INTRODUCTION
Species interactions have been widely studied across terrestrial and marine habitats as they play important roles in structuring communities, with the outcomes of these interactions having implications for patterns of biodiversity and ecosystem functioning (Reed & Foster, Reference Reed and Foster1984; Bertness et al., Reference Bertness, Leonard, Levine, Schmidt and Ingraham1999; O'Connor et al., Reference O'Connor, Crowe and McGrath2006; Bonanomi et al., Reference Bonanomi, Incerti and Mazzoleni2011). Facilitation (sensu Bertness & Callaway, Reference Bertness and Callaway1994) occurs when neighbouring organisms modify physical or biotic conditions, which leads to positive effects for one or both of the organisms; these interactions can influence diversity, community structure and productivity, and have the potential to result in cascading effects through the community (Bruno & Bertness, Reference Bruno, Bertness, Bertness, Gaine and Hay2001; Stachowicz, Reference Stachowicz2001; Bruno et al., Reference Bruno, Stachowicz and Bertness2003; Gouhier et al., Reference Gouhier, Menge and Hacker2011). Mechanisms of facilitation operating in the marine environment include provision of secondary substrata, reduced or enhanced predation or herbivory, reduced competition, and the amelioration of physical stress (see Bulleri, Reference Bulleri2009 for review). Bulleri (Reference Bulleri2009) suggested that amelioration of physical stress appears to be the most common mechanism of facilitation in intertidal habitats, whilst reduced consumer pressure (associational defence) is the most important facilitation mechanism in subtidal systems.
Subtidal rocky reefs usually support complex and highly diverse communities as a result of habitat diversity and complexity (Sebens, Reference Sebens, Moore and Seed1985; Dayton, Reference Dayton1985a; Schiel & Hickford, Reference Schiel and Hickford2001). Seaweeds are one of the most important components of shallow rocky reef habitats, because of their productivity (Mann, Reference Mann1973) and their ability to modify the physical environment, which can facilitate or exclude species from the understorey (Bertness et al., Reference Bertness, Leonard, Levine, Schmidt and Ingraham1999). Sponges are also an important group of organisms in rocky reef communities throughout the world (Dayton et al., Reference Dayton, Robilliard, Paine and Dayton1974; Ayling, Reference Ayling1983; Bell & Barnes, Reference Bell and Barnes2000a; Bell, Reference Bell2008), as they fulfil many important roles including filtering nutrients and providing food and habitat for other species (Bell, Reference Bell2008). Although sponges are common in many habitats, they are often less abundant in high-light habitats, where seaweeds are abundant due to their requirement for light. This situation has led to some debate as to the degree to which sponges and algae are competing for space when they co-occur (e.g. Knott et al., Reference Knott, Underwood, Chapman and Glasby2004; Preciado & Maldonado, Reference Preciado and Maldonado2005; Cárdenas et al., Reference Cárdenas, Davy and Bell2012).
The generally higher abundance of seaweeds compared with sponges in high-light environments has led to the hypothesis that seaweeds constitute important spatial competitors of sponges, and are able to outcompete sponges in high-light environments (Kennelly & Underwood, Reference Kennelly and Underwood1993; Ginn et al., Reference Ginn, Logan and Thomas2000; Bell & Barnes, Reference Bell and Barnes2000b; Bell, Reference Bell2002, Reference Bell2007). Previous research from rocky reefs in New Zealand has shown that a combination of inclination and abundance of canopy and turf-forming algae explained a large proportion of the variability in the distribution and abundance of sponges (Cárdenas et al., Reference Cárdenas, Davy and Bell2012). This study found that although most sponge species were negatively correlated with algal abundance, some species were positively correlated with the abundance of canopy-forming algae. This positive correlation between some sponges and canopy-forming algae suggests that some species might actually benefit from the presence of canopy-forming algae (Wright et al., Reference Wright, Benkendorff and Davis1997; Ávila et al., Reference Ávila, Blancas-Gallangos, Riosmena-Rodríguez and Paul-Chávez2010; Flukes et al., Reference Flukes, Johnson and Wright2014).
Although many studies have assessed the effect of algal canopies on understorey algae and sessile assemblages (e.g. Eckman & Duggins, Reference Eckman and Duggins1991; Benedetti-Cecchi, Reference Benedetti-Cecchi2001; Bulleri et al., Reference Bulleri, Benedetti-Cecchi, Acunto, Cinelli and Hawkins2002; Connell, Reference Connell2003a, Reference Connellb; Edgar et al., Reference Edgar, Barrett, Morton and Samson2004), few have specifically evaluated the effect of canopy-forming algae on sponge assemblages. Algal canopies have the potential to affect understorey sponge assemblages by modifying the local environment. For example, algal canopies may result in shading (Toohey & Kendrick, Reference Toohey and Kendrick2008), which may benefit sponges containing photosymbionts, which are sensitive to elevated light levels (Wulff, Reference Wulff2012; Flukes et al., Reference Flukes, Johnson and Wright2014), reduced sedimentation and decreased water movement (Duggins et al., Reference Duggins, Eckman and Sewell1990; Connell, Reference Connell2003b; Russell, Reference Russell2007), enhanced nutrient supply (Duggins & Eckman, Reference Duggins and Eckman1997; Morrow & Carpenter, Reference Morrow and Carpenter2008), and they also provide shelter and secondary settlement space for some species (Smith, Reference Smith1996; Wright et al., Reference Wright, Benkendorff and Davis1997; Ávila et al., Reference Ávila, Blancas-Gallangos, Riosmena-Rodríguez and Paul-Chávez2010). Furthermore, canopy-forming algae may also affect sponges and other subcanopy species as a result of physical abrasion by fronds that can inhibit the recruitment of algae and invertebrates (Velimirov & Griffiths, Reference Velimirov and Griffiths1979; Jenkins et al., Reference Jenkins, Norton and Hawkins1999; Leonard, Reference Leonard1999; Fowler-Walker et al., Reference Fowler-Walker, Gillanders, Connell and Irving2005). However, even though the combination of decreased light and reduced sedimentation resulting from the presence of the algal canopy can facilitate recruitment for some species, physical abrasion seems to overpower any positive effect in structuring invertebrate and turf-algal assemblages (Connell, Reference Connell2003b; Russell, Reference Russell2007).
The habitat-forming kelp Ecklonia radiata (C. Agardh) dominates large areas on shallow-water rocky reefs of New Zealand and temperate Australia, providing food and shelter for many species (Choat & Schiel, Reference Choat and Schiel1982). Ecklonia radiata forests alter the local physical environment (including light, sediment and wave exposure), influencing the structure of assemblages through canopy-understorey interactions (Wernberg et al., Reference Wernberg, Kendrick and Toohey2005). Factors such as abundance, density and morphology of Ecklonia are critical in creating small-scale variation in diversity and structure of understorey assemblages (Schiel, Reference Schiel1988; Fowler-Walker et al., Reference Fowler-Walker, Gillanders, Connell and Irving2005; Smale et al., Reference Smale, Kendrick and Wernberg2011). Earlier studies have reported conflicting effects of canopy removal on sponges, although only a few individual sponge species have been considered and these studies have mostly focused on negative impacts of the algal canopy (but see Wright et al., Reference Wright, Benkendorff and Davis1997). For example, Kennelly (Reference Kennelly1987b; Reference Kennelly1989) found no relationship between shade or scour produced by E. radiata on the growth and abundance of the sponge Myxilla sp. in Australia, while Kennelly & Underwood (Reference Kennelly and Underwood1993) suggested that turf algae prevented settlement and affected the abundance of some sponge species after E. radiata was experimentally removed in kelp forests of New South Wales, Australia. More recently, Fowler-Walker et al. (Reference Fowler-Walker, Gillanders, Connell and Irving2005) found that sponge abundance was negatively correlated with E. radiata morphology on rocky reefs of temperate Australia, as plants with short stipes and long laminae caused more abrasion on the understorey organisms. In contrast, Wright et al. (Reference Wright, Benkendorff and Davis1997) reported a complete shift in the sponge assemblage inside vs outside the Ecklonia canopy, where four Chrondropsis spp. dominated the substratum beneath the canopy. Considering these major differences between sponge assemblages inside vs outside Ecklonia canopies reported by Wright et al. (Reference Wright, Benkendorff and Davis1997), here we explore the potential for some sponge species to benefit from the presence of the dominant habitat-forming kelp E. radiata through facilitation as a result of habitat modification. We predict that the removal of the E. radiata canopy would affect the structure of subcanopy assemblages, and hypothesized that if the canopy facilitates sponge assemblages there would be changes in assemblage structure and a reduction in sponge species richness and abundance when the canopy is removed. Furthermore, we investigated the biological and physical variables that best explained variability in sponge assemblages after canopy removal.
MATERIALS AND METHODS
Study sites
The Wellington south coast, which is located on the southern tip of the North Island of New Zealand, is an energetic environment that is subjected to regular southerly swells that move onto the Wellington shelf for more than 80% of the time (Carter & Lewis, Reference Carter and Lewis1995). The subtidal algal assemblage on the Wellington south coast is dominated by Ecklonia radiata, and a mixture of Carpophyllum spp., Lessonia variegata and Landsburgia quercifolia (Shears & Babcock, Reference Shears and Babcock2007). The understorey is dominated by crustose coralline algae and the overall abundance of sessile invertebrates has been reported to be low (Shears & Babcock, Reference Shears and Babcock2007). However, a recent study has described a diverse sponge assemblage in the area (Berman & Bell, Reference Berman and Bell2010), with the highest abundance occurring on vertical walls, but with some species, such as Haliclona sp., Polymastia spp. and Cliona sp., being positively correlated with the presence of the algal canopy (Cárdenas et al., Reference Cárdenas, Davy and Bell2012).
In order to test the effect of removal of the dominant laminarian E. radiata (hereafter Ecklonia) on subcanopy assemblages, specifically on sponge assemblages, we conducted algal clearances at two sites on the south coast of Wellington – Palmer Head (41°20′46″S 174°49′19″E) and Breaker Bay (41°19′58″S 174°49′53″E) – which are approximately 2 km apart. A third site (Moa Point) was part of the original experimental design, however it was not possible to survey experimental plots after T1 (second sampling interval) due to the rapid spread of Caulerpa sp. in cleared plots, which produced substantial changes in the structure of the subcanopy assemblages within 6 weeks following canopy removal. Both sites are characterized by the presence of steep rock walls, which form narrow channels (~2.5–5 m wide). At each site, we haphazardly selected six walls of similar size, aspect, slope, inclination (~45°–100°) and direction (in relation to light exposure and swell-action). The walls were separated by at least 20 m, and located at depths of 6–9 m. Walls had a high cover of Ecklonia, with adult plant densities ranging between 8–14 plants m−2 (mean = 8.1, SD = 3.1). The mean total plant length was 56.7 cm ± 7.5 SD. Ecklonia plants had short stipes with a mean length of 12.1 ± 3.6 cm.
Experimental design and data analysis
In July 2010 (winter), we cleared 3 × 3 m areas (hereafter plots) of Ecklonia on five randomly selected walls and a sixth wall was left undisturbed and acted as a control. The experimental design was unbalanced as we only had one control wall at each site due to the lack of walls and also as a result of problems produced by urchins grazing on others. The size of the plots ensured the exclusion of any effect of the surrounding canopy (i.e. edge effects) on the cleared plots (Kennelly, Reference Kennelly1987a, Reference Kennellyb; Kennelly, Reference Kennelly1989). Plants were removed from the holdfast either by hand or using a knife when necessary to simulate the effect of storms, which in most cases, remove the entire plant from the substratum. Holdfasts were carefully removed to avoid damage to neighbouring sponges. An initial survey of subcanopy assemblages was conducted before the algal canopy was removed, where quadrats were randomly placed avoiding holdfasts. Subsequent surveys were conducted approximately every 6–10 weeks until April 2012 (N = 10, experimental duration was 85 weeks). Control and removal plots were photographed at each survey interval and Ecklonia recruits were carefully removed. On each survey date, the two sites were sampled during the same day. Sampling dates were: T0 (0 weeks) = 15 July 2010, T1 (6 weeks) = 25 August 2010, T2 (19 weeks) = 27 October 2010, T3 (27 weeks) = 17 January 2011, T4 (34 weeks) = 18 March 2011, T5 (42 weeks) = 13 May 2011, T6 (54 weeks) = 5 August 2011, T7 (65 weeks) = 12 October 2011, T8 (72 weeks) = 2 December 2011, T9 (85 weeks) = 2 April 2012.
Five 25 × 25 cm quadrats were randomly placed within the central (2 × 2) portion of each plot (to mitigate edge effects only the centre of the 3 × 3 m area was used) and photographed. Photo-quadrats were analysed with CPCe v3.5 (Coral Point Count with Excel extensions) (Kohler & Gill, Reference Kohler and Gill2006) by superimposing a grid of 100 points onto each image, and determining the percentage cover of sessile organisms, macroalgae, bare rock and settled sediment (estimated as the area of the quadrat covered in sediment in each picture). During the initial survey, species forming a canopy were moved aside to allow the substrate below them to be photographed. All taxa in photoquadrats were identified to the lowest taxonomic level possible. For analysis, species of algae that were often not identifiable to species level from the photographs were grouped under broad categories of algae. Categories used in the analysis were crustose coralline algae (CCA), erect coralline algae (ECA), red algae and brown algae.
Ambient irradiance was measured with a Diving-PAM (Walz GmbH, Germany) within the canopy and outside the canopy (N = 16; four readings on 3 consecutive days). The mean ambient irradiance underneath the canopy was 5.75 µmol quanta m−2 s−1 (±2.05 SD) whereas the mean value in absence of canopy was 91.66 ± 20.5 µmol quanta m−2 s−1.
Effect of canopy removal on the structure of subcanopy assemblages
Non-metric multidimensional scaling (nMDS) was used to show relative changes over time in cleared and control plots. Data were averaged to obtain a centroid for each sampling time on each plot, and consecutive sampling times are linked by lines representing the trajectory of assemblage change at an individual plot through time (Clarke et al., Reference Clarke, Somerfield, Airoldi and Warwick2006). The same procedure was used to show relative changes on sponge assemblages over time in cleared and control plots.
To test the effect of canopy removal on the richness and abundance of sponges over time, a repeated measures permutational analysis of variance (PERMANOVA) was performed. Tests were based on Bray-Curtis similarity matrices and four root-transformed data, using site (treated separately; two levels, random), treatment (two levels, fixed), and time (10 levels, random) as variables. Statistical differences were tested using 9999 permutations under a reduced model. Pair-wise post-hoc tests were generated using 9999 permutations of raw data. Monte Carlo tests were used when the number of unique permutations was low (Anderson et al., Reference Anderson, Gorley and Clarke2008).
Similarity percentages analysis (SIMPER) (Clarke, Reference Clarke1993) was used to identify taxa contributing most to assemblage dissimilarity between canopy removal and control plots. PERMANOVA tests were performed to test the effect of treatment and time for each dominant taxon (treated as a univariate measure). The percentage cover of organisms was averaged across quadrats for comparisons between treatments. Tests were performed based on Euclidean distance matrices of fourth root-transformed percentage cover data. Statistical differences were further analysed by pair-wise tests based on 9999 permutations of raw data. Monte Carlo tests were used when the number of unique permutations was low (Anderson et al., Reference Anderson, Gorley and Clarke2008). PERMANOVA was chosen for univariate analyses because it does not assume a normal distribution of errors. The same procedure was used to examine the effect of sediment accumulation on benthic assemblages between canopy removal and control plots, as an increase in the cover of settled sediment may affect the composition of understorey and sessile invertebrates (Connell, Reference Connell2003a). All analyses were performed in PRIMER v6 (Clarke & Gorley, Reference Clarke and Gorley2006; Anderson et al., Reference Anderson, Gorley and Clarke2008).
Biotic and abiotic associations of variation in sponge assemblages after canopy removal
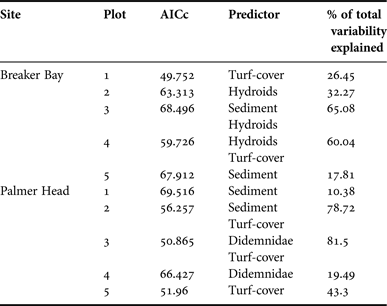
In order to identify factors that best explained changes in sponge abundance after canopy removal, we assessed whether changes in sponge abundance were correlated with the percentage cover of other sessile organisms, increases in turf algae, or with changes in settled sediment, using a permutational distance-based multiple regression technique (DistLM) (McArdle & Anderson, Reference McArdle and Anderson2001). This was performed for each removal plot at each site. DistLM carries out a partitioning of variation in a data set described by a resemblance matrix according to a multiple regression model. This technique makes no prior assumptions about the nature of the response variable distribution and therefore normality does not have to be satisfied (Anderson et al., Reference Anderson, Gorley and Clarke2008). DistLM analyses model the relationship between abundance data (sponge abundance) and one or more variables (predictor variables). Predictor variables tested were: (1) incremental changes in the abundance of turf algae per sampling period. Turf algae included all algal taxa except coralline algae; (2) percentage cover of settled sediment; and (3) cover of other dominant benthic groups (based on SIMPER). Models incorporating all possible combinations of predictor variables were generated using the Best procedure within DistLM. All tests were performed on Bray–Curtis dissimilarity matrices using 9999 permutations. We used modified Akaike's Information Criterion (AICc) to identify the model that best explained the maximum amount of variation in the sponge data (Burnham & Anderson, Reference Burnham and Anderson2002; Hobbs & Hilborn, Reference Hobbs and Hilborn2006). AICc is recommended for analyses with small sample size. AICc values indicate the goodness of a model fit to the data, penalized for increasing the number of factors. Models with the lowest AICc are considered the most parsimonious (Symonds & Moussalli, Reference Symonds and Moussalli2011).
RESULTS
Effect of canopy removal on the structure of sponge assemblages
At both Breaker Bay and Palmer Head, ordination plots showed a divergence in sponge assemblages between control and removal plots following canopy removal (Supplementary Figures S1 and S2). This means that assemblages in control and removal plots became less similar over time after canopy removal.
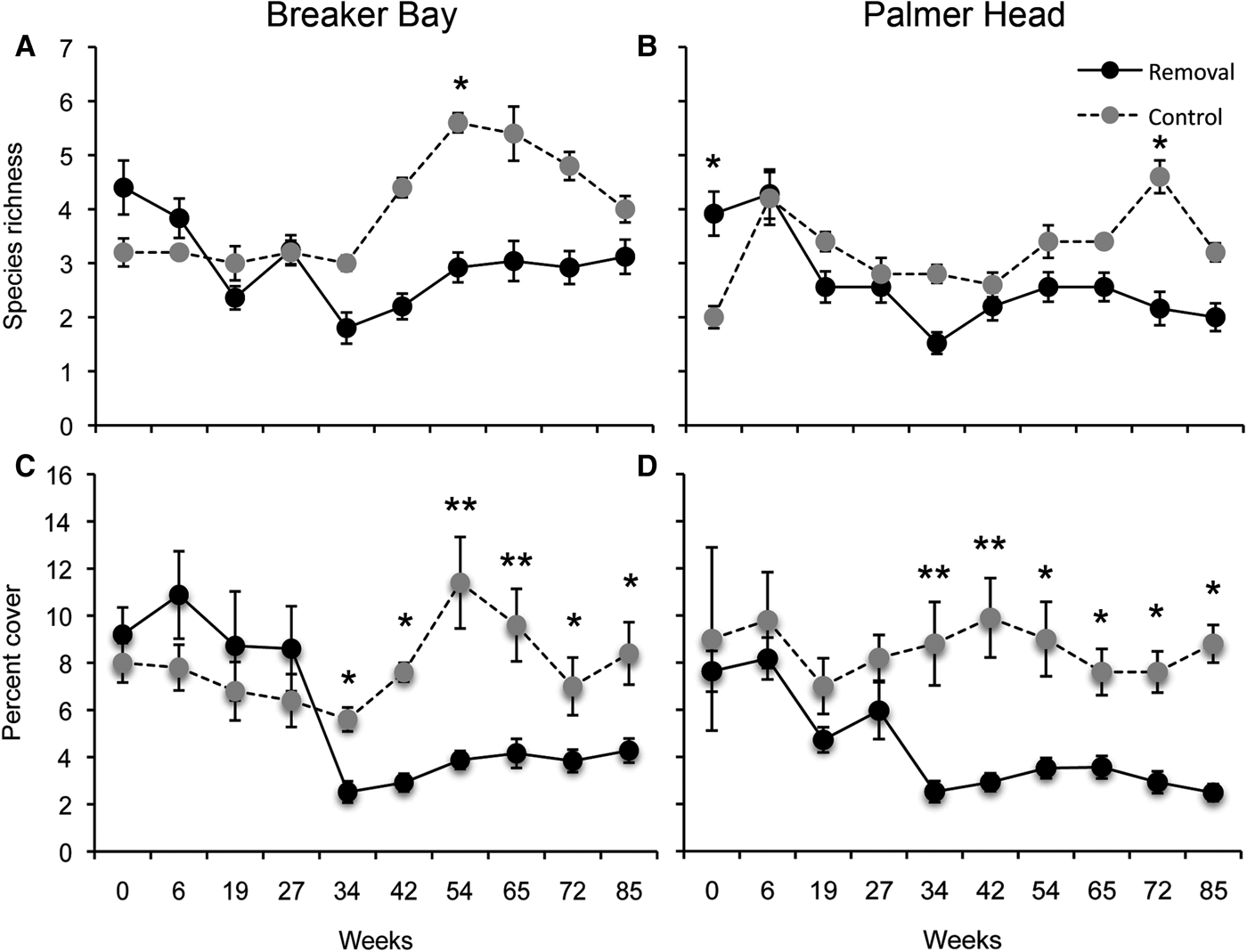
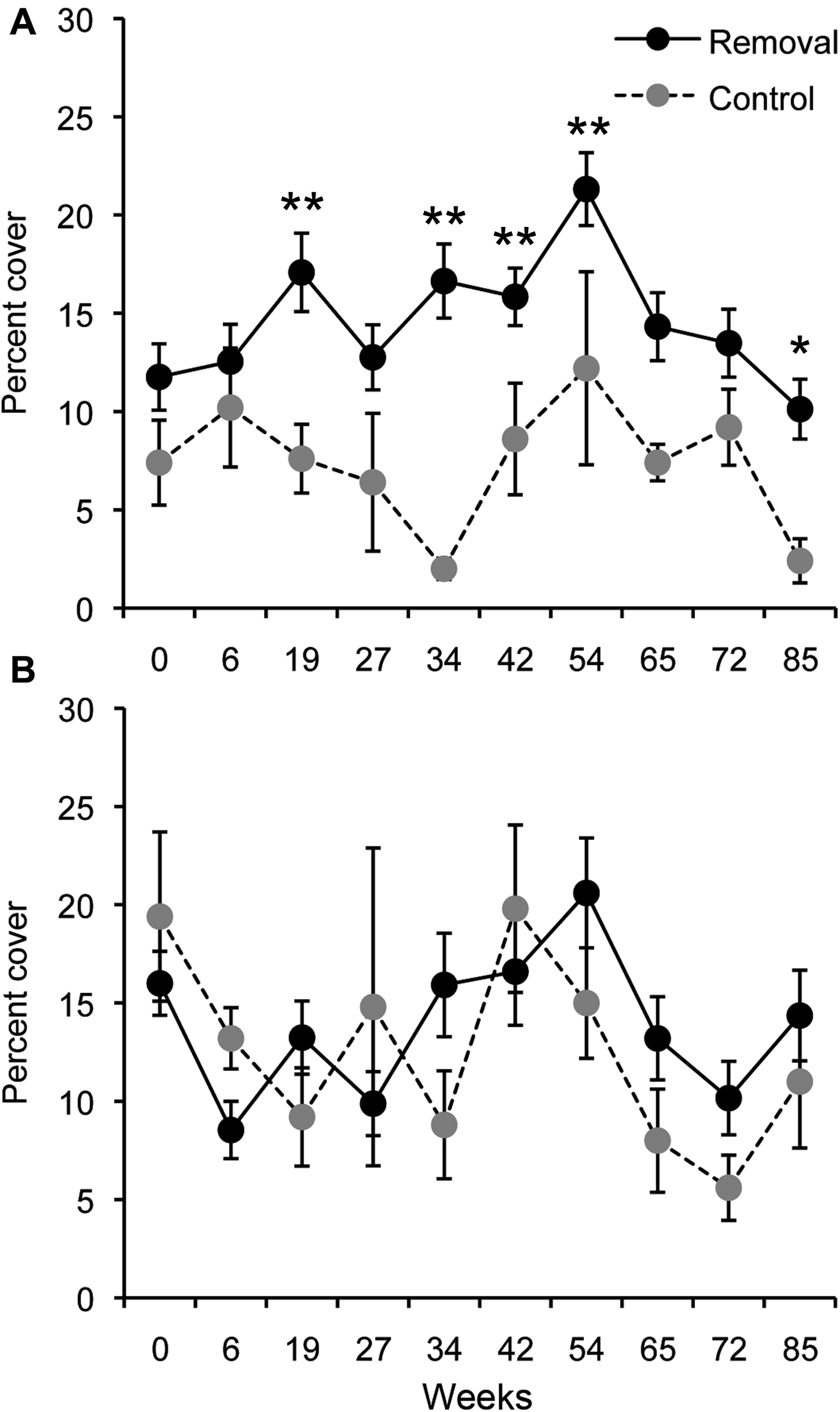
The removal of the canopy resulted in a reduction in sponge species richness, at both sites, typically staying below those of the control plots for the duration of the experiment (PERMANOVA Treatment P = 0.001, Treatment × Time P = 0.001; Figure 1). Differences in the number of sponge species per quadrat between control and removal plots increased within 34 weeks following canopy removal, remaining lower than in control plots until the end of the experiment (Figure 1), however these differences were only significant after 54 and 72 weeks following canopy removal at Breaker Bay and Palmer Head, respectively (Figure 1).

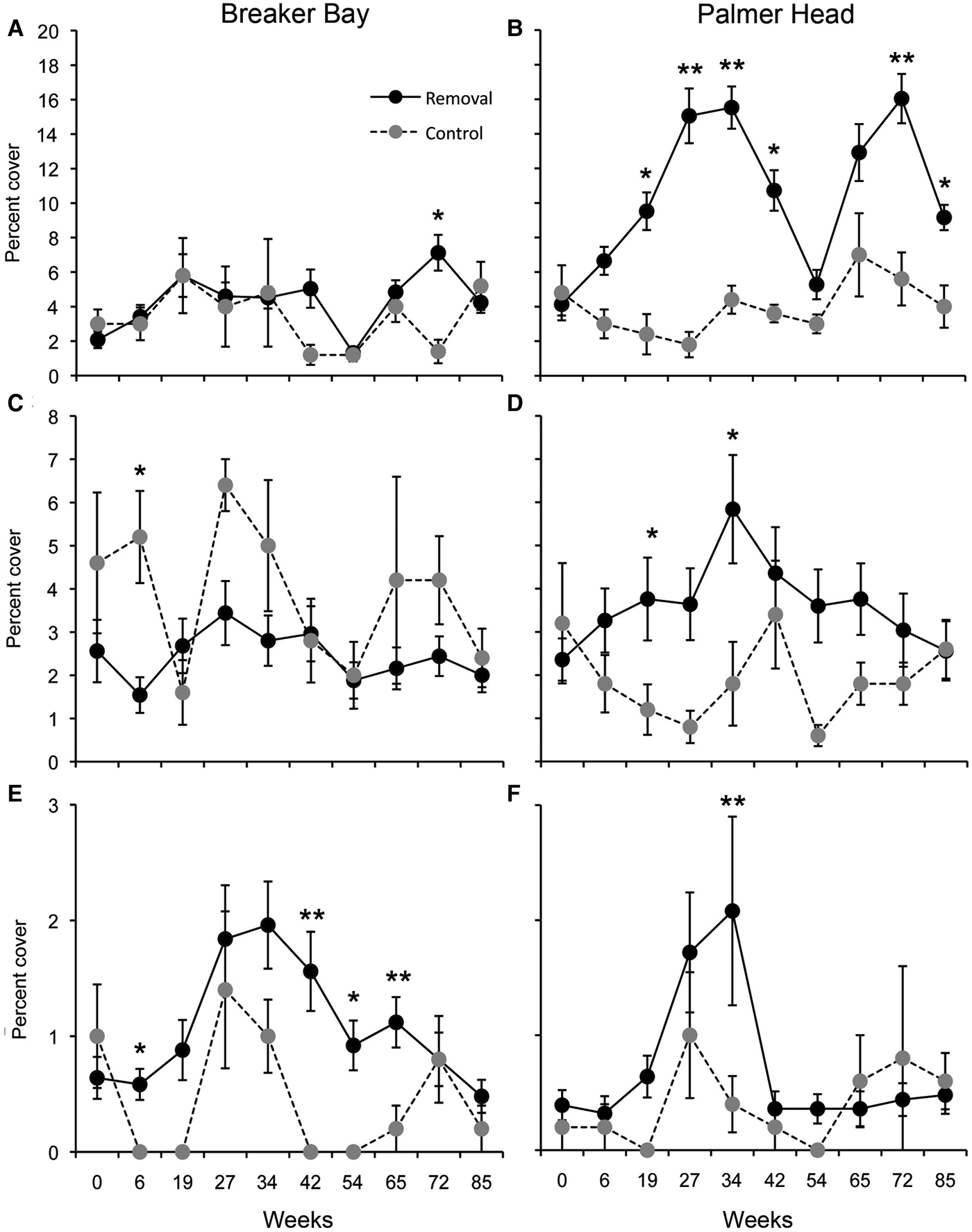
Fig. 1. Effect of removal of Ecklonia radiata on: (A, B) mean sponge species richness; and (C, D) mean percentage cover of sponges, at Breaker Bay and Palmer Head. Values are means (±SE) of five quadrats from each experimental plot. *P < 0.05, **P < 0.001.
The mean percentage cover of sponges decreased after canopy removal at both sites, decreasing more than three times within 34 weeks after canopy removal and remained significantly different until the end of the experiment (PERMANOVA Treatment P = 0.001, Treatment × Time P = 0.01; Figure 1). At Breaker Bay, sponge abundance decreased from 9.2 to 2.5%, whereas at Palmer Head sponge abundance decreased from 7.6 to 2.5% after 34 weeks (Figure 1).
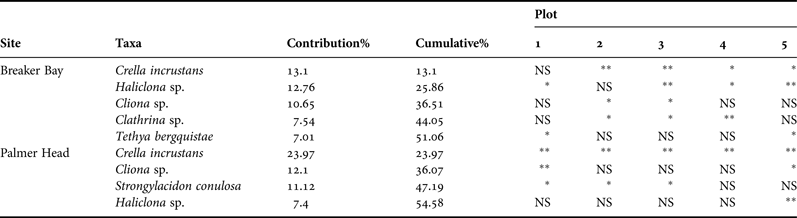
Differences in sponge abundance between control and removal plots at Breaker Bay were mainly driven by Crella incrustans (13.10%), Cliona sp. (12.76%), Strongylacidon conulosa (10.65%), Haliclona sp. (7.54%) and Tethya bergquistae (7.01%). At Palmer Head, four species were responsible for 51% of the differences in the sponge assemblages between treatments: Crella incrustans (23.97%), Cliona sp. (12.10%), Strongylacidon conulosa (11.12%) and Haliclona sp. (7.40%). All these species, with the exception of T. bergquistae and Cliona sp., decreased in abundance after canopy removal. The effect of removal on different sponge species is shown in Table 1.
Table 1. Sponge taxa contributing to 50% of the observed differences in sponge assemblages between treatments, as determined by SIMPER. PERMANOVA analyses to test the effect of canopy removal were based on Euclidean distances using 9999 permutations of the raw data.

*P < 0.05, **P < 0.001, NS, non-significant.
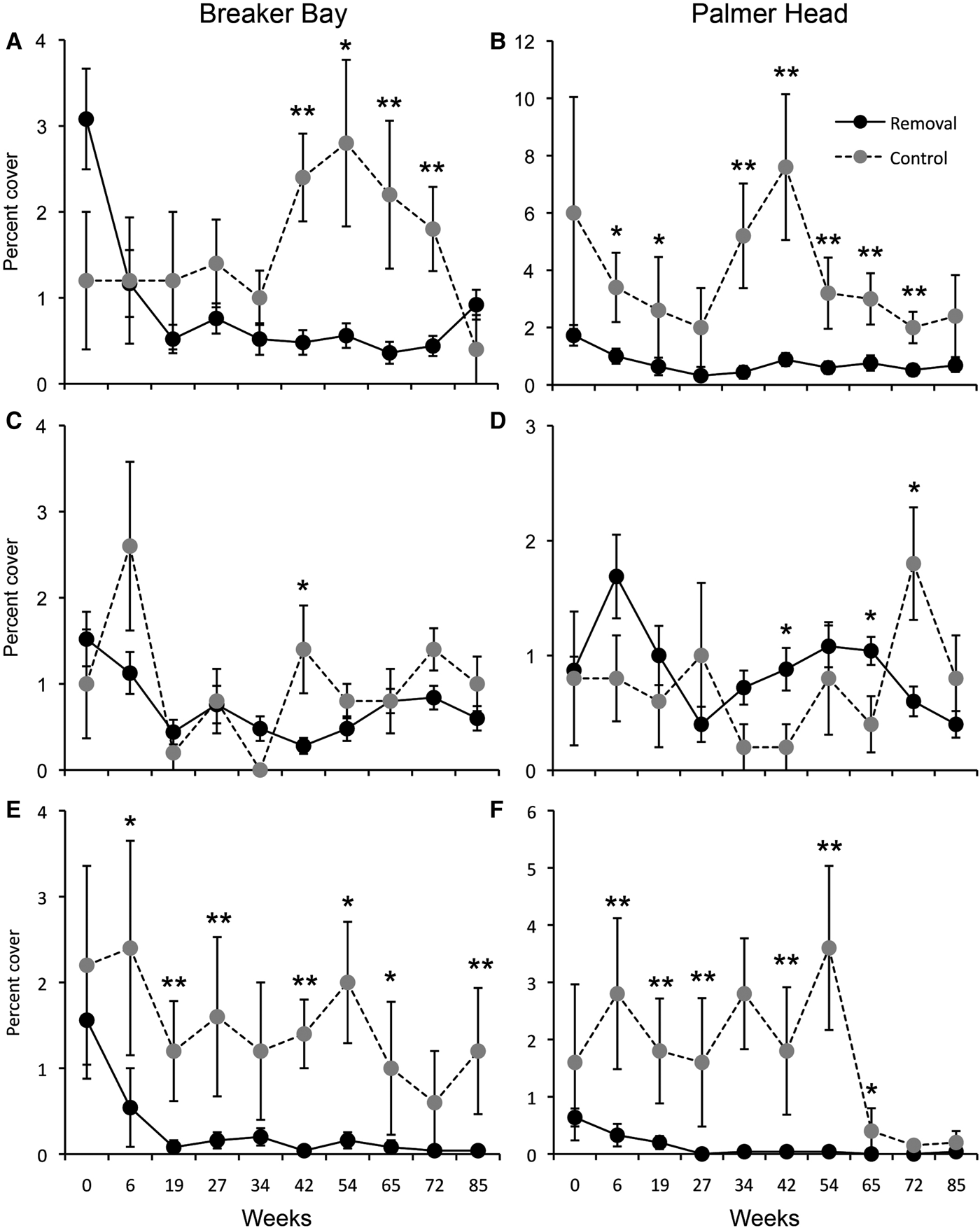
The removal of the canopy had a significant effect on the abundance of C. incrustans (Table 1). Its abundance declined dramatically following canopy removal at both sites and remained low for the rest of the experiment (Figure 2), being significantly different between treatments in nine of 10 experimental plots (Table 1). The effect of removal on other sponge species was site dependent. Species such as Haliclona sp. and S. conulosa showed a significant response to canopy removal at Breaker Bay and Palmer Head, respectively. At Breaker, the abundance of Haliclona sp. was three and 15 times lower in weeks 6 and 19 than at the start of the experiment (Figure 2). The response of Haliclona sp. to canopy removal was significant in four of five plots at Breaker Bay, whereas a significant response was found in one plot at Palmer Head. In contrast, at Palmer Head the abundance of S. conulosa declined from 0.6% (±0.15) to 0.04% (±0.2) within 6–19 weeks after removal and remained lower than that in the control plot until the end of the experiment (Figure 2).

Fig. 2. Responses of: (A, B) Crella incrustans; (C, D) Cliona sp; (E) Haliclona sp; and (F) Strongylacidon conulosa, to removal of Ecklonia radiata at Breaker Bay and Palmer Head. Values are means (±SE) of five quadrats from each experimental plot. *P < 0.05, **P < 0.001. Note: scale for Y-axis differs between panels.
Taxon specific responses to Ecklonia removal
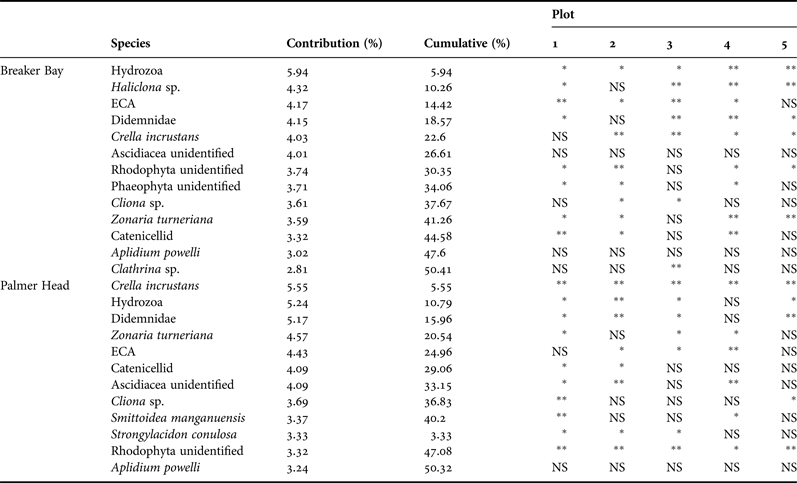
SIMPER analyses identified several taxa as the main contributors to observed differences in community structure between control and removal plots. At Breaker Bay, taxa including hydroids (5.94%), Haliclona sp. (4.32%), ECA (4.17%), Didemnidae (4.15%) and Crella incrustans (4.03%) accounted for more than 22% of the difference between canopy removal and control assemblages (Table 2). Of these taxa, hydroids and didemnids increased their abundance after removal, whereas the abundance of the other three groups/species decreased after canopy removal. At Palmer Head, taxa including C. incrustans (5.55%), hydroids (5.24%), Didemnidae (5.17%), Zonaria turneriana (4.57%) and erect crustose algae (4.43%) accounted for almost 25% of the difference in community composition between control and removal plots (Table 2). The effect of canopy removal on different taxa is shown in Table 2. In general, sponge species decreased in abundance while brown and red algae increased in abundance after canopy removal. The removal of Ecklonia had a significant effect on abundance of brown and red algae in the majority of the experimental plots.
Table 2. Subcanopy taxa contributing to 50% of observed differences in subcanopy assemblage structure between treatments, as determined by SIMPER. PERMANOVA analyses to test the effect of canopy removal were based on Euclidean distances using 9999 permutations of raw data.

*P < 0.05, **P < 0.001, NS, non-significant.
Changes in the abundance of algae in response to canopy removal were variable and depended on taxa and site. Red non-calcareous algae (Rhodophyta) showed a consistent response through time between removal and control plots at Palmer Head, being 20 times more abundant in control plots after 27 weeks post removal and remaining at this level until near the end of the experiment (Figure 3). In contrast, the response of red algae to canopy removal was not consistent at Breaker Bay (Figure 3, Table 2), where the abundance of non-calcareous red algae in control plots remained similar to that in removal plots during most of sampling times, except after 72 weeks following canopy removal (Figure 3). The response of ECA and other brown algae to canopy removal was also variable (Figure 3, Table 2). At Palmer Head, the abundance of ECA increased in removal plots after removal. The abundance of other brown algae also increased after removal, being significantly higher at specific times at both sites.

Fig. 3. Responses of: (A, B) red algae; (C, D) ECA; and (E, F) other brown algae, to removal of Ecklonia radiata at Breaker Bay and Palmer Head. Values are means (±SE) of five quadrats from each experimental plot. *P < 0.05, **P < 0.001.
The effect of canopy removal on the percentage cover of settled sediment was variable between sites (Figure 4). At Breaker Bay, sediment cover (composed mainly of fine silt) increased after removal. In contrast, sediment cover was highly variable at Palmer Head. However, changes in the coverage of sediment were significant in a few plots at both sites (PERMANOVA P < 0.05 in plots 1 and 2 at Palmer Head, and plots 3 and 5 at Breaker Bay).

Fig. 4. Mean percentage cover of sediment at: (A) Breaker Bay and (B) Palmer Head in response to removal of Ecklonia radiata. Values are means (±SE) from five quadrats on each experimental plot. *P < 0.05, **P < 0.001.
Biotic and abiotic associations of variation in sponge assemblages after canopy removal
In general, increased abundance of turf algae after canopy removal was one of the best predictors explaining the decrease in sponge abundance through time in most of the experimental plots (Table 3). The abundance of turf algae was negatively correlated with sponge abundance, especially at Palmer Head, explaining a considerable amount of the variation (26–60%). Settled sediment was also an important predictor in several plots. It explained between 10–17% of the variability; in a couple of cases, it was part of a two–factor model together with turf algae and hydroids, explaining 79 and 65% of the variation, respectively (Table 3). Other groups including hydroids and didemnid ascidians were the best predictors in some plots (Table 3).
Table 3. Results of distance-based permutational multivariate multiple regression analyses (DistLM) for associations between the decrease in sponge abundance after canopy removal and predictor variables. Model selection was based on models with lowest modified Akaike's Information Criterion (AICc) for each response variable. All tests were based on 9999 permutations.

DISCUSSION
The role of negative interactions, such as competition, predation and physical stress have been extensively studied in marine communities (Connell, Reference Connell1983; Dayton, Reference Dayton1985b; Bertness & Leonard, Reference Bertness and Leonard1997; Sousa, Reference Sousa, Bertness, Gaines and Hay2000; Barott et al., Reference Barott, Williams, Vermeij, Harris, Smith, Rohwer and Sandin2012). Such negative interactions have been traditionally considered to be the main forces structuring communities, however, over the last two decades the role of positive interactions has received increased interest, particularly because of the role that facilitation can play in enhancing biodiversity and promoting species coexistence (see Bruno et al., Reference Bruno, Stachowicz and Bertness2003; Thomsen et al., Reference Thomsen, Wernberg, Altieri, Tuya, Gulbransen, McGlathery, Holmer and Silliman2010; Gouhier et al., Reference Gouhier, Menge and Hacker2011). Here, we present data suggesting that Ecklonia radiata is important in facilitating some sponge species such as Crella incrustans. Our results also highlight the likely role that light, indirectly influencing turf algal abundance, has in explaining the spatial variation of some sponge species.
The removal of Ecklonia led to substantial increases in the area occupied by turf algae, an effect that is generally consistent with previous studies (Kennelly, Reference Kennelly1987b; Benedetti-Cecchi, Reference Benedetti-Cecchi2001; Benedetti-Cecchi et al., Reference Benedetti-Cecchi, Pannacciulli, Bulleri, Moschella, Airoldi, Relini and Cinelli2001; Flukes et al., Reference Flukes, Johnson and Wright2014). Ecklonia forests modify the physical environment, altering light regimes and sediment cover, which can have wide effects on the diversity, abundance and structure of understorey algae (Wernberg et al., Reference Wernberg, Kendrick and Toohey2005; Smale et al., Reference Smale, Kendrick and Wernberg2011). Although the responses of other subcanopy algae were variable (and often site-specific), there appeared to be a general increase in the percentage cover of understorey algae over the course of the experiment in our study plots, and we suggest that Ecklonia appears to have a positive impact on some sponge species by influencing the abundance of turf algae through light reduction. In contrast, species such as C. incrustans typically declined in abundance following canopy removal. The negative effect on C. incrustans produced by the algal canopy removal may be the result of displacement by competitively superior turf algal species (produced by canopy removal), however the mechanism(s) involved remain unclear and further experiments are required to clarify the effect of direct contact between turf algae and C. incrustans. Although similar patterns were recorded for other sponge species such as Haliclona sp. and S. conulosa, their responses were highly localized with respect to site and plot. This situation might be explained by differences in kelp density occurring at the start of the experiment (Melville & Connell, Reference Melville and Connell2001) or might be associated with the morphology, size and ecology of these two species.
Increased sedimentation and ambient irradiance beyond an acceptable level for sponges or changes in water flow may also have some influence in the decline in sponges once the canopy is removed. The increase in the percentage cover of settled sediment after canopy removal explained a large amount of the variability observed in the sponge assemblages in some plots. The increased accumulation of sediment (induced by increased abundance of hydroids) may have a direct negative impact in some areas by inhibiting the settlement and growth of sponges occurring underneath the canopy (Eckman & Duggins, Reference Eckman and Duggins1991).
Relationships between canopy-forming species and understorey assemblages usually involve both positive (e.g. provision of shelter and increased supply of nutrients) and negative interactions (e.g. abrasion by fronds and reduced light) (Dayton, Reference Dayton1975; Foster & Schiel, Reference Foster and Schiel1985; Melville & Connell, Reference Melville and Connell2001; Morrow & Carpenter, Reference Morrow and Carpenter2008). Recently, Smale et al. (Reference Smale, Burrows, Moore, O'Connor and Hawkins2013) suggested a positive effect of abrasion produced by kelp on sponges in the northern hemisphere. In this case, the whiplash effect provided by fronds of Laminaria digitata facilitates the sponge Halichondria panicea, which without the abrasion provided by the kelp would be outcompeted by the algal understorey. Positive and negative interactions are sometimes hard to separate. For example, shade provided by the canopy negatively affects understorey algae, which compete for space with sessile invertebrates, which then provides an advantage to the sessile organisms (Arkema et al., Reference Arkema, Reed and Schroeter2009; Benedetti-Cecchi et al., Reference Benedetti-Cecchi, Pannacciulli, Bulleri, Moschella, Airoldi, Relini and Cinelli2001; Connell, Reference Connell2003b). However, a reduction in harsh physical conditions as a result of the presence of the algal canopy can have strong direct positive effects on some subcanopy species (Bertness et al., Reference Bertness, Leonard, Levine, Schmidt and Ingraham1999). The absence of the canopy may have left sponges more exposed to big storms and regular southerly swells, which are relatively common in the area (Carter & Lewis, Reference Carter and Lewis1995). In this regard, the decrease in sponge abundance in removal plots around weeks 34–42 may be related to strong southerly swells produced by a big storm that occurred at that time of the year (NIWA National Climate Database, 2013).
The results of our experiment support the existence of positive interactions and highlight the importance of the Ecklonia canopy for some sponge species, which is consistent with a previous study from New South Wales, Australia (Wright et al., Reference Wright, Benkendorff and Davis1997). These authors reported major differences between the sponge assemblages inside and outside Ecklonia forests, suggesting that four Chondropsis spp. seemed to be positively influenced by conditions provided by Ecklonia. As an ecosystem engineer, Ecklonia can control (directly or indirectly) the availability of resources to other organisms by causing physical state changes in biotic or abiotic factors (Jones et al., Reference Jones, Lawton and Shachak1997). Ecklonia forests appear to provide suitable conditions for species such as Crella incrustans. Connell (Reference Connell2003a) suggested that sponges might tolerate physical abrasion under existing canopies due to their capacity to feed without projecting their filter feeding appendages outside their bodies. However, it is possible that the early life stages of most sponge species may be affected by abrasion produced by fronds and also by light, which may increase post-settlement mortality due to competition with turf algae (Miller & Etter, Reference Miller and Etter2008), explaining why only a few species are able to survive underneath the canopy.
Negative interactions between turf algae and sponges have been suggested in a number of observational and experimental studies (Kaandorp & de Kluijver, Reference Kaandorp and de Kluijver1992; Kennelly & Underwood, Reference Kennelly and Underwood1993; Turon et al., Reference Turon, Tarjuelo and Uriz1998; Bell & Barnes, Reference Bell and Barnes2000a, Reference Bell and Barnesb). Our results partially support this view as decreases in sponge abundance (especially C. incrustans) in removal plots coincided with increases in the abundance of brown and red algae. This apparent negative correlation between sponges and algal abundance appears to be the most likely factor driving changes in several of the plots. The removal of Ecklonia indirectly affected sponges, as increased light availability after removal seemed to favour understorey algae (5.75 vs 91.66 µmol quanta m−2 s−1 underneath the canopy and when the canopy was absent, respectively), which may outcompete small encrusting sponges. Increased light availability also affected the temporal variability of turf-forming algae (e.g. red and brown algae), which was greater in magnitude in removal compared with control plots. Consequently, these temporal changes of greater magnitude may have affected encrusting species such as C. incrustans in removal plots. Although previous research has reported detrimental effects of light in sciaphilic sponges (with absence of cyanobacteria) transplanted to high-light environments (Wilkinson & Vacelet, Reference Wilkinson and Vacelet1979), a direct effect due to increased light levels on adult of C. incrustans and other species we studied is unlikely as it was demonstrated by a recent study on other temperate sponge species (Cárdenas et al., Reference Cárdenas, Bell, Davy, Hoggard and Taylor2014).
The positive interaction occurring between the Ecklonia canopy and C. incrustans is a good example of the complexity of interactions between biotic and abiotic factors, showing how environmental factors can determine the outcomes of ecological interactions occurring in a specific habitat. In this case, the canopy-forming species seems to facilitate the presence of C. incrustans, and potentially other sponge species, by altering immediate physical factors (e.g. light and sediment accumulation). At the same time, the canopy may indirectly facilitate sponges by influencing the abundance of understorey algae, and hence competition with sponges, through light reduction. Thus, interactions between sponges, understorey algae and other organisms (such as ascidians) remain in balance when the canopy is present. Our experiment, however, provides no mechanistic understanding for the role of physical factors; hence further experimental work is needed to clarify the mechanisms involved. In addition, the variability in the responses in plots is not surprising as rocky walls are highly dynamic environments where available space is limited (Sebens, Reference Sebens, Moore and Seed1985) and each individual species satisfies its requirements for space, light, water-flow, protection from abrasion and sediment, and protection from predators (Wright et al., Reference Wright, Benkendorff and Davis1997; Davis et al., Reference Davis, Fyfe, Turon and Uriz2003). Furthermore, differences in unmeasured physical factors (particularly irradiance regimes and water-flow) may have resulted in differences in algal abundance in different plots. The subcanopy seems to be a dynamic environment even when canopy is intact, being influenced by the availability of certain species to colonize patches within a particular forest at any particular time. Therefore, high levels of patchiness occurring in the understorey along with the variability and timing of disturbances (e.g. canopy removal) are critical with regard to what subsequently happens to subcanopy assemblages. This situation may also explain the lack of consistency in the responses of Haliclona sp. and S. conulosa to canopy removal. The responses of these species will require further studies to clarify the role that Ecklonia plays in influencing their abundance. Physical and biological factors may co-vary and each of the factors can have separate effects (positive or negative) on different species, which in many cases are very difficult to separate.
The results of our study must be considered with respect to the limitations of this experiment. Although our study was carried out using one control plot, we found a similar effect of canopy removal on C. incrustans in most of the experimental plots at both sites. However, we could not find consistent responses on other subcanopy sponges (e.g. Haliclona sp. and S. conulosa). Further replicated field and laboratory experiments will be required to confirm the pattern observed in C. incrustans and also to clarify the effect of individual factors on different species and their influence on interactions between sponges and understorey algae.
Finally, our study shows the importance of Ecklonia in structuring subcanopy assemblages and the potential effect of canopy loss on sponge assemblages in particular. This, in turn, could affect the flow of energy in these habitats, since sponges can play an important role linking pelagic and benthic habitats on temperate rocky reefs (e.g. Perea-Blázquez et al., Reference Perea-Blázquez, Davy and Bell2012). The increased sediment loads occurring in coastal regions (Syvitski et al., Reference Syvitski, Vörösmarty, Kettner and Green2005) and the predicted levels of ocean warming (Flukes et al., Reference Flukes, Johnson and Wright2014) may have an extensive impact on canopy-forming species, resulting in secondary negative cascading effects on the structure of the entire community that can affect sponges and other suspension feeders, and hence alter community function (Naranjo et al., Reference Naranjo, Carballo and García-Gómez1996; Airoldi, Reference Airoldi2003; Roberts et al., Reference Roberts, Davis and Cummins2006).
ACKNOWLEDGEMENTS
We are grateful to D.K. Lekan, S.W. Geange, D. Diaz-Guisado and P.J. Mensink for their assistance in the field. S.W. Geange, T. Jones and two anonymous reviewers provided many helpful comments on this manuscript. The authors also thank the skippers at the Victoria University Coastal Ecology Lab (VUCEL) for help and support during diving activities. C.A.C. was funded by a CONICYT-VUW PhD Scholarship.