygotes were preincubated with melatonin (0, 1, 10, and 50 μM) in dimethylsulfoxide (DMSO) or DMSO alone (0.1%) for 1 h. After preincubation, melatonin or DMSO was maintained and H2O2 was added for 15 min, and then washed out and the embryos were again cultured in medium containing melatonin or DMSO until day 7.
Terminal-deoxynucleotidyl transferase mediated nick end labelling (TUNEL) assay
Embryo cell apoptosis was determined according to the manuscript’s instructions. Briefly, blastocysts were washed three times (5 min each time) in phosphate-buffered saline (PBS) (Beyotime, China) containing 0.2% polyvinyl alcohol (PVA), fixed in Immunol Staining Fix Solution (Beyotime, China) for 30 min, and the embryos were then permeabilized with 0.1% Triton X-100 in PBS for 10 min, and then equilibrated for 8 min after three washes. Samples were then incubated with rTdT incubation buffer (45 μl equilibration buffer, 5 μl nucleotide mix, and 1 μl rTdT enzyme) in the dark for 1 h at 37°C. The tailing reaction was terminated in 2× standard saline citrate for 15 min.
Immunofluorescence staining of embryos
Embryos were washed three times (5 min each) in PBS containing 0.2% PVA, and fixed in Immunol Staining Fix Solution (Beyotime, China) for 1 h. All steps were performed at room temperature unless otherwise stated. Embryos were permeabilized with 0.2% Triton X-100 in PBS for 30 min. After three washes, they were blocked in the Immunol Staining Blocking Solution (Beyotime, P0102) for 12 h at 4°C and then incubated with the first antibodies for 12 h at 4°C. Antibody against Caudal Type Homeobox 2 (CDX2; BioGenex, USA) was diluted 1:200 using Immunol Staining Primary Antibody Dilution Solution (Beyotime). After three washes, the embryos were treated with secondary antibodies of Alexa Fluor 555-labelled Goat Anti-Mouse IgG (Beyotime). Samples were analyzed using a Nikon eclipse Ti-S microscope equipped with a 198 Nikon DS-Ri1 digital camera (Nikon, Japan). The experiments were replicated three times. In each replicate, 10 to 15 embryos per group were processed.
Detection of caspase-3 activity
Caspase-3 activity was determined using a caspase assay kit (Beyotime, China), which detected production of the chromophore p-nitroanilide after its cleavage from the peptide substrate DEVD-p-nitroanilide and LEHD-p-nitroanilide.
Quantitative real-time PCR
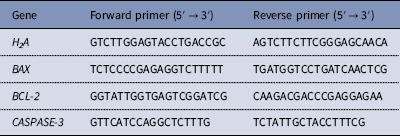
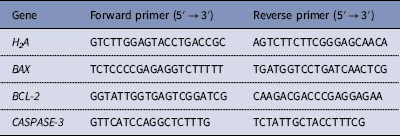
A single day 7 blastocyst was used per sample, and 5–8 embryos were used from each group. Total RNA from the embryos was isolated using the Cells-to-Signal Kit (Ambion Co., USA) according to the manufacturer’s protocol. The mRNA levels were quantified using SYBR Premix ExTaq™ II (TaKaRa, Japan) on an ABI PRISM 7700 Sequence Detection System (Applied Biosystems, Inc., Carlsbad, CA, USA). Samples were denatured at 95°C for 1 min and then subjected to 40 cycles of amplification (95°C, 5 s; 60°C, 30 s). Fold changes in each gene were calculated using the 2−ΔΔCT method. All primers used in this study are shown in Table 1.
Table 1 Primers for qRT-PCR
Determination of ROS products
To measure ROS levels, zygotes were exposed to H2O2 for 15 min, and then each group was incubated in SOF supplemented with dichlorodihydrofluorescein diacetate (DCFH-DA; 2 mM, Molecular Probes) for 30 min at 37°C as previously described.
Determination of adenosine triphosphate (ATP) content
The levels of ATP were measured with a luminometer (Bioluminat Junior, Berthold, Germany) and an ATP Bioluminescence Assay Kit (Beyotime).
Detection of mitochondrial membrane potential (MMP)
MMP was determined by staining the zygotes with 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolyl-carbocyanine iodide (JC-1; BioVision; MA, USA). The zygotes were incubated in SOF supplemented with JC-1 (1.25 μM) for 20 min at 37°C following exposure to H2O2.
Oxidant and antioxidant capability assay
In total, 30–50 blastocysts from each group were lysed for 5 min, vortexed for 5 min, then frozen at −80°C and thawed at 37°C three times. The mixture was centrifuged at 10,000 g for 10 min at 4°C and placed on ice. The level of malondialdehyde (MDA), total antioxidant capacity (T-AOC), and glutathione (GSH) and the activities of glutathione peroxidase (GSH-Px) and catalase (CAT) were determined according to the manufacturer’s instructions (Nanjing Jiancheng Biochemistry Reagent Co., Nanjing, Jiangsu, China).
Statistical analysis
Data [mean ± standard deviation (SD)] were analyzed using SPSS software (SPSS Inc., Chicago, IL, USA). Differences were analyzed using one-way analysis of variance (ANOVA) or the t-test. For statistical analysis of the percentage values, the χ2 test was performed. Differences with a P-value <0.05 were considered to be statistically significant.
Results
Melatonin protected the development of yak preimplantation embryos from H 2 O 2 -induced oxidative stress
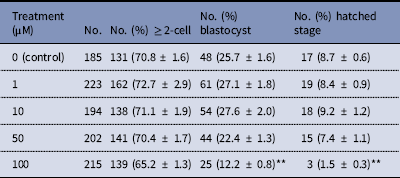
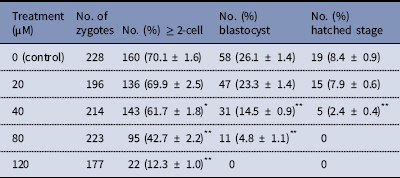
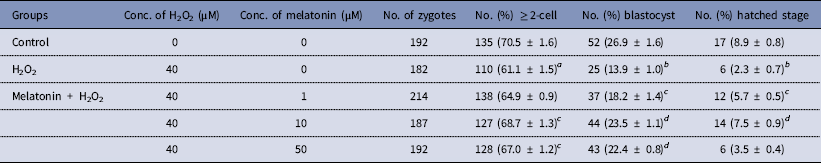
The dose effect of melatonin on embryonic development was first investigated. Hence, we used melatonin at different concentrations and compared the development of melatonin-treated embryos to controls cultured in DMSO. Table 2 shows that melatonin at 1, 10, and 50 μM, had no significant effect on the embryonic development, while melatonin at 100 μM reduced the rate of blastocysts formation. To induce oxidative stress, H2O2 was used to treat yak zygotes. When zygotes were treated with 40 μM H2O2, the rates of cleavage stage embryo and blastocyst formation decreased significantly (Table 3). In subsequent experiments, the effect of melatonin on the development of preimplantation embryos was evaluated following treatment of zygotes with 75 μM H2O2. Table 4 shows that the two-cell, blastocyst and hatched stage formation almost recovered to the levels in the control group at 10 μM melatonin, but when the concentration of melatonin reached 50 μM, the percentage of developmental embryos decreased, especially in the hatched stage. These findings indicated that the effect of melatonin on H2O2-injured embryos was not linear.
Table 2 Development of preimplantation yak embryos in the presence of melatonin
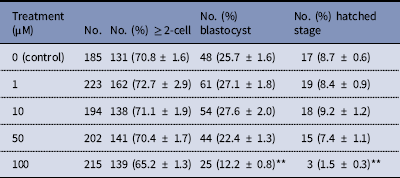
**P < 0.01 versus control; χ2 test was used.
Table 3 Development of preimplantation yak embryos after treatment with H2O2

*P < 0.05, **P < 0.01 versus control; χ2 test was used.
Table 4 Protective effect of melatonin on the development of preimplantation yak embryos exposure to H2O2
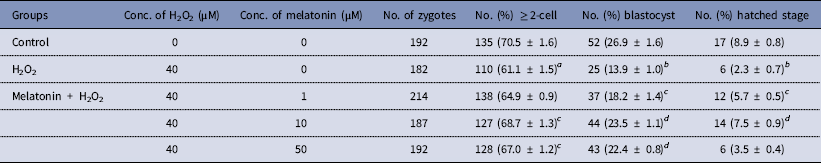
a P < 0.05, b P < 0.01 versus control; c P < 0.05, d P < 0.01 versus H2O2; χ2 test was used.
Melatonin protected the quality of blastocysts developed from zygotes exposed to H2O2
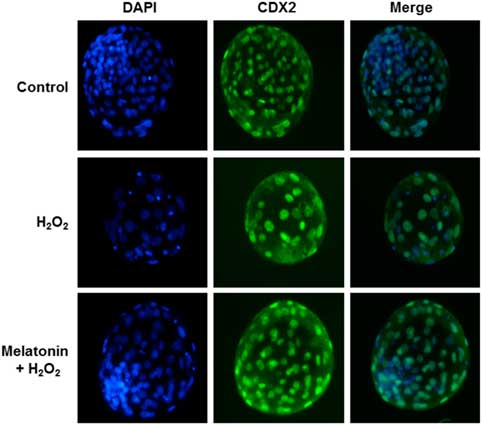
To determine whether the improvement in embryo development reflected enhanced blastocyst quality, we determined the cell numbers and ratio of inner-cell-mass cells (ICM)/total cell number (TCN) in blastocysts in each group; these parameters were the criteria used to assess blastocyst quality. As shown in Fig. 1 and Table 4, zygotes treated with H2O2 showed decreased numbers of total and ICM cells, as well as the ICM:TCN ratio of blastocysts, when compared with the control group. However, melatonin prevented these effects.
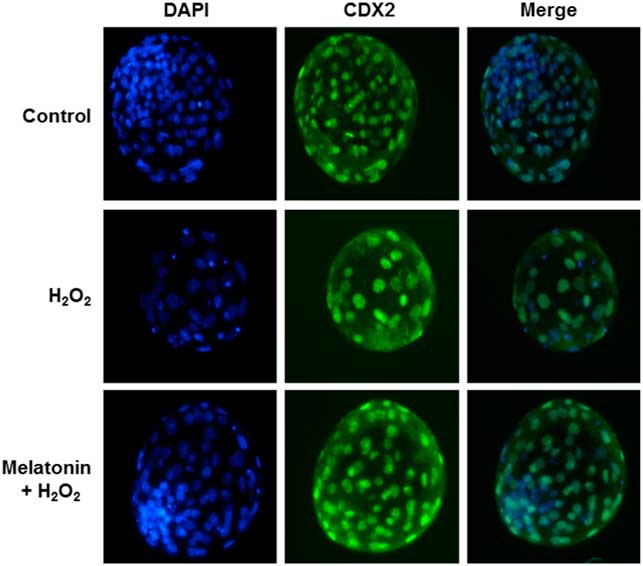
Figure 1 Immunostaining of Caudal Type Homeobox 2 (CDX2). Representative images of yak blastocysts was stained with 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI) and CDX2 (a marker for trophectoderm) after treatment with H2O2 (40 μM) or H2O2 (40 μM) plus melatonin (10 μM) during the zygote stage. Scale bar = 100 μm. n = 29, 34, and 33 in the Control, H2O2 and Melatonin + H2O2 group, respectively.
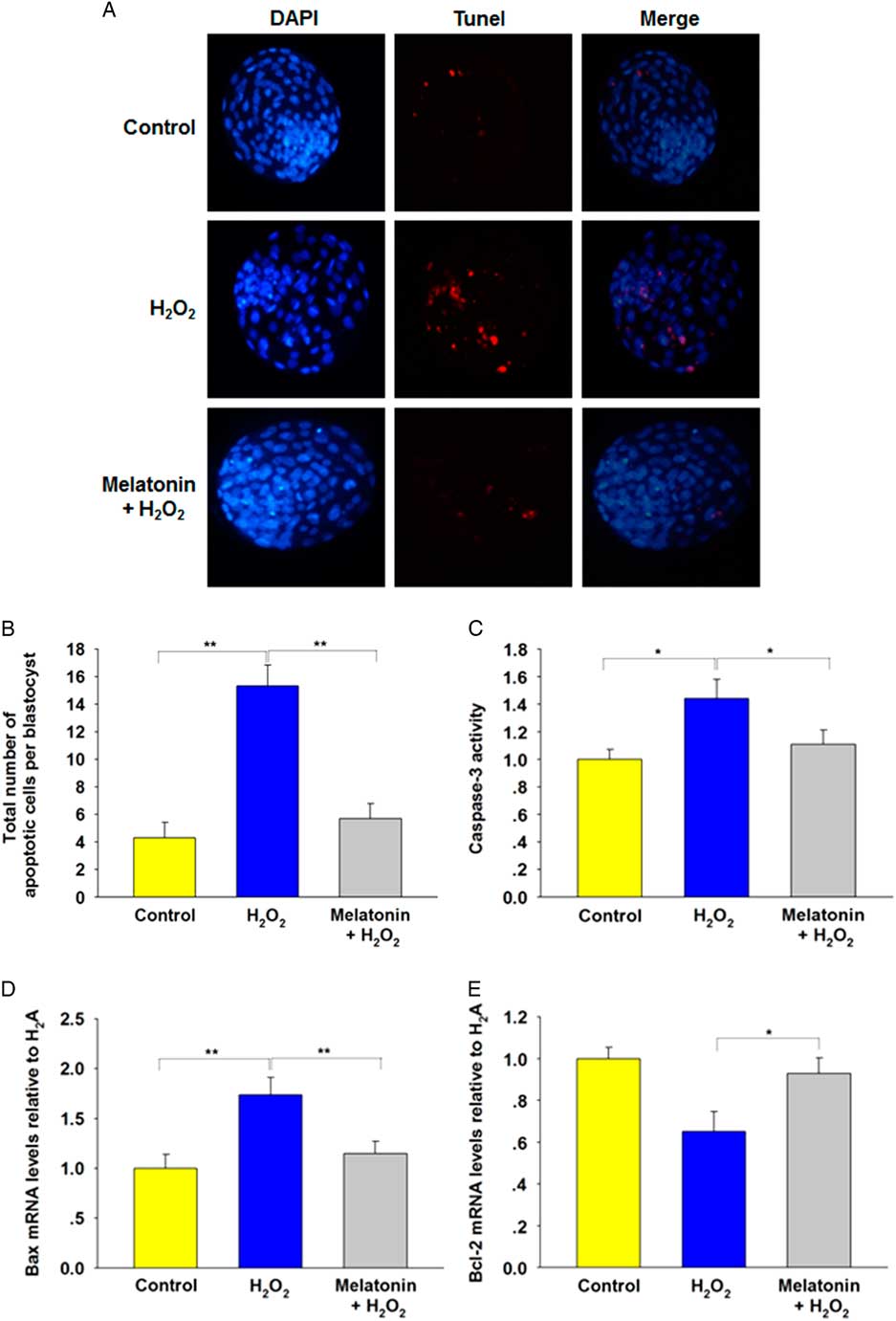
Apoptotic cell death is another criterion for evaluating blastocyst quality. As shown in Fig. 2(A , B), H2O2 treatment induced apoptosis in blastocysts compared with the control group. The number of TUNEL-positive cells decreased significantly in blastocysts derived from groups treated with all concentrations of melatonin. To determine the cause of the reduced apoptosis rate, we analyzed the relative expression levels of apoptosis-related genes. The results showed that H2O2 upregulated caspase-3 activity (Fig. 2C ) and expression of the proapoptotic gene Bax (Fig. 2D ), and downregulated antiapoptotic gene Bcl-2 (Fig. 2E ), which was preserved by melatonin. Collectively, these data suggested a protective effect of melatonin against apoptosis in blastocysts.
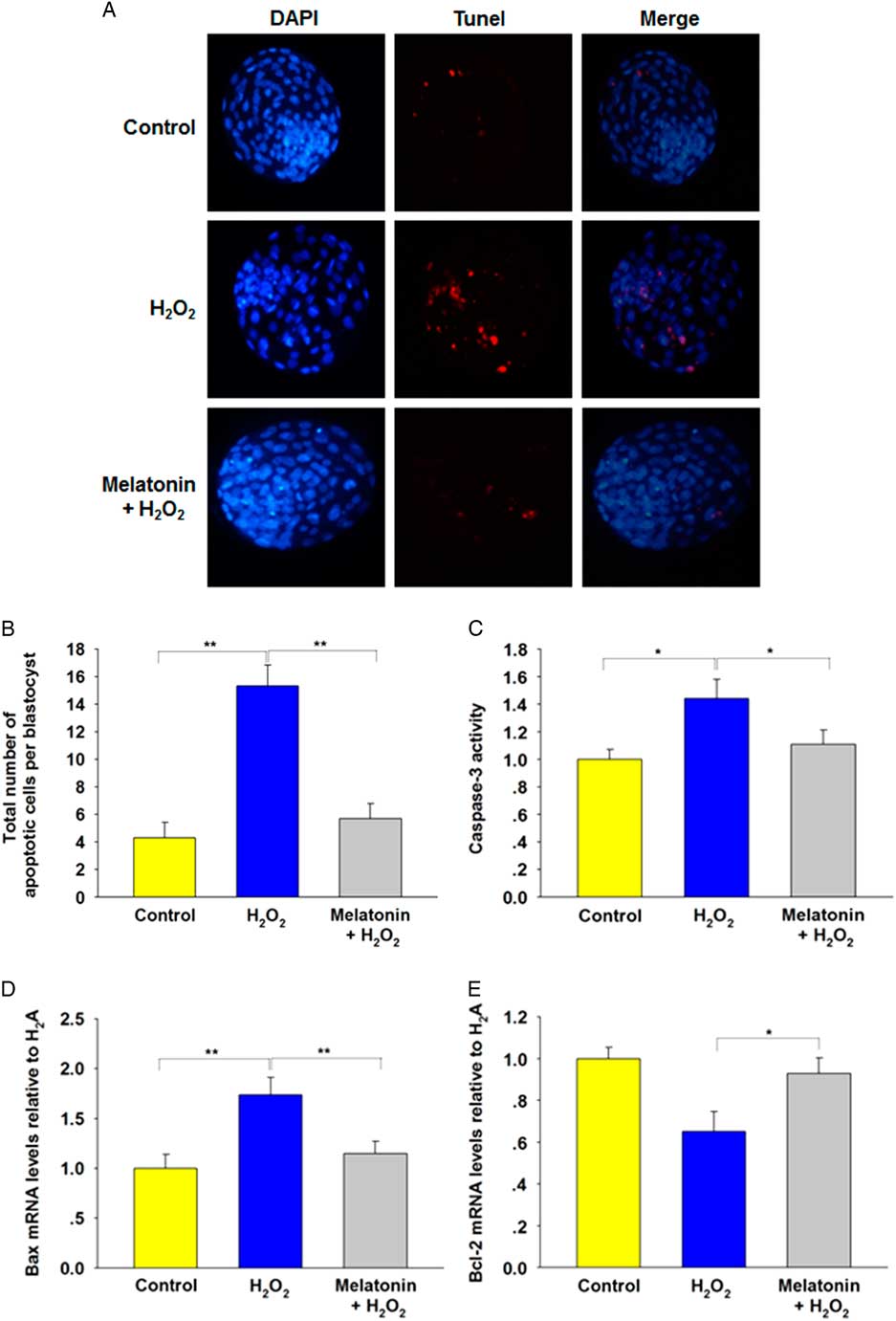
Figure 2 Incidence of apoptosis in blastocysts. The treatment concentrations of H2O2 and melatonin during zygote stage were 40 μM and 10 μM respectively. (A) TUNEL assay of blastocysts (red). Each sample was counterstained with DAPI to visualize DNA (blue). Scale bar = 100 μm. n = 34, 31, and 36 in the Control, H2O2 and Melatonin + H2O2 group, respectively. (B) Total number of apoptotic cells in each blastocyst. Data are presented as mean ± standard deviation (SD). (C) Caspase-3 activity. (D) Relative expression levels of Bax. (E) Relative expression levels of Bcl-2. *P < 0.05, **P < 0.01.
Melatonin reduced H2O2-induced intracellular ROS levels in zygotes
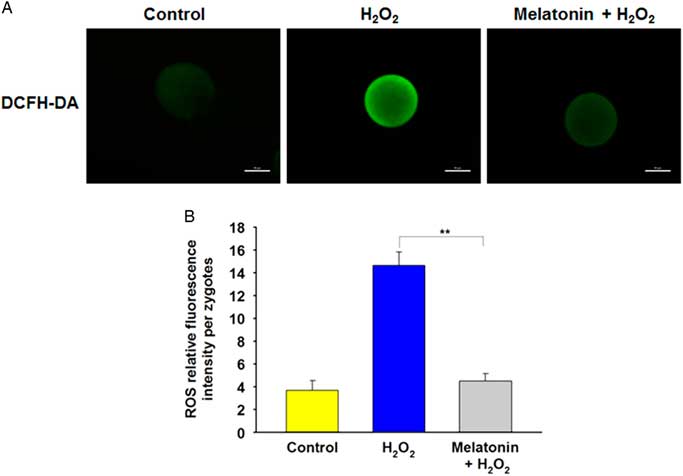
To determine whether melatonin improved resistance to oxidative stress through regulation of ROS levels, the DCFH fluorescent reaction was performed to evaluate ROS production in zygotes. Compared with the control group, the DCFH fluorescence intensity was much higher in the H2O2-treated group, whereas it was markedly weakened in the presence of melatonin (Fig. 3A , B), indicating that melatonin can prevented the increase in ROS induced by H2O2.
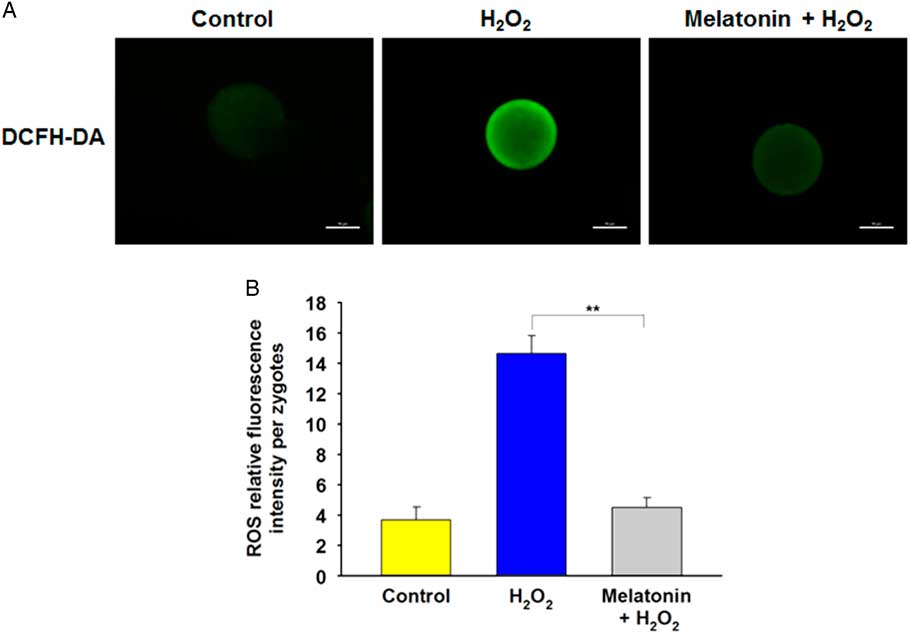
Figure 3 Melatonin decreases H2O2-induced intracellular ROS level in yak zygotes. (A) Representative photomicrographs of yak zygotes from H2O2 (40 μM), or H2O2 (40 μM) plus melatonin (10 μM) group stained with DCHFDA to detect ROS levels. (B) Quantification of relative fluorescence intensity of ROS per zygote. Scale bar = 100 μm. Data are presented as the mean ± standard deviation (SD) from three independent experiments. n = 27, 31, and 28 in the Control, H2O2 and Melatonin + H2O2 group, respectively.
Melatonin prevented mitochondrial dysfunction induced by H2O2 in zygotes
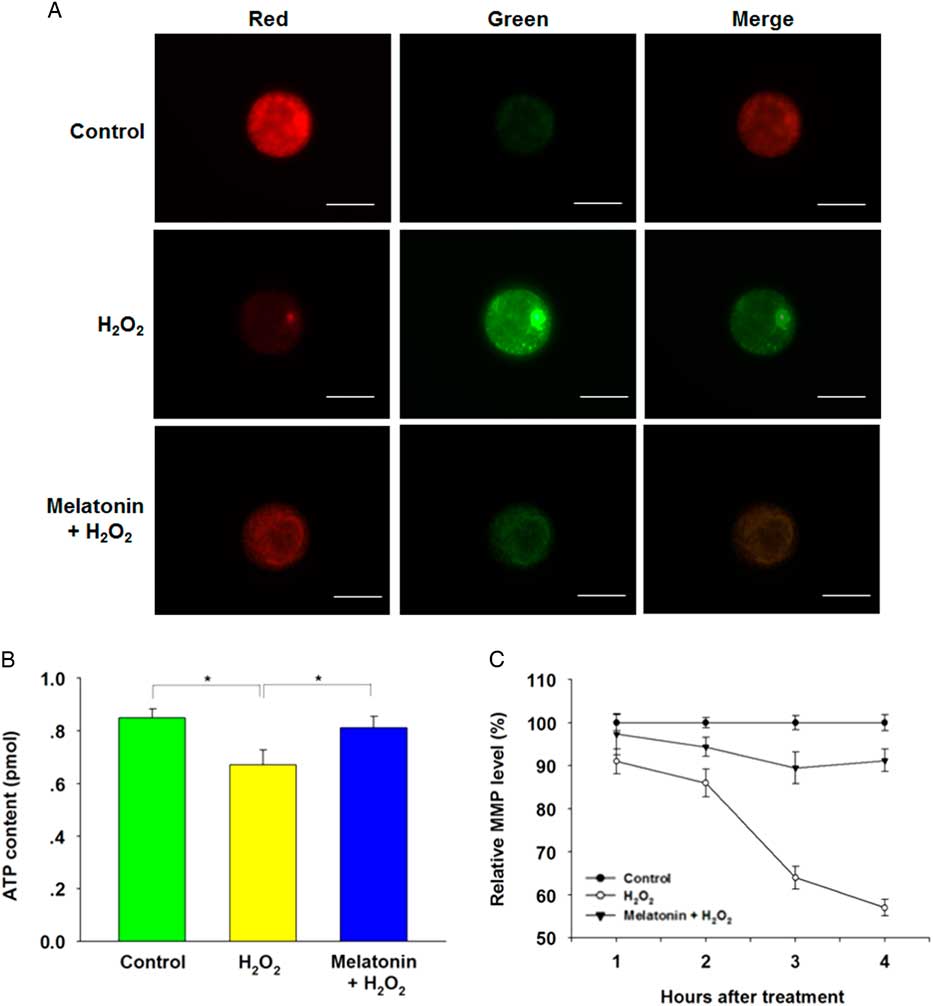
Mitochondria are the major organelles that produce ROS, and mitochondrial dysfunction compromises embryo development. Therefore, we further investigated the ATP content and MMP in zygotes after H2O2 exposure. Melatonin treatment significantly recovered the decreased ATP content induced by H2O2 (Fig. 4B ). Moreover, the MMP depolarized rapidly in zygotes following treatment with H2O2, as shown by enhanced green fluorescence and attenuated red fluorescence compared with the control group. However, exposure to melatonin significantly recovered MMP level (Fig. 4A , C), suggesting that melatonin may maintain mitochondrial function in zygotes following oxidative stress.
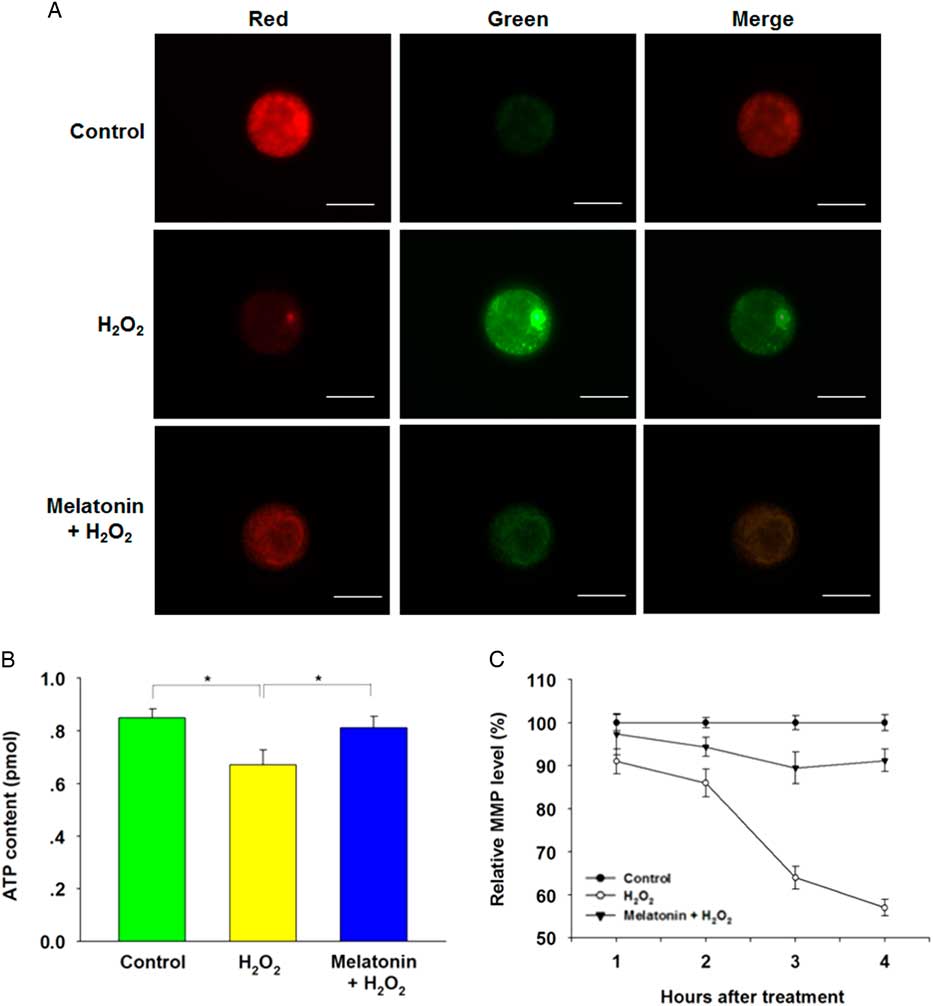
Figure 4 Melatonin prevented H2O2-induced mitochondrial dysfunction in yak zygotes. The treatment concentration of H2O2 and melatonin were 40 μM and 10 μM respectively. (A) Representative images of inner mitochondrial membrane potential (MMP) in yak zygotes. Zygotes were stained with JC-1. Red fluorescence represented J-aggregates (high polarized mitochondria) and green fluorescence represented monomer form of JC-1 (low polarized mitochondria). (B) ATP content in yak zygotes after treated with H2O2, or H2O2 plus melatonin. (C) The ratios of intensity of red /green fluorescence (MMP) were analyzed from the images. The average of MMP from zygotes in the control group at each time point was set at 100%. Scale bar = 100 μm. Data are presented as mean ± standard deviation (SD) from three independent experiments. n = 19 for each group.
Melatonin protected preimplantation embryos from oxidative damage by preserving antioxidative enzymes
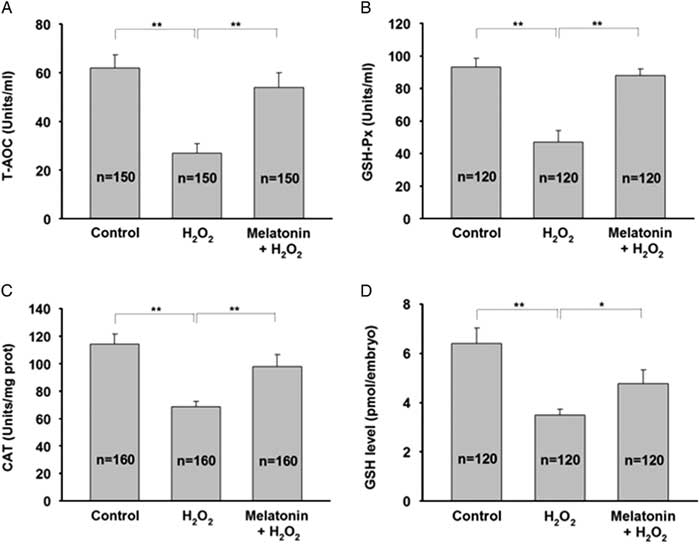
H2O2 is a free radical generator and can lead to a decrease in antioxidant capacity, therefore we first determined the T-AOC in zygotes treated with H2O2 in the presence or absence of melatonin. H2O2 treatment decreased T-AOC and melatonin efficiently prevented this effect (Fig. 5A ). In order to further investigate the effect of melatonin on antioxidant cellular defences, we evaluated the enzymatic and non-enzymatic antioxidants. Figure 5B , C shows that melatonin exposure significantly restored the decreased activity of GSH-Px and CAT induced by H2O2. In addition, H2O2 treatment resulted in a significant decrease in the level of GSH and the main non-enzymatic antioxidants in embryos, whereas melatonin treatment completely restored GSH level (Fig. 5D ). These data suggested that melatonin may protect preimplantation embryos from oxidative injury by maintaining antioxidative enzymes.
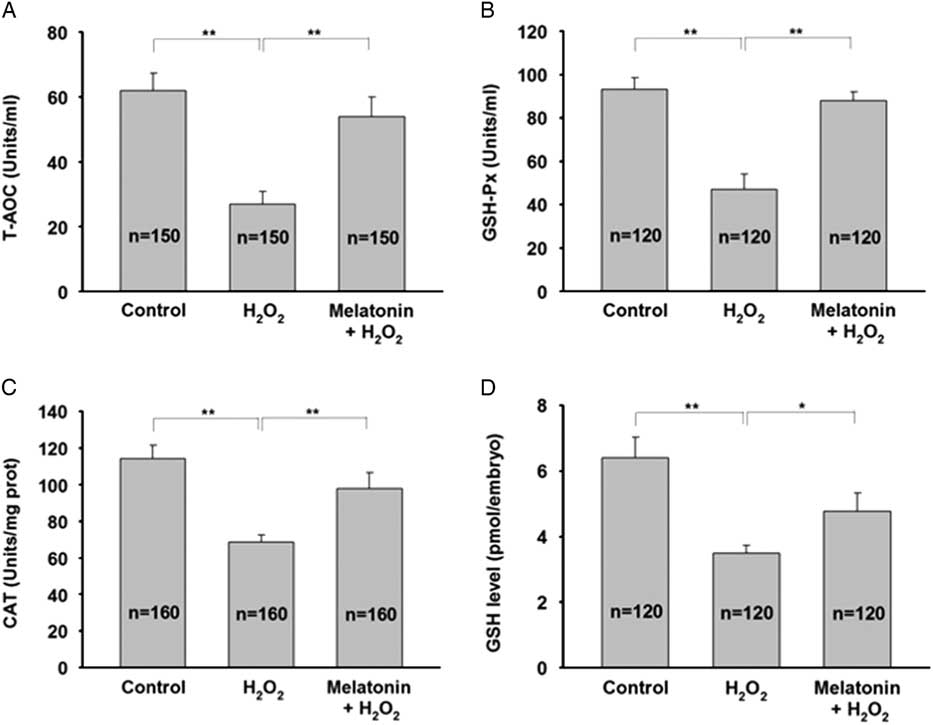
Figure 5 Melatonin attenuates H2O2-induced oxidative injury in yak zygotes by sparing antioxidative enzymes. Zygotes were pretreated with 10 μM melatonin for 1 h followed by addition of 40 μM H2O2 for 15 min, and then cultured in the presence of melatonin until day 7. (A) T-AOC activity, (B) GSH-Px activity, (C) CAT activity, and (D) GSH level were measured. Data are presented as mean ± standard deviation (SD) from three independent experiments. n indicates the total number of treated zygotes. *P < 0.05, **P < 0.01.
Discussion
During the development of preimplantation embryos in vitro, oxidative stress has a deleterious effect on fertilization and embryo quality (Kim et al., Reference Kim, Kim, Lee, Lee, Jin and Lee2017). The positive effects of antioxidants have been reported in relation to several reproductive aspects (Barbato et al., Reference Barbato, Talevi, Braun, Merolla, Sudhakaran, Longobardi and Gualtieri2017; Do et al., Reference Do, Kim, Park, Yoon, Park, Yang, Jung, Kwon, Kang, Song, Kim, Chang and Koo2017). The current study confirmed that the addition of melatonin significantly improved the development of yak zygotes to the blastocyst stage in the presence of H2O2 and decreased apoptosis in blastocysts, which suggested that melatonin had a protective effect against oxidative stress in yak preimplantation embryos.
In physiological conditions, cells can maintain their dynamic balance between ROS production and elimination. Low concentrations of ROS have been shown to reduce DNA fragmentation and improve the developmental potential of embryos (Takahashi et al., Reference Takahashi, Inaba, Somfai, Kaneda, Geshi, Nagai and Manabe2013). However, an imbalance in these processes results in oxidative stress, and is associated with defective embryo development (Cheng et al., Reference Cheng, Zhang, Chen, Jin, Cui, Butte, Chan and Hirschi2011). The antioxidative capacity of melatonin has been shown in several other cell types, but this study is the first to report the effects of melatonin on the development of yak preimplantation embryos under oxidative stress (Goswami and Haldar, Reference Goswami and Haldar2014; Czechowska et al., Reference Czechowska, Celinski, Korolczuk, Wojcicka, Dudka, Bojarska and Reiter2015; Zhou et al., Reference Zhou, Zhao, An, Zhang, Jiang and Yang2015). Melatonin exposure greatly improved the quality of H2O2-treated embryos, indicated by reduced oxidative damage, reduced apoptosis and increased blastocyst formation. The beneficial effects of melatonin may be attributed to its amphiphilic nature, thereby enabling it to penetrate all morphophysiological barriers and enter all subcellular compartments to scavenge intracellular ROS.
In addition, the current study demonstrated that not only the production rate but also embryo quality was increased by melatonin treatment. A high cell number/blastocyst ratio indicated that the embryo efficiently proceeded through mitosis and was likely to undergo to easy implantation resulting in pregnancy. The blastocysts produced following melatonin treatments had higher mean cell number/blastocysts than those in the H2O2-treated group. The ratio of ICM/TCN is another criterion for evaluating blastocyst quality (Moreno et al., Reference Moreno, Neira, Dubreil, Liegeois, Destrumelle, Michaud, Thorin, Briand-Amirat, Bencharif and Tainturier2015). The present results showed that H2O2 treatment decreased ICM cells and the ratio of ICM/TCN compared with the control group. Under oxidative stress, melatonin minimized the negative influence of H2O2 on embryo quality. Notably, we found that pretreatment with melatonin (100 µM) exhibits adverse effects on early yak embryo development in vitro, as shown by embryo development arrest. This is not surprising because relatively high concentrations of melatonin has the anti-proliferative effect in human leukemia, breast cancer and colon cancer cells. In fact, a current study also demonstrated that relatively low concentrations of melatonin exhibit beneficial effects on bovine oocyte maturation in vitro, while melatonin retards bovine oocyte maturation and embryo development at high concentrations (Tian et al., Reference Tian, Wang, He, Zhang, Tan, Reiter, Xu, Ji and Liu2014).
In addition to scavenging ROS, another powerful antioxidant property of melatonin involves maintaining stable mitochondrial function (Areti et al., Reference Areti, Komirishetty, Akuthota, Malik and Kumar2017; Maarman et al., Reference Maarman, Andrew, Blackhurst and Ojuka2017; Sharafati-Chaleshtori et al., Reference Sharafati-Chaleshtori, Shirzad, Rafieian-Kopaei and Soltani2017). During early embryo development, mitochondrial dysfunction may contribute to both cell cycle arrest and apoptosis (Chowdhury et al., Reference Chowdhury, Ray, Chatterjee and Roy2017; Liu et al., Reference Liu, Zhang, Chen, Yu, Li, Dan, Zhao and Zhou2017). As important parameters of mitochondrial function, MMP and ATP content have been used as indicators of cell apoptosis (Abe et al., Reference Abe, Sakairi, Beeson and Kopp2013). In the present study, we found that administration of H2O2 led to dissipation of MMP and ATP content, and this effect was blocked by melatonin, which was consistent with the results of a previous study. Moreover, the results of the TUNEL assays supported our findings on the mRNA transcripts for genes related to apoptosis. Melatonin treatment was found to strongly downregulate caspase-3 activity and Bax expression. The expression of these genes was also lower in the melatonin treatment group compared with the H2O2 control. Bcl-2 is an important member of the anti-apoptotic family and plays a critical role in the regulation of apoptosis (Pan et al., Reference Pan, Cui, Baloch, Fan, He, Li, Zheng, Zhang, Lv and Yu2015). In the current study, we observed that the Bcl-2 expression level in granulosa cells under thermal stress was significantly upregulated by melatonin treatment. These results indicated that melatonin was probably preventing apoptosis not only due to its antioxidant effects but also its ability to regulate gene expression.
In embryogenesis, GSH-Px, an enzymatic antioxidant, is highly expressed in most cells and tissues and protects the embryos from oxidative stress (Huo et al., Reference Huo, Liu, Gao, Xu, Zhu and Cao2017; Hamdy et al., Reference Hamdy, Shabaan, Abdel Latif, Abdel-Aziz and Amin2017; Delwing-de Lima et al ., Reference Delwing-de Lima, Sasso, Dalmedico, Delwing-Dal Magro, Pereira and Wyse2017). In addition to GSH-Px, CAT is another enzymatic antioxidant expressed in embryos and its vital role as an antioxidant has been demonstrated using knock-out and transgenic approaches in mice (Rahman et al., Reference Rahman, Halade, Bhattacharya and Fernandes2009; Avdesh et al., Reference Avdesh, Wong, Martins and Martin-Iverson2011). In addition to enzymatic defences, GSH has a non-enzymatic antioxidative defence and detoxifies H2O2 via the action of GSH-Px, and plays a prominent role in maintaining the redox status in embryos (Takahashi, Reference Takahashi2012). Our results showed that melatonin not only strongly restored T-AOC and GSH-Px activity to levels similar to those in the controls under oxidative stress, but also partially recovered CAT activity. Moreover, we found that melatonin maintained intracellular GSH at high levels, which was consistent with the results of a previous study. Collectively, these data showed that melatonin may protect yak embryos against ROS by sparing antioxidative enzymes.
In summary, our results showed that under oxidative stress, yak zygotes exposed to melatonin maintained stable mitochondrial function, increased T-AOC, GSH-Px and CAT activities and decreased ROS levels, thereby improving embryo quality in vitro. These results provided a potential method for preventing oxidative damage in yak preimplantation embryos.
In conclusion, the results obtained from the studies provided new information regarding the mechanisms by which melatonin protects the development of yak preimplantation embryos from oxidative stress, which provides an important reference for in vitro embryo production of yak.
Financial support
This work was funded by the State Key Program (No. 31530075) of National Natural Science Foundation of China and China Postdoctoral Science Foundation Grant (No. 2016M590978).
Conflicts of interest
The authors indicate no competing financial interest.
Ethical standards
All animal experiments and treatments were approved and supervised by the Animal Care Commission of the College of Veterinary Medicine, Northwest A&F University. Ovaries of slaughtered cattle were obtained from Datong Abattoir, a local slaughterhouse located in Qinghai, China.