Introduction
Migraine varies in its frequency, severity, and impact; treatment should consider these variations and the patient’s needs and goals.Reference Silberstein 1 Treatment begins with a proper diagnosis and addressing the impact of the headache.Reference Silberstein, Saper and Freitag 2 Education about adverse events, duration of therapy, and expectations is important.Reference Lipton and Silberstein 3 Comorbidity is the association of two disorders more likely to occur by coincidence. Migraine comorbid disorders are listed in Table 1. Migraine treatment may be acute (abortive) or preventive (prophylactic), and patients may need both. Successful prevention reduces attack frequency. It may also decrease attack duration or severity and enhance the response to acute treatments, improve function, reduce disability,3 and reduce healthcare costs.Reference Silberstein, Winner and Chmiel 4
Table 1 Migraine comorbid disease

Acute Treatment
Medications are usually the acute treatment of choice. In a longitudinal study, 91.7% of 11,388 people with episodic migraine reported using pharmacologic treatment for their acute migraine attacks.Reference Chu, Buse, Bigal, Serrano and Lipton 5 The objectives of acute treatment are to treat attacks early; to achieve quick, complete pain relief; to minimize or eliminate adverse events; to restore function; to decrease recurrence and the need for rescue treatment; and to reduce medical resource use.Reference Silberstein 6
Acute pharmacologic treatment includes both migraine-specific medications, such as triptans and dihydroergotamine, and nonspecific medications such as acetylsalicylic acid (ASA), acetaminophen, and nonsteroidal anti-inflammatory drugs (NSAIDs). It also includes medications for relief of associated symptoms, such as nausea. Adjunctive medications include antiemetics (eg, metoclopramide or prochlorperazine) and corticosteroids.
General Principles
-
1. The most effective strategy for patients with attacks of different severity is a “step care within attack” strategy. Early administration of treatment is most appropriate for consistently moderate or severe attacks that respond well to treatment. This recommendation should be guided by the frequency of the headache. For those with near-daily, daily, or continuous headache, caution is needed to avoid acute medication overuse.
-
2. The route of administration depends on the prior response to oral therapy, the temporal characteristics of the attack, and the presence and timing of nausea and vomiting. Early nausea and vomiting during an attack may impair absorption and bioavailability, diminishing the efficacy and/or consistency of acute medications.Reference Aurora, Kori, Barrodale, Nelsen and McDonald 7 Non-oral routes of administration include nasal spray, suppository, subcutaneous or transcutaneous injection, and inhalation.
-
3. Adjunctive medications are useful for patients who respond partially to a single medication. Patients who do not consistently achieve an adequate response to a triptan, for example, may require concomitant therapy to treat nausea (antiemetic), or to achieve a more sustained response by reducing the risk of recurrence within a 24- to 48-hour period (NSAID).Reference Jakubowski, Levy, Goor-Aryeh, Collins, Bajwa and Burstein 8 – Reference Rapoport 11
-
4. All patients need rescue therapy when acute therapy is not effective, even if they typically respond to their usual treatment.Reference Silberstein 12 – Reference Bigal and Ho 15
Acute Treatment Guidelines
According to an evidence-based guideline from the American Headache Society (Table 2), all currently available triptans, in various formulations, are effective (Level A) for the acute treatment of migraine for moderate or severe pain at the time of treatment. Dihydroergotamine nasal spray is effective (Level A), and ergotamine and intravenous ergotamine are probably effective (Level B) for acute treatment.
Table 2 Acute treatment evidence
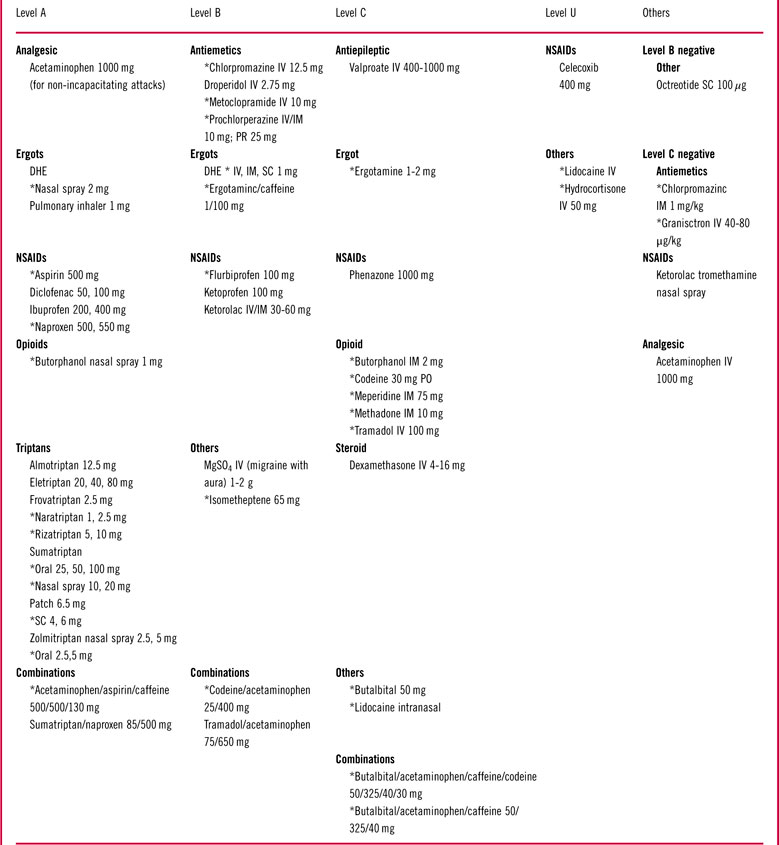
Level A: Medications are established as effective for acute migraine treatment based on available evidence.
Level B: Medications are probably effective for acute migraine treatment based on available evidence.
Level C: Medications are possibly effective for acute migraine treatment based on available evidence.
Level U: Evidence is conflicting or inadequate to support or refute the efficacy of the medications for acute migraine.
Level B negative: Medication is probably ineffective for acute migraine.
Level C negative: Medication is possibly ineffective for acute migraine.
NS=nasal spray.
Effective nonspecific medications include aspirin (500 mg), acetaminophen (1000 mg), diclofenac (50 or 100 mg), ibuprofen, metamizole (dipyrone) (1 mg), naproxen (500 or 550 mg), rofecoxib (25 mg), butorphenol nasal spray, codeine, and a combination of acetaminophen/aspirin/caffeine (Level A). Ketoprofen, IV ketorolac, or magnesium; isometheptene compounds; and tramadol/acetaminophen are probably effective (Level B). There is not enough information available to determine if celecoxib (400 mg) is effective in migraine (Level U). The antiemetics prochlorpromazine, droperidol, chlorpromazine, and metoclopramide are probably effective (Level B).
Dexamethasone is probably effective when given with rizatriptan (10 mg) (Level B). There is inadequate evidence for intravenous valproic acid (Level U). Butalbital is possibly effective (Level C).
Emerging Acute Therapies
While the triptans have significantly advanced acute migraine treatment, approximately one-fifth of migraineurs have cardiovascular contraindications that limit their use. Their efficacy is limited when considering the most robust patient-centered outcomes. In addition, triptans induce latent central sensitization and may promote the development of medication overuse headache (MOH).Reference De, Ossipov and Wang 16 Therefore, there is a large unmet treatment need for safe and effective acute migraine drugs that do not constrict vascular beds or induce MOH. In addition, new formulations of older drugs are being developed. U.S. Food and Drug Administration (FDA)-approved new formulations include sumatriptan needle-free injection (brand name Sumavel); sumatriptan epipen-like injection (brand name Alsuma); sumatriptan auto-injectors (Dr. Reddy’s Zembrace, Sun, generic); and breath-powered powder sumatriptan intranasal treatment (brand name Onzetra).
Sumatriptan iontophoretic patch (brand name Zecuity) is off the market because of adverse events (AEs). Awaiting FDA approval are rizatriptan dissolvable film (RHB-103, VersaFilm) and dihydroergotamine (DHE) oral inhalation (brand name Semprana).
New medication devices in development include an intracutaneous microneedle system of zolmitriptanReference Kellerman, Lickliter, Mardell and von Stein 17 and sumatriptan (Sofusa Dose Disc System Skin Patch), zolmitriptan oral inhalation (CVT-427), and sumatriptan oral spray (SUD-001).
5-HT 1F Receptor Agonists
Serotonin (5-hydroxytryptamine [5-HT]), a biogenic amine, was identified, crystallized, and named by Rapport and Page.Reference Rapport, Green and Page 18 There are 7 types of 5-HT receptors, 5-HT1–7. All are G protein-coupled receptors except the 5-HT3 receptor, which is a ligand-gated cation channel.Reference McCorvy and Roth 19 The 5-HT1 subfamily consists of 5-HT1A, 5-HT1B, 5-HT1D, 5-HT1E, and 5-HT1F.Reference McCorvy and Roth 19
Ergotamine targets 5-HT1A-1F; 5-HT2A-C, D1-5 and α1 and α2 adrenergic receptor types.Reference Silberstein 20 The anti-migraine effects are due to agonist activity at the 5-HT1B, 5-HT1D, and possibly 5-HT1F receptors on trigeminal nerve terminals.Reference Ramírez Rosas, Labruijere, Villalón and Maassen VanDenBrink 21 Ergotamine causes vasoconstriction, which can cause hypertension and coronary vasoconstriction. Retroperitoneal fibrosis, pleuropulmonary fibrosis, and cardiac valvulopathy can occur with chronic use.
The triptans have high 5-HT1B and 5-HT1D receptor affinity. 5-HT1B receptors mediate vasoconstriction, and 5-HT1D receptors mediate inhibition of neuronal impulse transmission.Reference Rapoport 11 In addition, several triptans have affinity for the 5-HT1F receptors subtype, which does not mediate vasoconstion.Reference Ramadan, Skljarevski, Phebus and Johnson 22 Spiral and vestibular ganglion cells in rodents and primates express the 5-HT1A, 5-HT1B, 5-HT1D, and 5-HT1F receptors. Hence, actions of ganglion cells might partly explain the efficacy of these agents in vestibular migraine and their vestibular AEs.Reference Furman, Marcus and Balaban 23
Lasmiditan (COL-144) is a highly selective, potent 5-HT1F receptor agonist.Reference Ferrari, Farkkila and Reuter 24 In preclinical animal models, it inhibited dural plasma protein extravasation and reduced trigeminal nucleus caudalis c-Fos expression, following trigeminal ganglion stimulation.Reference Nelson, Phebus and Johnson 25 Lasmiditan had no vasoconstrictor effect on the rabbit saphenous vein.Reference Nelson, Phebus and Johnson 25 An intravenous formulation was effective in a proof-of-concept, dose-finding study. Intravenous lasmiditan, at a starting dose of 2.5 mg, was evaluated for the acute treatment of migraine in 130 subjects in a hospital setting.Reference Ferrari, Farkkila and Reuter 24 Forty-two subjects received placebo and 88 received lasmiditan, in doses ranging from 2.5–45 mg. Of subjects treated in the 10, 20, 30, and 45 mg lasmiditan dose groups, 54–75% showed a 2-hour headache response, compared to 45% in the placebo group (P < 0.0126). AEs occurred in 65% of lasmiditan and in 43% of placebo subjects. Dizziness, paresthesia, and limb heaviness occurred more often with lasmiditan.
Oral lasmiditan (50, 100, 200, and 400 mg) was studied in a multicenter, double-blind, parallel-group, dose-ranging, acute migraine study.Reference Farkkila, Diener and Geraud 26 Lasmiditan was superior to placebo, and showed a dose response effect at 2 hours. Treatment-emergent AEs were dose-dependent, and mild or moderate in intensity. Adverse events included vertigo, dizziness, paresthesia, fatigue, and somnolence.
Controlled phase 3 trials have been completed. 27 In the SAMURAI trial, 2,231 patients were randomized to lasmiditan (100 mg, 200 mg) or placebo; a second dose was permitted for rescue or recurrence. The primary endpoint was 2-hour pain freedom. The key secondary endpoint was the relief of the most bothersome symptoms (MBS) at 2 hours (before dosing, subjects noted whether nausea, phonophobia, or photophobia were present and which was “most bothersome”). About half of subjects found photophobia was their MBS (about one-quarter for nausea and vomiting). For the groups lasmitidan 100 mg, lasmitidan 200 mg, and placebo, 28.2%, 32.2%, and 15.3%, respectively, were free of pain at 2 hours and 40.9%, 40.7%, and 29.5%, respectively, were free of MBS at 2 hours (all < 0.001 compared to placebo). Headache pain relief was 59.4%, 59.5%, and 42.2%, respectively (p < 0.001). AEs were dose-dependent and included vertigo, dizziness, paresthesia, fatigue, and somnolence. In this trial, lasmiditan met its primary and secondary endpoints, and many subjects had cardiovascular risk factors or conditions. The GLADIATOR phase 3 long-term, open-label trial is ongoing. 28
The SPARTAN trial, in addition to 100 mg and 200 mg lasmiditan groups, included a 50 mg group to find the lowest effective dose in acute migraine.Reference Farkkila, Diener and Geraud 26 The primary and secondary outcome measures are like those of the SAMURAI trial. This trial was completed in June 2017. 29
CGRP Receptor Antagonists (Gepants)
The CGRP family of neuropeptides consists of CGRP, calcitonin (CT), adrenomedullin (AM), and amylin (AMY). Human CGRP comes in 2 types: α and β. α-CGRP results from alternative splicing of the calcitonin gene; it is the main subtype in trigeminal neurons.Reference Ho, Edvinsson and Goadsby 30 The canonical CGRP receptor has three parts: (1) calcitonin-like receptor, (2) receptor activity–modifying protein type 1, and (3) a receptor component protein.Reference Eftekhari and Edvinsson 31 CGRP receptors are present on trigeminal ganglia, primary dural sensory afferents, the periaquedactual gray (PAG), and on meningeal blood vessels.Reference Ho, Edvinsson and Goadsby 30 The adrenomedullin receptors, AM1 and AM2, consist of CLR coupled with either RAMP2 or RAMP3, respectively. The calcitonin receptor (CTR) consists of only CTR. Amylin receptors are created by linking CTR with a RAMP; amylin AMY1-3 receptors consist of CTR plus RAMP1, 2, or 3, respectively. The amylin 1 receptor (CTR with RAMP1) also responds to CGRP.Reference Walker and Hay 32
CGRP is important in migraine pathophysiology. CGRP infusion triggers attacks of migraine that are indistinguishable from spontaneous attacks in migraineursReference Hansen, Hauge, Olesen and Ashina 33 ; triptans inhibit the release of CGRPReference Amrutkar, Ploug, Hay-Schmidt, Porreca, Olesen and Jansen-Olesen 34 ; migraine pain relief parallels the decline in circulating CGRP levelsReference Goadsby and Edvinsson 35 ; and its levels are increased in external jugular venous blood during an acute migraine attack.Reference Goadsby and Edvinsson 35 The most compelling pieces of evidence are the results of several trials that evaluated the selective CGRP receptor antagonists (gepants) for acute migraine treatment. Gepants are not vasoconstrictors and have minimal adverse events. Gepants block both the canonical CGRP receptor and the Amylin 1 receptor. Five gepants are effective for acute migraine treatment.Reference Ho, Ferrari and Dodick 36 – Reference Marcus, Goadsby, Dodick, Stock, Manos and Fischer 40 Olcegepant, while effective, could only be given intravenously and was abandoned.Reference Olesen, Diener and Husstedt 38 Olcegepant (2.5 mg IV) had a response rate of 66%, compared with 27% for placebo. Telcagepant (orally available) had 6 positive phase III trials.Reference Ho, Edvinsson and Goadsby 30 , Reference Ho, Dahlof and Silberstein 41 , Reference Tfelt-Hansen 42 AEs were similar to placebo, but it acted slower than triptans: 26% of subjects were pain free at 2 hours; placebo 11%; rizatriptan (10 mg) 41%; and almotriptan (12.5 mg) 35%.Reference Tfelt-Hansen 42 Telcagepant development has been stopped because of significant elevations in liver transaminase levels. Another gepant, MK-3207, was terminated because of asymptomatic liver enzyme abnormalities.Reference Hewitt, Aurora and Dodick 39 Boehringer Ingelheim compared BI 44370 TAReference Diener, Barbanti, Dahlof, Reuter, Habeck and Podhorna 37 (50, 200, and 400 mg) to eletriptan (40 mg) and placebo. More patients had pain freedom at 2 hours, which was significant in the 400 mg group (27.4 %) and the eletriptan group (34.8 %), compared to placebo. BMS-927711 (10, 25, 75, 150, 300, or 600 mg) was tested in a double-blind, placebo-controlled, dose-ranging studyReference Marcus, Goadsby, Dodick, Stock, Manos and Fischer 40 using an adaptive design in comparison to sumatriptan 100 mg or placebo. Significantly more patients in the BMS-927711 75 mg (31.4%, p < 0.002), 150 mg (32.9%, p < 0.001), and 300 mg (29.7%, p < 0.002) groups and the sumatriptan group (35%, p < 0.001) were free of pain at 2 hours compared to placebo (15.3%). No serious treatment-related AEs were reported, and no patients discontinued because of AEs. Biohaven has acquired BMS-927711 (now called rimegepant) for acute migraine and BHV-3500 for migraine prevention from Bristol-Myers Squibb Company.
Recently Allergan acquired 2 CGRP small molecule receptor antagonists from Merck: ubrogepant (MK-1602) for acute migraine treatment and atogepant (MK-8031) for migraine prevention. These different chemical entities are believed to not cause the liver AEs of prior gepants. Voss et al Reference Voss, Lipton and Dodick 43 studied ubrogepant for the acute migraine treatment in a Phase 2b randomized, double-blind, placebo-controlled trial. Ubrogepant (1 mg, 10 mg, 25 mg, 50 mg, 100 mg) was compared to placebo in a 1:1 ratio. Ubrogepant 100 mg was significantly superior to placebo for pain freedom (25.8% versus 8.9%), but not for headache response at 2 hours. Overall AEs were similar to placebo. Further trials are underway. In aggregate, these studies confirm that gepants are effective for acute migraine treatment and, like lasmiditan, lack vasoconstrictor activity.
Preventive Treatment
Principles
Recent guidelinesReference Silberstein 44 – Reference Carville, Padhi, Reason and Underwood 49 have established criteria for considering migraine preventive treatments and their efficacy (Table 3):
-
1. Disabling attacks despite appropriate acute treatment
-
2. Frequency (≥4 attacks or ≥8 headache days/month)
-
3. Acute treatment failure, overuse, or bothersome AEs
-
4. Patient choice
-
5. Hemiplegic migraine; basilar migraine; frequent, prolonged, or uncomfortable aura symptoms; or migrainous infarctionReference Silberstein 44 , Reference Lipton, Diamond, Freitag, Bigal, Stewart and Reed 45 , Reference Lipton, Bigal and Diamond 50
Table 3 Classification of migraine preventive therapies

Abbreviations: ACE=angiotensin-converting-enzyme; Ca++ blockers=calcium channel blockers; MRM=menstrually related migraine; SSNRI=selective serotonin–norepinephrine reuptake inhibitor; SSRI=selective serotonin reuptake inhibitor; TCA=tricyclic antidepressant.
A preventive treatment is successful when it decreases migraine by half.
General guidelines for instituting preventive therapy
-
∙ Start at a low dose and slowly increase until it is effective, the maximum dose is reached, or there are intolerable AEs.
-
∙ Consider comorbidity disorders (Table 1).Reference Silberstein, Holland, Freitag, Dodick, Argoff and Ashman 46 , Reference Olerud, Gustavsson and Furberg 51 – Reference Schoenen, Dodick and Sandor 58
-
∙ Do not use contraindicated drugs (coexistent or comorbid illnesses).
-
∙ Have an adequate trial (2–6 months).
-
∙ Have realistic goals.
-
∙ Periodically revaluate therapy.
-
∙ Women need to be aware of drug effects on a fetus.Reference Silberstein 59
-
∙ Involve patients; discuss their treatment and their expectations.
-
∙ Discuss AEs.
Monotherapy is a treatment goal but is often not attainable. Polytherapy may enable therapeutic adjustments based on the status of coexistent disorders.
New Preventive Medications
Small molecule CGRP antagonists (gepants)
Gepants block both the canonical CGRP receptor and the amylin 1 receptor. Ho et al Reference Ho, Connor and Zhang 60 evaluated telcagepant in a migraine preventive trial. The trial was terminated due to hepatotoxicity concerns. Telcagepant was effective, but the aminotransferase elevations led to its discontinuation. Allergan acquired the rights to 2 new CGRP small molecule receptor antagonists from Merck, including atogepant (MK-8031), for the prevention of migraines. Biohaven acquired 2 new gepants from Bristol-Myers Squibb Company including BHV-3500 for migraine prevention.
Monoclonal antibodies (mABs)
Monoclonal antibodies (mABs) to CGRP and its receptor now exist. How do they differ from small molecules? MABs function extracellularly, while small molecules function both extra- and intracellularly. They are more specific, do not affect QT intervals, and have limited, off-target toxicity. They do not cross the blood–brain barrier and have few central nervous system AEs. mABs are large molecules and cannot be administered orally. Their half-life is weeks, allowing for long-dosing intervals. They are not eliminated through the liver or kidneys. Humanized mABs contain 85% to >90% human protein. Fully human or human mABs contain both heavy and light chains from human origins.Reference Silberstein, Lenz and Xu 61
Three mABs that target CGRP, and one that targets the canonical CGRP receptor, are being developed. Lilly is developing Galcanezumab (LY2951742), a humanized monoclonal antibody against CGRP. Galcanezumab blocks capsaicin-induced increases in skin blood flow. Capsaicin stimulates dermal neurons to release CGRP, resulting in increased dermal blood flow. CGRP mAB receptor antagonist effects last for at least a week.Reference Zhu, Zhang and Zhou 62 The time to Cmax after subcultaneous (SC) ranges from 7 to 13 days, with a 28 days elimination half-life. A phase 2 study in episodic migraine has been completed. Patients (18–65) were randomly assigned (1:1) to galcanezumab (n=108) or placebo (n=110) (SC) every 2 weeks for 12 weeks. The primary endpoint (mean change migraine headache days/28-day period) was assessed at 9–12 weeks. The mean change in migraine headache days was –4·2 (SD 3.1; 62.5% decrease), with galcanezumab group compared with –3.0 (SD 3·0; 42.3% decrease) with placebo. AEs more frequent than placebo included injection site pain, erythema, upper respiratory tract infections, and abdominal pain. Galcanezumab thus is beneficial in migraine prevention and provides support for the role of CGRP in the pathogenesis of migraine.Reference Dodick, Goadsby, Spierings, Scherer, Sweeney and Grayzel 63 There are a number of ongoing trials:
-
∙ EVOLVE-2 study (NCT02614196): a phase 3, randomized, D-B, P-C study in episodic migraine
-
∙ REGAIN study (NCT02614261): a phase 3, randomized, D-B, P-C study in chronic migraine
-
∙ Studies in episodic (NCT02397473) and chronic (NCT02438826) cluster headache 64
Teva acquired fremanezumab (TEV-48125, formerly LBR-101) from Labrys. It is a humanized mAB against isoforms (α and β) of CGRP.Reference Bigal, Walter and Rapoport 65 In phase 1 studies, it was given to 94 subjects (0.2–2000 mg) once (day 1) intravenously (IV), or up to 300 mg given twice (day 1 and day 14). The drug was very well-tolerated, with about 1.4 treatment-emergent AEs compared to 1.3 on placebo. Overall treatment-related AEs occurred in 21.2% of the active group and in 17.7% of the placebo group.
Bigal et al Reference Bigal, Dodick and Rapoport 66 studied fremanezumab in the prevention of high-frequency episodic migraine (phase 2b). They randomly assigned patients to 3 28-day treatment cycles of subcutaneous (SC) fremanezumab (225 mg or 675 mg) or placebo. Migraine days decreased from baseline to weeks 9–12 by –3.46 days in the placebo group, compared to –6.27 days for 225 mg, and –6.09 days for the 675 mg group. In this trial, fremanezumab was safe, well-tolerated, and effective as a preventive treatment of high-frequency episodic migraine.Reference Bigal, Dodick and Rapoport 66 Bigal et al then studied 2 doses of fremanezumab in chronic migraine prevention. They randomly assigned patients to 3 28-day treatment cycles of SC fremanezumab (675 mg in the first and 225 mg in the second and third treatment cycles), fremanezumab (900 mg each treatment cycle), or placebo. The mean decrease from baseline in headache-hours during weeks 9–12 was –59.84 hours in the 675/225 mg group, –67.51 hours in the 900 mg group, compared to –37.10 hours for placebo. Most AEs were mild (injection-site pain and pruritus). Fremanezumab SC was tolerable and effective.Reference Bigal, Edvinsson and Rapoport 67
Trials underway include the following:
-
∙ A multicenter, randomized, double-blind, placebo-controlled, parallel-group study comparing the efficacy and safety of 2 dose regimens for episodic migraine prevention (NCT02629861)
-
∙ A second study for chronic migraine prevention (NCT02621931)
-
∙ A randomized, double-blind, placebo-controlled, parallel-group study of 2 dose regimens for episodic cluster headache prevention (NCT02945046)
-
∙ A second study for chronic cluster headache (NCT02964338) 64
Alder developed eptinezumab (ALD403) a desialylated, humanized IgG1 mAB that binds to both α and β forms of human CGRP. The plasma elimination after T ½ is 31 days. Dodick et al Reference Dodick, Goadsby and Silberstein 68 studied eptinezumab for migraine prevention in a phase 2 trial. Patients (18–55) who had 5 to 14 migraine days/28-days were randomized to IV eptinezumab 1000 mg or placebo. Safety was assessed 12 weeks later. The primary endpoint was the change from baseline to weeks 5–8 in migraine day frequency. AEs occurred in 57% of patients in the eptinezumab group and 52% in the placebo group. The mean change in migraine days was –5.6 (SD 3.0) for the eptinezumab group compared with –4.6 (3.6) for placebo (difference –1·0, 95% CI –2·0 to 0·1; one-sided p=0·0306). No safety issues were found. In this study, eptinezumab was effective in high-frequency, episodic migraine prevention.
Dodick et al Reference Dodick, Silberstein and Lipton 69 studied single IV infusions of eptinezumab 300 mg, 100 mg, 30 mg, and 10 mg versus placebo in the prevention of chronic migraine. Subjects were randomized, and received either a single infusion of eptinezumab 300 mg, 100 mg, 30 mg, 10 mg, or placebo by a 1-hour IV infusion. The study met the primary 12-week post-infusion efficacy endpoint: % difference in patients achieving a 75% reduction in migraine days from baseline: eptinezumab 300 mg (33%) and 100 mg (31%) versus placebo (21%) (weeks 1–12). Significantly more patients had a 50% reduction in migraine days from baseline for eptinezumab 300 mg, 100 mg, and 30 mg versus placebo (weeks 1–12). Eptinezumab was safe, well-tolerated, and met the primary endpoint of ≥ 75% reduction in migraine days compared to placebo.
Erenumab (AMG 334), developed by Amgen, is a human mAB of the IgG2 subtype against the canonical CGRP receptor, not CGRP. The CGRP receptor is a G protein-coupled receptor that is composed of the calcitonin receptor-like receptor and receptor activity-modifying protein 1 (RAMP1) subunits. Erenumab has high affinity (Kd 20 pM) competitively and reversible receptor binding. Its estimated elimination T ½ is 21 days.
Sun et al Reference Sun, Dodick and Silberstein 70 studied erenumab in migraine prevention in a double-blind, placebo-controlled, phase 2 trial. Patients (18–60) who had 4 to 14 migraine days per month were randomized (3:2:2:2 ratio) to monthly SC placebo, erenumab 7 mg, 21 mg, or 70 mg. The primary endpoint, the mean change in monthly migraine days (baseline to the last 4 weeks of the 12-week, double-blind treatment phase), was –3.4 days with erenumab 70 mg versus –2.3 days with placebo (difference –1.1 days, p=0.021). The mean reductions in monthly migraine days with the 7 mg (–2.2) and the 21 mg (–2.4) doses were not significantly different from placebo. AEs occurred in 54% of those who received placebo compared to 50% to 54% in the erenumab groups. Erenumab 70 mg is a potential therapy for prevention of episodic migraine. Two trials are underway for rrenumab in episodic migraine prevention: ARISE (NCT02483585) and STRIVE (NCT02456740). 64
Tepper et al Reference Tepper, Ashina and Reuter 71 studied erenumab in subjects with chronic migraine (18–65 years). Patients received erenumab (70 mg or 140 mg) or placebo every month by SC injection. The primary endpoint was the change from baseline in monthly migraine days. The secondary endpoints were the proportion of patients with ≥ 50% reduction in monthly migraine days, change from baseline in acute migraine-specific medication use days, and change from baseline in cumulative headache hours. Both erenumab doses [70 mg (–6.64) and 140 mg (16.63)] had statistically significant clinically meaningful reduction in monthly migraine days compared with placebo (–4.8). Erenumab 70 mg (39.9%) and 140 mg (41.1%) also showed statistically significant improvements in ≥ 50% responder rate compared to placebo (23.5%). Safety and tolerability were like placebo. One trial is underway for erenumab in chronic migraine prevention (NCT20120295). 64
Orexin receptor antagonists (rexants)
The orexins (A and B) are a pair of hypothalamic neuropeptides that may be involved in nociception. Both neuropeptides are cleaved from the same precursor, preproorexin, and act on 2 G-coupled receptors termed the orexin 1 (OX1R) and 2 (OX2R) receptors. Orexin A shows equal efficacy for both receptors, while orexin B is relatively selective for the OX2R. Filorexant, a dual orexin receptor antagonist, was not effective in a phase 2a trial for migraine prevention.Reference Chabi, Zhang and Jackson 72 However, preclinical evidence suggests that orexin A is anti-nociceptive, whereas orexin B was pro-nociceptive. Perhaps antagonism of the OX2R or agonism of the OX1R may be beneficial.Reference Bartsch, Levy, Knight and Goadsby 73
Nitric oxide synthase inhibitors (NOS)
NOS produces nitric oxide triggering CGRP release. NOS inhibitors (neuronal and inducible) were not effective for acute or preventive migraine treatment.Reference Hoivik, Laurijssens and Harnisch 74 , Reference Palmer, Guillard, Laurijssens, Wentz, Dixon and Williams 75
Botulinum toxin (BoNT) for migraine
Seven BoNT serotypes (A, B, C1, D, E, F, and G) are produced by Clostridium botulinum. All inhibit acetylcholine release, but they differ in targets, characteristics, and potencies.Reference Aoki and Guyer 76 , Reference Mauskop 77 Botulinum toxin type A (BoNTA) is the most commonly studied serotype.Reference Aoki and Guyer 76 BoNT is available as onabotulinumtoxinA (botulinum toxin type A), abobotulinumtoxinA (another type A), and BoNTB (rimabotulinumtoxinB).
BoNT is approved for chronic migraine prevention and may be effective in high-frequency episodic migraine. The mechanism of action of BoNT in headache is still uncertain.
Conclusion
Pharmacologic treatment is a cornerstone of migraine management. Preventive medication can not only reduce attack frequency, but can also improve acute treatment response and quality of life. Many migraine patients need prevention, but few get it. Many preventive medications are available, and guidelines for their selection and use have been established. Comorbid medical and psychological illnesses must be considered when choosing preventive drugs,Reference Palmer, Guillard, Laurijssens, Wentz, Dixon and Williams 75 but there are no characteristics predictive of response to acute or preventive treatment.
Optional Posttest and CME Certificate
CME Credit Expires: November 30, 2020
Posttest Study Guide
NOTE: The posttest can only be submitted online. The below posttest questions have been provided solely as a study tool to prepare for your online submission. Faxed/mailed copies of the posttest cannot be processed and will be returned to the sender. If you do not have access to a computer, contact NEI customer service at 888-535-5600.
-
1. According to treatment guidelines, ergotamine is considered:
-
A. Effective (Level A)
-
B. Probably effective (Level B)
-
C. Possibly effective (Level C)
-
D. Unknown (Level U)
-
-
2. Lasmiditan, in Phase 3 trials for the treatment of acute migraine, acts at what receptors?
-
A. 5HT1B
-
B. 5HT1F
-
C. 5HT2C
-
D. 5HT3
-
-
3. Preventive treatment for migraine is considered successful when it reduces migraine by:
-
A. One third
-
B. One half
-
C. Two thirds
-
D. Three fourths
-
-
4. Monoclonal antibodies (mABs) to CGRP and its receptors function:
-
A. Extracellularly
-
B. Intracellularly
-
C. Both extra- and intracellularly
-
Optional Online Posttest and CME Certificate Instructions
There is no posttest fee nor fee for CME credits.
-
1. Read the article.
-
2. Complete the posttest and evaluation, available only online at www.neiglobal.com/CME (under “CNS Spectrums”).
-
3. Print your certificate (passing score = 70% or higher).
Questions? call 888-535-5600, or email CustomerService@neiglobal.com





