Introduction
Mood disorders and cardiovascular diseases (CVD) are among the highest sources of morbidity and premature mortality worldwide (GBD 2015 DALYs & HALE Collaborators, 2016). Moreover, their high comorbidity (Van der Kooy et al. Reference Van der Kooy, van Hout, Marwijk, Marten, Stehouwer and Beekman2007; Glaus et al. Reference Glaus, Vandeleur, Gholam-Rezaee, Castelao, Perrin, Rothen, Bovet, Marques-Vidal, von Kanel, Merikangas, Mooser, Waterworth, Waeber, Vollenweider and Preisig2013) is associated with even greater disability adjusted life years than either disorder alone (Charlson et al. Reference Charlson, Moran, Freedman, Norman, Stapelberg, Baxter, Vos and Whiteford2013). An increasingly recognized mechanism to explain comorbidity of CVD with mood disorders is common underlying low-grade inflammation. Specifically, there is a consistent association between circulating levels of pro-inflammatory cytokines, such as interleukin (IL)-1β, IL-6 and tumor necrosis factor (TNF)-α, and the acute phase reactant C-reactive protein (CRP) with both mood disorders (Howren et al. Reference Howren, Lamkin and Suls2009; Dowlati et al. Reference Dowlati, Herrmann, Swardfager, Liu, Sham, Reim and Lanctot2010; Modabbernia et al. Reference Modabbernia, Taslimi, Brietzke and Ashrafi2013; Glaus et al. Reference Glaus, Vandeleur, von Kanel, Lasserre, Strippoli, Gholam-Rezaee, Castelao, Marques-Vidal, Bovet, Merikangas, Mooser, Waeber, Vollenweider, Aubry and Preisig2014) and CVD (Baune et al. Reference Baune, Stuart, Gilmour, Wersching, Arolt and Berger2012). Alternatively, such inflammatory processes could also provoke mood disorders or CVD through, for instance, stress- induced perturbation of the Hypothalamic-Pituitary-Adrenal (HPA) axis (Rosmond & Bjorntorp, Reference Rosmond and Bjorntorp2000; Watson & Mackin, Reference Watson and Mackin2006). Conversely, mood disorders could induce inflammation through similar mechanisms (Rosenblat et al. Reference Rosenblat, Cha, Mansur and McIntyre2014).
Although the bulk of prospective research on inflammatory markers and CVD has focused on major depression, comparably few studies have examined the extent to which associations extend to other mood disorders, such as bipolar disorder (BPD) (Van der Kooy et al. Reference Van der Kooy, van Hout, Marwijk, Marten, Stehouwer and Beekman2007; Modabbernia et al. Reference Modabbernia, Taslimi, Brietzke and Ashrafi2013). In a recent meta-analysis of 25 cross-sectional studies and two longitudinal analyses (changes in CRP levels before and after treatment for mania and depression), BPD disorder was associated with increased levels of CRP (Fernandes et al. Reference Fernandes, Steiner, Molendijk, Dodd, Nardin, Goncalves, Jacka, Kohler, Karmakar, Carvalho and Berk2016). In another meta-analysis of 30 cross-sectional studies, BPD was associated with increased levels of IL-6 and TNF-α (Modabbernia et al. Reference Modabbernia, Taslimi, Brietzke and Ashrafi2013).
Moreover, few longitudinal studies have examined depressive subtypes (Rothermundt et al. Reference Rothermundt, Arolt, Fenker, Gutbrodt, Peters and Kirchner2001; Kaestner et al. Reference Kaestner, Hettich, Peters, Sibrowski, Hetzel, Ponath, Arolt, Cassens and Rothermundt2005; Yoon et al. Reference Yoon, Kim, Lee, Kwon and Kim2012; Lamers et al. Reference Lamers, Vogelzangs, Merikangas, de Jonge, Beekman and Penninx2013; Glaus et al. Reference Glaus, Vandeleur, von Kanel, Lasserre, Strippoli, Gholam-Rezaee, Castelao, Marques-Vidal, Bovet, Merikangas, Mooser, Waeber, Vollenweider, Aubry and Preisig2014; Rudolf et al. Reference Rudolf, Greggersen, Kahl, Huppe and Schweiger2014) that have been shown to be highly heterogeneous (Schmidt et al. Reference Schmidt, Shelton and Duman2011; Lopresti et al. Reference Lopresti, Maker, Hood and Drummond2014) and even fewer studies have considered the well-established comorbidity among mental disorders (Stewart et al. Reference Stewart, Rand, Muldoon and Kamarck2009; Kivimaki et al. Reference Kivimaki, Shipley, Batty, Hamer, Akbaraly, Kumari, Jokela, Virtanen, Lowe, Ebmeier, Brunner and Singh-Manoux2014) or current v. past disorders (Whooley et al. Reference Whooley, de Jonge, Vittinghoff, Otte, Moos, Carney, Ali, Dowray, Na, Feldman, Schiller and Browner2008).
With respect to directionality, longitudinal studies of the links between mood disorders and inflammation have predominantly been performed in patients with a major depressive disorder (MDD) (Valkanova et al. Reference Valkanova, Ebmeier and Allan2013; Chocano-Bedoya et al. Reference Chocano-Bedoya, Mirzaei, O'Reilly, Lucas, Okereke, Hu, Rimm and Ascherio2014; Kivimaki et al. Reference Kivimaki, Shipley, Batty, Hamer, Akbaraly, Kumari, Jokela, Virtanen, Lowe, Ebmeier, Brunner and Singh-Manoux2014; Tully et al. Reference Tully, Baumeister, Bengel, Jenkins, Januszewski, Martin and Wittert2015). Recent meta-analyses (Valkanova et al. Reference Valkanova, Ebmeier and Allan2013) and more recent studies (Chocano-Bedoya et al. Reference Chocano-Bedoya, Mirzaei, O'Reilly, Lucas, Okereke, Hu, Rimm and Ascherio2014; Tully et al. Reference Tully, Baumeister, Bengel, Jenkins, Januszewski, Martin and Wittert2015) have shown that these associations between inflammatory factors and mood disorders may be partially attributable to potential confounders, including body mass index (BMI) and smoking. Similar findings regarding mediation by BMI and behavioral cardiovascular risk factors (CVRFs) have also emerged from prospective studies of inflammation as a potential consequence of mood disorders (Matthews et al. Reference Matthews, Schott, Bromberger, Cyranowski, Everson-Rose and Sowers2007; Gimeno et al. Reference Gimeno, Kivimaki, Brunner, Elovainio, De Vogli, Steptoe, Kumari, Lowe, Rumley, Marmot and Ferrie2009; Stewart et al. Reference Stewart, Rand, Muldoon and Kamarck2009; Duivis et al. Reference Duivis, de Jonge, Penninx, Na, Cohen and Whooley2011). However, to date, the few studies that have examined the bi-directional associations simultaneously have yielded conflicting results (Gimeno et al. Reference Gimeno, Kivimaki, Brunner, Elovainio, De Vogli, Steptoe, Kumari, Lowe, Rumley, Marmot and Ferrie2009; Stewart et al. Reference Stewart, Rand, Muldoon and Kamarck2009; Matthews et al. Reference Matthews, Schott, Bromberger, Cyranowski, Everson-Rose and Sowers2010; Duivis et al. Reference Duivis, de Jonge, Penninx, Na, Cohen and Whooley2011). The aggregate findings from these studies highlight the importance of incorporating CVRFs including diabetes (Marques-Vidal et al. Reference Marques-Vidal, Schmid, Bochud, Bastardot, von Kanel, Paccaud, Glaus, Preisig, Waeber and Vollenweider2012a ), overweight (Marques-Vidal et al. Reference Marques-Vidal, Velho, Waterworth, Waeber, von Kanel and Vollenweider2012b ; Kiecolt-Glaser et al. Reference Kiecolt-Glaser, Derry and Fagundes2015), smoking (Yanbaeva et al. Reference Yanbaeva, Dentener, Creutzberg, Wesseling and Wouters2007), chronic alcohol consumption (Achur et al. Reference Achur, Freeman and Vrana2010) and physical inactivity (Hamer, Reference Hamer2007; Kiecolt-Glaser et al. Reference Kiecolt-Glaser, Derry and Fagundes2015) along with depression and anxiety (e.g. Agosti & Levin, Reference Agosti and Levin2006; Mezuk et al. Reference Mezuk, Eaton, Albrecht and Golden2008; Chaiton et al. Reference Chaiton, Cohen, O'Loughlin and Rehm2009; Anthenelli, Reference Anthenelli2010; Luppino et al. Reference Luppino, de Wit, Bouvy, Stijnen, Cuijpers, Penninx and Zitman2010; Glaus et al. Reference Glaus, Vandeleur, Gholam-Rezaee, Castelao, Perrin, Rothen, Bovet, Marques-Vidal, von Kanel, Merikangas, Mooser, Waterworth, Waeber, Vollenweider and Preisig2013; Lasserre et al. Reference Lasserre, Glaus, Vandeleur, Marques-Vidal, Vaucher, Bastardot, Waeber, Vollenweider and Preisig2014; Kiecolt-Glaser et al. Reference Kiecolt-Glaser, Derry and Fagundes2015), when examining the association with inflammation.
Our previous cross-sectional findings from a large community cohort revealed that there were significantly elevated levels of high sensitivity CRP (hsCRP) among those with the atypical subtype of depression and BPDs, but these associations were explained by concurrent CVRFs, such as BMI, diabetes, and hypertension (Glaus et al. Reference Glaus, Vandeleur, von Kanel, Lasserre, Strippoli, Gholam-Rezaee, Castelao, Marques-Vidal, Bovet, Merikangas, Mooser, Waeber, Vollenweider, Aubry and Preisig2014). In the present study, we extend these earlier cross-sectional analyses from our large community-based cohort in Lausanne, Switzerland, to examine the directionality, specificity, timing, and potential causal v. common etiologic mechanisms for associations between mood disorders and markers of a chronic low-grade inflammation by addressing the following aims: (1) to evaluate the 5-year prospective associations between mood disorders including their subtypes, and changes in circulating levels of specific inflammatory markers (IL-6, TNF-α, hsCRP) to determine the specificity of the associations; (2) to assess the directionality of links between inflammatory markers and mood disorders; (3) to examine whether the markers comprise state v. trait indices by comparing remitted v. current disorders, as well as incident cases; and (4) to examine the role of health behaviors, CVRFs, and treatment factors that may influence this association.
Based on our previous cross-sectional findings (Glaus et al. Reference Glaus, Vandeleur, von Kanel, Lasserre, Strippoli, Gholam-Rezaee, Castelao, Marques-Vidal, Bovet, Merikangas, Mooser, Waeber, Vollenweider, Aubry and Preisig2014) as well as those of other studies (Lamers et al. Reference Lamers, Vogelzangs, Merikangas, de Jonge, Beekman and Penninx2013; Hickman et al. Reference Hickman, Khambaty and Stewart2014; Rudolf et al. Reference Rudolf, Greggersen, Kahl, Huppe and Schweiger2014), we hypothesized that there would be an association between the atypical subtype of depression and increased levels of inflammatory markers, whereas other subtypes of MDDs were not expected to be associated with increased inflammation.
Methods and materials
Study sample
We drew the data for the present investigation from CoLaus|PsyCoLaus (Firmann et al. Reference Firmann, Mayor, Vidal, Bochud, Pecoud, Hayoz, Paccaud, Preisig, Song, Yuan, Danoff, Stirnadel, Waterworth, Mooser, Waeber and Vollenweider2008; Preisig et al. Reference Preisig, Waeber, Vollenweider, Bovet, Rothen, Vandeleur, Guex, Middleton, Waterworth, Mooser, Tozzi and Muglia2009), a cohort study designed to prospectively assess the associations between mental disorders and CVD or CVRFs in the community. The sample was randomly selected from the civil register of the city of Lausanne (Switzerland) in 2003. Sixty-seven percent of participants between 35 and 66 years-of age (n = 5535), who underwent the physical exam between 2003 and 2006, also accepted the psychiatric evaluation, resulting in a sample of 3719 individuals at baseline (Preisig et al. Reference Preisig, Waeber, Vollenweider, Bovet, Rothen, Vandeleur, Guex, Middleton, Waterworth, Mooser, Tozzi and Muglia2009) (see Fig. 1, flow chart). The mean interval between the physical and psychiatric evaluations at baseline was 1.3 years (s.d. 0.5). Five years later, 3191 participated in the physical exam (85.8% participation). Among them, 61 participants were excluded due to missing information on inflammatory markers both at baseline and follow-up, nine subjects were excluded due to missing information on mental disorders and three subjects were excluded due to missing information on CVRFs. The final sample used to examine associations between mood disorders at baseline and inflammation at follow-up consisted of 3118 participants in total (53.7% women; mean age: 51.0, s.d. 8.8 years): 2796 participants were analyzed for cytokines levels, and 2896 for hsCRP levels. The mean follow-up duration for the physical examination was 5.5 years (s.d. 0.6 years). The mean follow-up duration for the mental health assessment was also 5.5 years (s.d. 0.8). The number of participants with data on the psychiatric evaluation at follow-up was 2840. Among them, 258 participants were excluded due to missing information on inflammatory markers and two participants due to missing information on CVRFs. The resulting number of participants used to determine the association between inflammation at baseline and mental disorders at follow-up was 2580. The Institutional Ethics’ Committee of the University of Lausanne approved the CoLaus and the PsyCoLaus study. All participants provided written informed consent for the study protocol.
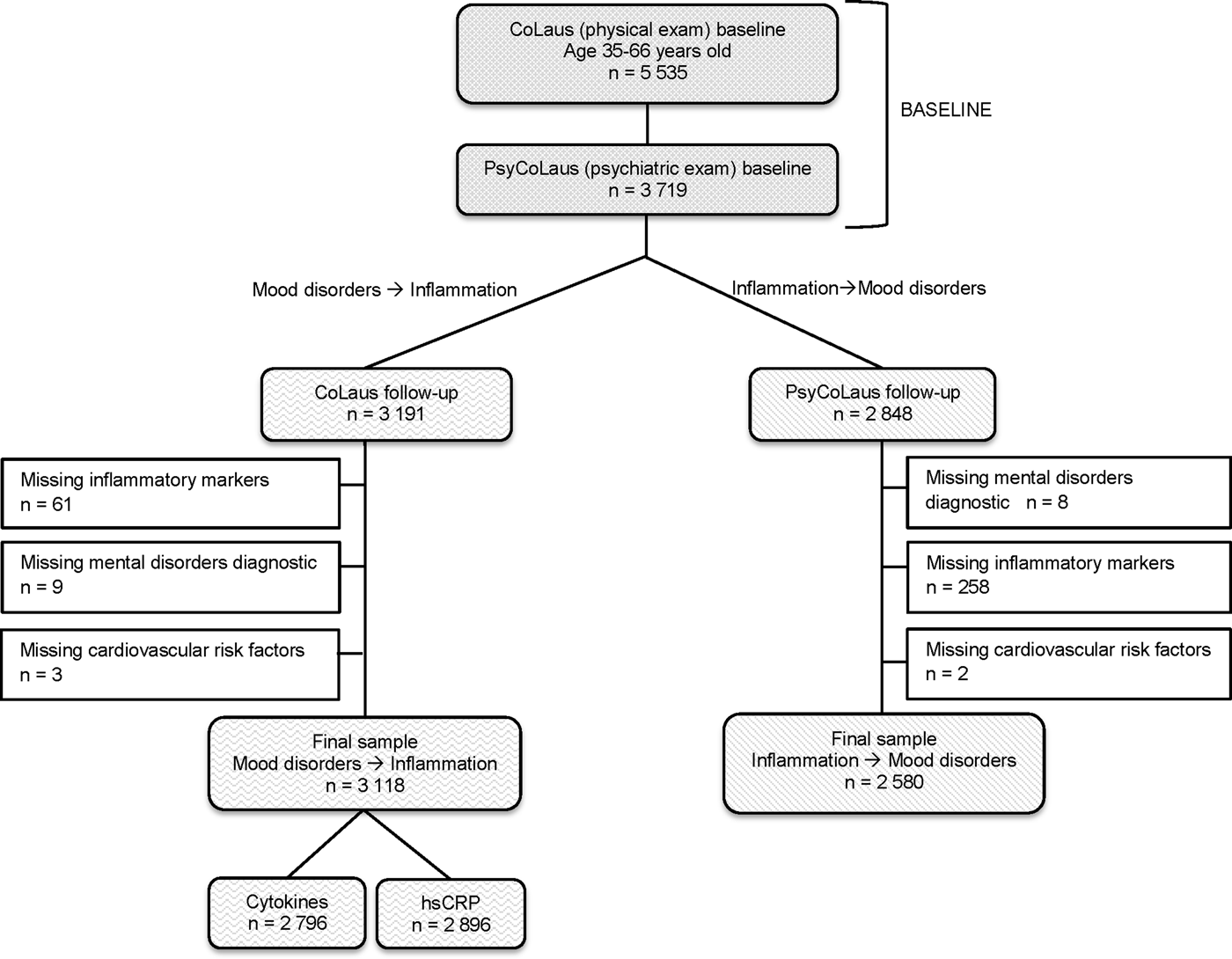
Fig. 1. Flowchart of the study for the association between mood disorders at baseline and inflammatory markers at follow-up and for the association between inflammatory markers at baseline and mood disorders at follow-up. Abbreviation: hsCRP, high-sensitivity C-Reactive Protein. ![]() Baseline assessment;
Baseline assessment; ![]() Follow-up assessment for the association between mood disorders at baseline and inflammatory markers at follow-up;
Follow-up assessment for the association between mood disorders at baseline and inflammatory markers at follow-up; ![]() Follow-up sample for the association between inflammatory markers at baseline and mood disorders at follow-up.
Follow-up sample for the association between inflammatory markers at baseline and mood disorders at follow-up.
Measurements
Mood disorders and comorbid disorders
Mental disorders at baseline and follow-up were assessed using the French version (Leboyer et al. Reference Leboyer, Barbe, Gorwood, Teherani, Allilaire, Preisig, Matthey, Poyetton and Ferrero1995) of the semi-structured Diagnostic Interview for Genetic Studies (DIGS), which was developed and validated by the National Institute of Mental Health (Nurnberger et al. Reference Nurnberger, Blehar, Kaufmann, York-Cooler, Simpson, Harkavy-Friedman, Severe, Malaspina and Reich1994). The French version of this instrument revealed adequate inter-rater and test–retest reliability for major mood (Preisig et al. Reference Preisig, Fenton, Matthey, Berney and Ferrero1999) and substance use disorders (Berney et al. Reference Berney, Preisig, Matthey, Ferrero and Fenton2002). The DIGS was completed with sections on generalized anxiety and phobia disorders using questions from the Schedule for Affective Disorders and Schizophrenia-Lifetime and Anxiety disorder version (SADS-LA (Endicott & Spitzer, Reference Endicott and Spitzer1978)), which also revealed satisfactory test–retest reliability (Leboyer et al. Reference Leboyer, Maier, Teherani, Lichtermann, D'Amato, Franke, Lepine, Minges and McGuffin1991; Rougemont-Buecking et al. Reference Rougemont-Buecking, Rothen, Jeanpretre, Lustenberger, Vandeleur, Ferrero and Preisig2008). Interviewers were required to be masters-level psychologists and were trained over a 2-month period. In order to provide ongoing supervision throughout the study, each interview and diagnostic assignment was reviewed by an experienced senior psychologist. Diagnoses were assigned according to the DSM-IV. Following Angst et al. (Reference Angst, Gamma, Benazzi, Silverstein, Ajdacic-Gross, Eich and Rossler2006), MDD was subtyped into: (1) MDD with at least one atypical and one melancholic episode or MDD with atypical and melancholic features simultaneously (combined type); (2) MDD with at least one atypical (but no melancholic) episode; (3) MDD with at least one melancholic (but no atypical) episode; and (4) MDD with neither atypical nor melancholic episodes (unspecified type). We defined MDD as current if it was present at the time of the physical evaluation.
Inflammatory markers
HsCRP was assessed during baseline and follow-up physical evaluations using immunoassay and latex HS (IMMULITE 1000-High, Diagnostic Products Corporation, LA, CA, USA), with maximum intra- and interbatch coefficients of variation of 1.3% and 4.6%, respectively (Firmann et al. Reference Firmann, Mayor, Vidal, Bochud, Pecoud, Hayoz, Paccaud, Preisig, Song, Yuan, Danoff, Stirnadel, Waterworth, Mooser, Waeber and Vollenweider2008). Subjects with a hsCRP level higher than 10 mg/l were excluded as such an elevation is likely to be attributable to acute infection (Pearson et al. Reference Pearson, Mensah, Alexander, Anderson, Cannon, Criqui, Fadl, Fortmann, Hong, Myers, Rifai, Smith, Taubert, Tracy and Vinicor2003). For the baseline and follow-up cytokine measurements, serum was preferred to plasma, as it has been shown that different anticoagulants may differentially affect absolute cytokine levels (Skeppholm et al. Reference Skeppholm, Wallen, Blomback and Kallner2008). Serum samples were stored at −80 °C before assessment and sent on dry ice to the laboratory. As previously described, cytokine levels were measured using a multiplexed particle-based flow cytometric cytokine assay (Marques-Vidal et al. Reference Marques-Vidal, Bochud, Bastardot, Luscher, Ferrero, Gaspoz, Paccaud, Urwyler, von Kanel, Hock, Waeber, Preisig and Vollenweider2011). Lower detection limits (LOD) for IL-6 and TNF-α were 0.2 pg/ml. Good agreement between signal and cytokine was found within the assay range (R 2 ⩾ 0.99).
Covariates
Data were collected on age, race (Caucasian v. non-Caucasian), marital status (living alone v. living with someone) and health-related behaviors at baseline including smoking (never, former, current) and physical inactivity (no or low v. at least 20 min twice a week). Information on socio-economic status (SES) and on psychotropic drug treatment was derived from the DIGS. The level of SES was assessed using the Hollingshead scale (Hollingshead, Reference Hollingshead1975). The section on psychotropic drug treatment covered all types of antidepressants, mood stabilizers (lithium and anti-epileptics) and antipsychotics. Moreover, information on aspirin and statin use was collected during the baseline physical evaluation (never v. occasional v. regular use). The following biological variables were measured during the baseline physical evaluation: BMI (weight in kilograms divided by height in meters square), diabetes (fasting blood glucose ⩾7 mmol/l or treatment for diabetes), dyslipidemia (HDL-cholesterol <1 mmol/l, or LDL-cholesterol ⩾4.1 mmol/l, or triglycerides ⩾2.2 mmol/l, or treatment with a lipid-lowering drug), hypertension (systolic blood pressure ⩾140 mmHg or diastolic blood pressure ⩾90 mmHg, or treatment for hypertension).
Statistical analysis
Statistical analyses were conducted using the Statistical Analysis System (SAS Institute Inc., Cary, NC, USA), version 9.3 for Windows. Age was standardized. Descriptive data for demographic characteristics, comorbid disorders, medication, health-related behaviors, physical risk factors for CVD and inflammatory marker levels were derived by lifetime mood disorders and χ2 and Kruskal–Wallis tests were used to determine the difference between mood disorders. The Benjamini–Hochberg false discovery method (Benjamini & Hochberg, Reference Benjamini and Hochberg1995) was employed to correct for multiple tests for all depressive subtypes except the pre-specified association with the atypical subtype of depression.
Associations between current and remitted mood disorders at baseline and changes in inflammatory markers at follow-up were determined using multiple linear regression models. Box–Cox transformation was applied to the response variable whenever a deviation from fundamental assumptions was observed. All three inflammatory markers were transformed: for the dependent variables, inflammatory markers were log-transformed, whereas for the independent variables, inflammatory markers were normalized and log-transformed. Values below the LOD of 0.2 [pg/ml] (i.e. 5% of values for IL-6, 0.5% of values for TNF-α) were considered as censored observations. IL-6 and TNF-α were analyzed with the qualitative and limited dependent variable model with the threshold of −1.65 [log (pg/ml)] as the meaningful lower bound of observed values.
A multiple linear regression model was performed on naturally log-transformed and standardized observed values. Three models of increasing complexity were computed. Model 1 included one single mood disorder (current and remitted) at a time as the independent variable, adjusted for the corresponding inflammatory marker at baseline. Model 2 included current and remitted mood disorders simultaneously in order to determine the associations of specific mood disorder with inflammatory markers at follow-up, adjusting for the length of follow-up, socio-demographic characteristics (sex, age, SES, race, and marital status), comorbid disorders (anxiety and substance use disorders), health-related behaviors and physical risk factors for CVD at baseline and the covariate of Model 1. In case of significant associations, additional models also adjusted for the occurrence of new episodes in order to test whether the associations persist after the offset of depressive episodes. Similar models adjusted for the occurrence of new physical risk factors for CVD during the follow-up in order to test whether the prospective associations between mood disorders and inflammation marker levels were independent of the occurrence of these new risk factors.
Associations between inflammatory markers at baseline and a history of mood disorders during the follow-up period were determined using logistic regression models. Two models were computed. Model 1 included all inflammatory markers simultaneously, adjusting for the corresponding history of mood disorder at baseline. Model 2 was further adjusted for the length of follow-up, socio-demographic characteristics, health-related behaviors and physical risk factors for CVD.
Results
Sample characteristics
The description of the cohort of 3118 participants used for the assessment of the prospective associations between mood disorders at baseline and the concentrations of inflammatory markers at follow-up is presented in Table 1. The groups (BPD, MDD subtypes and no history of mood disorders) differed with respect to sex, age, marital status, comorbid disorders, medication, current smoking status, BMI, and hypertension. Moreover, the groups also differed with respect to hsCRP levels at baseline and at follow-up.
Table 1. Sociodemographic, medication, health-related behaviors, physical risk factors for cardiovascular disorders and inflammatory markers by lifetime mood disorders at baseline (n = 3118)

s.d., standard deviation; MDD, major depressive disorder; IQR, Interquartile range (the 25% and 75% are provided).
Median and IQR of inflammatory markers were not logarithmically transformed (n IL-6, TNF-α = 2796, n hsCRP = 2896).
a χ2 test.
b Kruskal–Wallis test.
Statistically significant results are in bold.
p Values are in italic.
Associations between mood disorders at baseline and inflammatory markers at follow-up
Associations between current and remitted mood disorders at baseline and changes in levels of inflammatory markers are presented in Tables 2 and 3. When models were only adjusted for the corresponding inflammatory marker at baseline (Table 2), current MDD, as well as the current combined and atypical MDD subtypes were associated with increased hsCRP levels. Moreover, current combined MDD was associated with decreased IL-6 levels. No significant associations were found for remitted mood disorders with inflammatory markers. When the model was further adjusted for potential confounders, MDD was no longer significantly associated with hsCRP levels [β = 0.09, 95% confidence interval (CI) −0.02 to 0.21]. In contrast, current combined MDD was associated with increased hsCRP levels and decreased IL-6 levels and current atypical MDD was associated with increased hsCRP levels at follow-up (Table 3). Moreover, remitted melancholic MDD was associated with decreased IL-6 levels at follow-up in the fully adjusted model (Table 3). Additional analyses revealed that the occurrence of new mood episodes during the follow-up did not affect the size of the associations between current mood disorders at baseline and the hsCRP at follow-up. Similarly, the occurrence of incident physical risk factors for CVD did not account for the associations between current combined MDD or current atypical MDD at baseline and the hsCRP at follow-up.
Table 2. Associations between current and remitted mood disorders at baseline and inflammatory markers at follow-up

β, β-estimator; CI, confidence interval; MDD, major depressive disorder.
Models adjusted for the corresponding inflammatory marker at baseline, ‘No mood disorders’ considered as the comparison group.
a Multiple regression with logarithmically transformed cytokine (n = 2796) or hsCRP concentrations (n = 2896).
Statistically significant results are in bold.
p Values are in italic.
Table 3. Adjusted associations between current and remitted mood disorders at baseline and inflammatory markers at follow-up

β, β-estimator; CI, confidence interval; MDD, major depressive disorder.
Models adjusted for the corresponding inflammatory marker at baseline, length of follow-up, socio-demographic variables (age, gender, socio-economic status, ethnicity and marital status), medications, comorbid mental disorders (anxiety and substance use disorders), behavioral cardiovascular risk factors (physical activity and smoking status) and physical cardiovascular risk factors (BMI, diabetes, hypertension and dyslipidemia) at baseline. ‘No mood disorders’ considered as the comparison group.
Associations between inflammatory markers and a current diagnosis of bipolar disorder could not be assessed due to small sample size.
a Multiple regression with logarithmically transformed cytokine (n = 2796) or hsCRP concentrations (n = 2896).
Statistically significant results are in bold.
p Values are in italic.
Since an association was found between the atypical MDD subtype and increased hsCRP levels at follow-up, we also looked at the correlation between the age of onset of atypical MDD and inflammatory markers. Interestingly, later atypical MDD onset was correlated with increased hsCRP levels (Pearson correlation = 0.16, p value = 0.0214).
Associations between inflammatory markers at baseline and mood disorders at follow-up
Regarding the associations between inflammatory markers at baseline and mood disorders at follow-up, higher levels of TNF-α were associated with a decreased risk of MDD and of the MDD atypical and unspecified subtypes after controlling for a history of depression at baseline (Table 4). After controlling for all the covariates (Model 2), higher levels of TNF-α were still associated with a decreased risk of MDD and of the MDD unspecified subtype, but not with the MDD atypical subtype. Moreover, levels of IL-6 and hsCRP at baseline were not associated with any mood disorders at follow-up.
Table 4. Associations between inflammatory markers at baseline and mood disorders at follow-up (n = 2580)

IL-6, interleukin-6; TNF-α, tumor necrosis factor-α; hsCRP, high sensitivity C-Reactive Protein; OR, odds ratio; CI, confidence interval; MDD, major depressive disorder.
Model 1: adjusted for the corresponding mental disorder at baseline.
Model 2: adjusted for the corresponding mental disorder at baseline, length of follow-up, socio-demographic variables (gender, age, socio-economic status, ethnicity and marital status), medications, health-related behaviors (former smoker, current smoker and physical inactivity), and physical cardiovascular risk factors (body mass index, diabetes, dyslipidemia and hypertension) at baseline.
Statistically significant results are in bold.
p Values are in italic.
We also tested the associations between inflammatory markers at baseline and incident cases of mood disorders excluding subjects that already had a lifetime history of this disorder at baseline. However, no significant associations were found (results not shown).
However, none of these associations remained significant after correction for multiple testing that was conducted on all subtypes of depression except the pre-specified atypical subtype.
Additional analyses
In order to examine the potential influence of concurrent medication use on these associations, we examined the effects of mood stabilizers and antidepressants on changes in inflammatory marker levels over time. We found that lower IL-6 levels were associated with mood stabilizer use in those with BPD (β = −1.33, p value = 0.036), but no differences were found for antidepressant use in the MDD subgroups.
Discussion
The present study prospectively assessed the bi-directional 5-year associations between mood disorder and its subtypes with several inflammatory markers with serial adjustments for sociodemographic, comorbid mental disorders, medication use, health behaviors, and CVRFs, in a large community sample of adults. Our results showed differential associations depending on temporal ordering, current v. remitted status of the mood disorder, the specific type of mood disorder and different indices of inflammation. The major finding is that there is an association between the current atypical subtype of MDD at baseline with increased levels of hsCRP at follow up, whereas inflammatory levels at baseline were not associated with subsequent atypical MDD at follow-up. This unidirectional association indicates that this disorder may be causally related to increased inflammation, rather than inflammation comprising a vulnerability factor for mood disorders. Future studies with more closely timed follow-up assessments may inform potential mechanisms for elevation of hsCRP among those with this condition. Our finding of unidirectional associations between MDD and elevated hsCRP levels appear to be specific to the atypical subtype of MDD, which is characterized by somatic symptoms including sleep, energy and eating behavior (Rothermundt et al. Reference Rothermundt, Arolt, Fenker, Gutbrodt, Peters and Kirchner2001; Kaestner et al. Reference Kaestner, Hettich, Peters, Sibrowski, Hetzel, Ponath, Arolt, Cassens and Rothermundt2005; Stewart et al. Reference Stewart, Rand, Muldoon and Kamarck2009; Duivis et al. Reference Duivis, de Jonge, Penninx, Na, Cohen and Whooley2011; Copeland et al. Reference Copeland, Shanahan, Worthman, Angold and Costello2012; Lamers et al. Reference Lamers, Vogelzangs, Merikangas, de Jonge, Beekman and Penninx2013; Glaus et al. Reference Glaus, Vandeleur, von Kanel, Lasserre, Strippoli, Gholam-Rezaee, Castelao, Marques-Vidal, Bovet, Merikangas, Mooser, Waeber, Vollenweider, Aubry and Preisig2014; Hickman et al. Reference Hickman, Khambaty and Stewart2014; Rudolf et al. Reference Rudolf, Greggersen, Kahl, Huppe and Schweiger2014; Schmidt et al. Reference Schmidt, Lichtblau, Minkwitz, Chittka, Thormann, Kirkby, Sander, Mergl, Fasshauer, Stumvoll, Holdt, Teupser, Hegerl and Himmerich2014). This finding suggests that the atypical MDD is associated with more detectable immune dysfunction. This could be a primary characteristic of this disorder or reflect other indirect factors, such as greater disturbance of circadian systems, HPA systems or other relevant immune modulators. A similar link between cumulative depressive episodes and CRP at follow-up in a prospective study of a community sample of youth would confirm this explanation and further imply that the associations observed herein may be present across the lifespan (Copeland et al. Reference Copeland, Shanahan, Worthman, Angold and Costello2012).The specificity of the findings with respect to the atypical subtype rather than BPD (Modabbernia et al. Reference Modabbernia, Taslimi, Brietzke and Ashrafi2013), as shown in previous studies of adults and youth, further suggests that the somatic manifestations of depression may be etiologically related to inflammation. However, the power to test the association between BPD and inflammation was reduced because of the relatively low numbers of people with BPD in this sample. Interestingly, a previous case-control study (Cunha et al. Reference Cunha, Andreazza, Gomes, Frey, da Silveira, Goncalves and Kapczinski2008) that evaluated BPD patients in different phases of their illness found increased hsCRP levels in patients with a current manic episode compared with depressed, euthymic patients and healthy controls. Moreover, recent studies have shown that mania, and to a lesser extent depression, in BPD patients are associated with pro-inflammatory cytokines (Muneer, Reference Muneer2016). To our knowledge, no previous studies have examined depressive subtypes in BPD compared with MDD patients and healthy controls. Future prospective studies should further investigate the different state-related associations between mood disorder subtypes and inflammation.
The lack of association between inflammatory markers at baseline and subsequent mood disorders at follow-up suggests that inflammation may not be a risk factor for the onset of mood disorders. This result extends the findings of two previous longitudinal studies, which found that depression predicted higher inflammation, whereas inflammation did not predict depression (Stewart et al. Reference Stewart, Rand, Muldoon and Kamarck2009; Duivis et al. Reference Duivis, de Jonge, Penninx, Na, Cohen and Whooley2011). However, our results do not confirm findings from previous studies that focused on depressive symptoms rather than disorder (Gimeno et al. Reference Gimeno, Kivimaki, Brunner, Elovainio, De Vogli, Steptoe, Kumari, Lowe, Rumley, Marmot and Ferrie2009; Matthews et al. Reference Matthews, Schott, Bromberger, Cyranowski, Everson-Rose and Sowers2010; Valkanova et al. Reference Valkanova, Ebmeier and Allan2013; Tully et al. Reference Tully, Baumeister, Bengel, Jenkins, Januszewski, Martin and Wittert2015). These discrepancies could be due to methodological differences. Moreover, no depressive subtypes were analyzed.
Our findings were restricted to the hsCRP, rather than the pro-inflammatory cytokines. CRP has been shown to lead to broader manifestations of the metabolic syndrome, particularly among those with current disorders rather than symptoms alone (Gimeno et al. Reference Gimeno, Kivimaki, Brunner, Elovainio, De Vogli, Steptoe, Kumari, Lowe, Rumley, Marmot and Ferrie2009; Matthews et al. Reference Matthews, Schott, Bromberger, Cyranowski, Everson-Rose and Sowers2010; Valkanova et al. Reference Valkanova, Ebmeier and Allan2013). This further suggests that the physical symptoms of these conditions may induce a stress-like physiologic reaction in affected individuals. Accordingly, depression may contribute to a chronic low-grade inflammatory state, which, in turn, might lead to a vascular pathology and ultimately to atherosclerotic vascular disease (Wagner et al. Reference Wagner, Wagner, Glaus, Vandeleur, Castelao, Strippoli, Vollenweider, Preisig and von Kanel2015). Several potential pathophysiological mechanisms have been suggested to underlie the association between depression and higher inflammation, including HPA-axis activation and oxidative stress (Rosenblat et al. Reference Rosenblat, Cha, Mansur and McIntyre2014). However, future longitudinal studies are needed to confirm these potential mechanisms.
Our evaluation of medication use at index evaluation as a possible explanation for changes in inflammatory markers revealed that mood stabilizer use was associated with lower IL-6 levels at follow-up. This suggests that future studies should examine the specific influences of mood stabilizers not only on symptom relief but also on concomitant inflammatory factors. However, no such changes emerged for inflammatory markers and other mood disorder subtypes.
Our results should be interpreted in the context of several limitations. First, there was an interval of almost 1 year between the physical and the psychiatric evaluations. Although the timing of depressive episodes was elicited in our diagnostic interviews, we cannot exclude misclassification regarding the ‘current’ status of disorders at the time of the physical evaluation. Second, inflammatory markers were only assessed twice, once at baseline and 5.5 years later at follow-up, which may only partially reflect the dynamic temporal relationship between mood disorders and inflammatory markers over time. However, despite this limitation, our findings still indicate that there may be an enduring increase of inflammation in individuals with specific subtypes of mood disorders. Third, low-grade inflammation was assessed through hsCRP and the two pro-inflammatory cytokines IL-6 and TNF-α. We did not include additional pro-inflammatory (i.e. IL-1β, IL-2, IFN-γ) and anti-inflammatory (i.e. IL-4, IL-8, IL-10) cytokines, and other potential biomarkers of inflammation, such as cortisol, leucocytes and thrombocytes. Fourth, with only 55 subjects with a lifetime history of BPD at baseline we did not have sufficient power to detect differences in inflammation levels at follow-up as a function of the BPD status at baseline. Despite these limitations, this is one of the largest prospective studies of a community sample, with the hitherto longest follow-up period. It is the first study to investigate the full range of mood disorder subtypes.
These prospective data from a large community sample show that unidirectional association between current MDD subtypes with atypical features and increased hsCRP levels at a more than 5-year follow-up may be a consequence of this condition. Further prospective studies with repeated assessments of inflammatory markers earlier in the development of mood disorders are required to examine potential influences of medications on inflammatory processes.
Acknowledgements
The authors would like to express their gratitude to the Lausanne inhabitants who volunteered to participate in the PsyCoLaus study and to the collaborators who contributed to the coordination of the study and the collection of data. The authors also like to thank all the investigators of the CoLaus study, who made the psychiatric study possible, as well as many GSK employees who contributed to the execution of this study. The CoLaus|PsyCoLaus study was and is supported by research grants from GlaxoSmithKline, Verona, Italy, the Faculty of Biology and Medicine of Lausanne (Switzerland), the Swiss National Science Foundation (Switzerland) (to M.P., P.V. and G.W.; grants 3200B0-105993, 3200B0-118308, 33CSCO-122661, 33CS30-139468 and 33CS30-148401), and in part by the Intramural Research Program of the National Institute of Mental Health (ZIAMH002932). This study was also funded by the Swiss National Science Foundation (to J.G., grant P2LAP3_161895 and A.L., grant 323530_151479). The funders had no role in the design of the study; the collection, management, analysis, and interpretation of the data; the preparation, review or approval of the manuscript; or the decision to submit the manuscript for publication.
Declaration of Interest
None.







