The texture of yogurt is dependent on the heating process of raw milk, and milk subjected to high temperatures forms hard curd. The mechanism underlying this phenomenon has been shown in several studies (Cobos et al. Reference Cobos, Horne and Muir1995; Lucey and Singh, Reference Lucey and Singh1997; Lucey et al. Reference Lucey, Munro and Singh1998). When milk is heated at 80 °C or higher, the β-lactoglobulin (β-Lg) of whey binds to κ-casein on the surface of the casein micelles via disulphide (S–S) bonds, and then interacts with α-lactalbumin (α-La) (Oldfield et al. Reference Oldfield, Singh and Taylor1998). Therefore, both whey proteins form complexes with casein micelles (Singh & Creamer, Reference Singh and Creamer1991; Corredig & Dalgleish, Reference Corredig and Dalgleish1996; Jovanović et al. Reference Jovanović, Barać, Maćej and Djurdjević2005), thus increasing the casein micellar size (Anema & Li, Reference Anema and Li2003a, Reference Anema and Lib). Furthermore, this promotes the mutual association of casein micelles (Ono et al. Reference Ono, Yoshida, Tanaami and Ohkosi1999), resulting in the formation of firm curd (Cayot et al. Reference Cayot, Fairise, Colas, Lorient and Brulé2003; Guyomarc'h et al. Reference Guyomarc'h, Queguiner, Law, Horne and Dalgleish2003b). On the other hand, it has been reported that following S–S bond formation between β-Lg and κ-casein, whey protein/κ-casein complexes dissociate from the casein micelles (Guyomarc'h et al. Reference Guyomarc'h, Law and Dalgleish2003a, Reference Guyomarc'h, Renan, Chatriot, Gamerre and Famelart2007; Vasbinder et al. Reference Vasbinder, Alting and de Kruif2003). Moreover, the physical and chemical properties of the whey protein/κ-casein complexes have been investigated (Jean et al. Reference Jean, Renan, Famelart and Guyomarc'h2006; Guyomarc'h et al. Reference Guyomarc'h, Violleau, Surel and Famelart2010), and the interaction between the complexes and κ-casein-dissociated micelles has been reported to increase the acid coagulability (Donato et al. Reference Donato, Alexander and Dalgleish2007; Morand et al. Reference Morand, Guyomarc'h and Famelart2011). Furthermore, another previous study had examined the relationship between κ-casein dissociation and pH during heating (Anema, Reference Anema2007) and the influence of κ-casein dissociation on the acid milk gel (Anema and Li, Reference Anema and Li2015). However, regarding the acid coagulability, those reports mention only whey protein/κ-casein complexes and κ-casein dissociation.
Among caseins, κ-caseins are the only glycoproteins; they are negatively charged and are located on the surface of micelles, providing the electric charge that allows the micelles to stably exist in milk despite their ultrahigh molecular weight (Tuinier & De Kruif, Reference Tuinier and De Kruif2002).
In addition, a previous study had reported that the acid gelation of milk increased with the degree of hydrolysis of κ-casein by chymosin reaction (Gastaldi et al. Reference Gastaldi, Triak, Guillaume, Gontard and Cuq2003).
Therefore, it was inferred that κ-casein dissociated micelles affected the acid coagulability, because casein micelles are destabilised by dissociation of κ-casein from the micelles as a result of S–S bond formation with the whey proteins. However, all these previous studies focused on the influence of the whey protein/κ-casein complexes, the details of the relationship between κ-casein dissociated casein micelles and acid milk gel strength are still not completely understood.
In this study, the effect of casein micelles with heat-induced κ-casein-dissociation on the acid coagulability of milk was investigated.
Materials and methods
Preparation of defatted milk
Fresh raw milk was obtained from Holstein cows bred in the Fuji farm (Fujinomiya city, Shizuoka pref.) of Tokyo University of Agriculture. Fresh raw milk was heated to 40 °C, and milk fat was separated and removed at the same temperature using a cream separator to obtain native defatted milk.
N-acetylneuraminic acid and sodium 8-anilino-1-naphthalensulfonate (ANS) were purchased from Sigma Aldrich Inc., St. Louis, USA. Other chemicals were of analytical grade and were purchased from Kanto Chemical Co., Inc., Tokyo, Japan.
Preparation of casein micelles which κ-casein dissociated
In order to prepare casein micelles which κ-casein dissociated, casein micelles and β-Lg (essential for the dissociation of κ-casein) were fractionated. Native defatted milk was ultracentrifuged (33 000 g , 20 °C, 45 min) to obtain a precipitate, which was then washed with pure water to remove whey, thereby obtaining the casein micelle fraction (Fairise et al. Reference Fairise, Cayot and Lorient1999; Huppertz et al. Reference Huppertz, Fox and Kelly2004). The supernatant was treated by the method of Aschaffeburg & Drewry (Reference Aschaffeburg and Drewry1957), to obtain the β-Lg fraction. Both proteins were mixed with 20 mm phosphate buffer containing 2 mm calcium chloride (pH 6·8), to prepare to the protein concentrations equivalent to those in native defatted milk. The mixture was heated at 80 °C for 30 min to allow S–S bond formation between β-Lg and κ-casein. the κ-casein-dissociated casein micelles were then separated from the heated mixtures by gel filtration chromatography.
Gel filtration chromatography (GFC)
The unheated and heated mixtures were chromatographed at a flow rate of 0·2 ml/min using a Sephacryl S-500 HR column (GE Healthcare, Buckinghamshire, England, UK) (ϕ 1·2 cm × 27 cm) equilibrated with the buffer solution. Aliquots of 1·5 ml of the resultant eluate were sampled for protein determination by the Bradford method (Bradford, Reference Bradford1976).
Two-dimensional polyacrylamide gel electrophoresis (2D-PAGE)
The fractions obtained by gel filtration chromatography were subjected to 2D-PAGE (Blue Native-PAGE/SDS-PAGE) to confirm that κ-casein-dissociated micelles and β-Lg/κ-casein complexes had been fractionated. Blue Native-PAGE was performed according to the method described by Schagger & Von Jagow (Reference Schagger and Von Jagow1991) and Schagger et al. (Reference Schagger, Cramer and Vonjagow1994), using Native-PAGE Bis-Tris Gel System (3–12%) (Invitrogen, Waltham, MA, USA) as the migration gel and Native Mark Unstained Protein Standard (Invitrogen, Waltham, MA, USA) as the marker. After electrophoresis, the proteins were stained with Coomassie Brilliant Blue. The lanes containing the GFC peaks were cut out and treated with a reducing solution (SDS, 2-mercaptoethanol, and bromophenol blue) overnight for the reduction of the S–S bonds. Next, the gels obtained were loaded at the top of a stacking gel for two dimensions and fixed with agarose for a second separation by SDS-PAGE. Protein detection was performed with SYPRO® Ruby (Lonza, Rockland, ME, USA) stain (Berggren et al. Reference Berggren, Chernokalskaya, Steinberg, Kemper, Lopez, Diwu, Haugland and Patton2000) using Precision Plus Protein Standard Unstained (Bio-Rad Laboratories, Hercules, CA, USA) as the marker. The image analysis was conducted by analysing the images obtained by Chemi Doc (Bio-Rad Laboratories, Hercules, CA, USA).
Measurement of the zeta-potential and the particle size of casein micelles
In order to evaluate the electric charge on the surface of the casein micelles, the zeta potential of micelles at high salt concentrations was measured. The sample was adjusted to a protein content of 1·0 mg/ml with 20 mm phosphate buffer (containing 0·2 m sodium chloride, pH 6·8), and then analysed by the zeta potential analyser Mobius (Wyatt Technology, Santa Barbara, CA, USA) coupled with the pressuriser Atlas (Wyatt Technology, Santa Barbara, CA, USA). At the same time, the average particle radius was measured by dynamic light scattering (DLS), and the obtained data was analysed by the cumulant method. The zeta potential measurement was carried out before and after pressurisation to 3 × 106 Pa, applying a voltage of 2·5 V at a frequency of 10 Hz for 15 s. The measurement was repeated 10 times. The DLS measurement was performed for 5 s at 25 °C and repeated 3 times.
Determination of sialic acid in κ-casein
The amount of sialic acid in κ-casein was determined by the fluorimetric method (Matsuno & Suzuki, Reference Matsuno and Suzuki2008; Rao et al. Reference Rao, Sharama and Rajput2012). All the regent solutions were precooled in an ice bath before use. Twenty microliters of 10 mm periodic acid sodium was added to 200 µl of casein solution, the concentration of which was adjusted to 0·2 mg/ml. The solution was chilled in the ice bath for 45 min and then 100 µl of 50 mm sodium thiosulphate was added to terminate the reaction. To the reaction mixture were added 500 µl of 4·0 m ammonium acetate (pH 7·5) and 400 µl of 100 mm acetoacetanilide (including 50% ethanol). The mixture was then left standing for 10 min at room temperature. The fluorescence intensity of the solution was measured at 471 nm with an excitation wavelength of 388 nm (RF-5000; Shimadzu, Kyoto, Japan).The amount of sialic acid per milligram of protein was calculated based on the standard curve obtained from the fluorescence intensity of the control solution, which was N-acetylneuraminic acid.
Measurement of casein micellar surface hydrophobicity
Casein micellar surface hydrophobicity was determined with the method described by Hayakawa & Nakai (Reference Hayakawa and Nakai1985). Forty microliters of 2 mm ANS solution (20 mm phosphate buffer) was added to 2·0 ml of casein solution, the concentration of which was adjusted to 0·1 mg/ml, and then the solution was reacted in a dark place for 30 min. The fluorescence intensity of the solution was measured at 480 nm with an excitation wavelength of 380 nm (RF-5000; Shimadzu, Kyoto, Japan). Intensity of fluorescence per milligram of protein was indicated as the degree of surface hydrophobicity (F.I./mg protein).
Evaluation of the isoelectric point of casein micelles
The pH value of the casein solution was first adjusted to 6·8; lactic acid (1 m) was then gradually added to the casein solution to progressively lower its pH value. The casein solutions at the different pH values were centrifuged (1000 g, 20 °C, 30 min) to obtain the precipitation and the supernatant. The isoelectric point of casein micelles was calculated from the amount of protein in the supernatant. All values were calculated in comparison to the amount of protein in the supernatant of pH 6·8 (100%) of lactic acid non-addition.
Results and discussion
Fractionation and analysis of casein micelles and β-Lg/κ-casein complexes
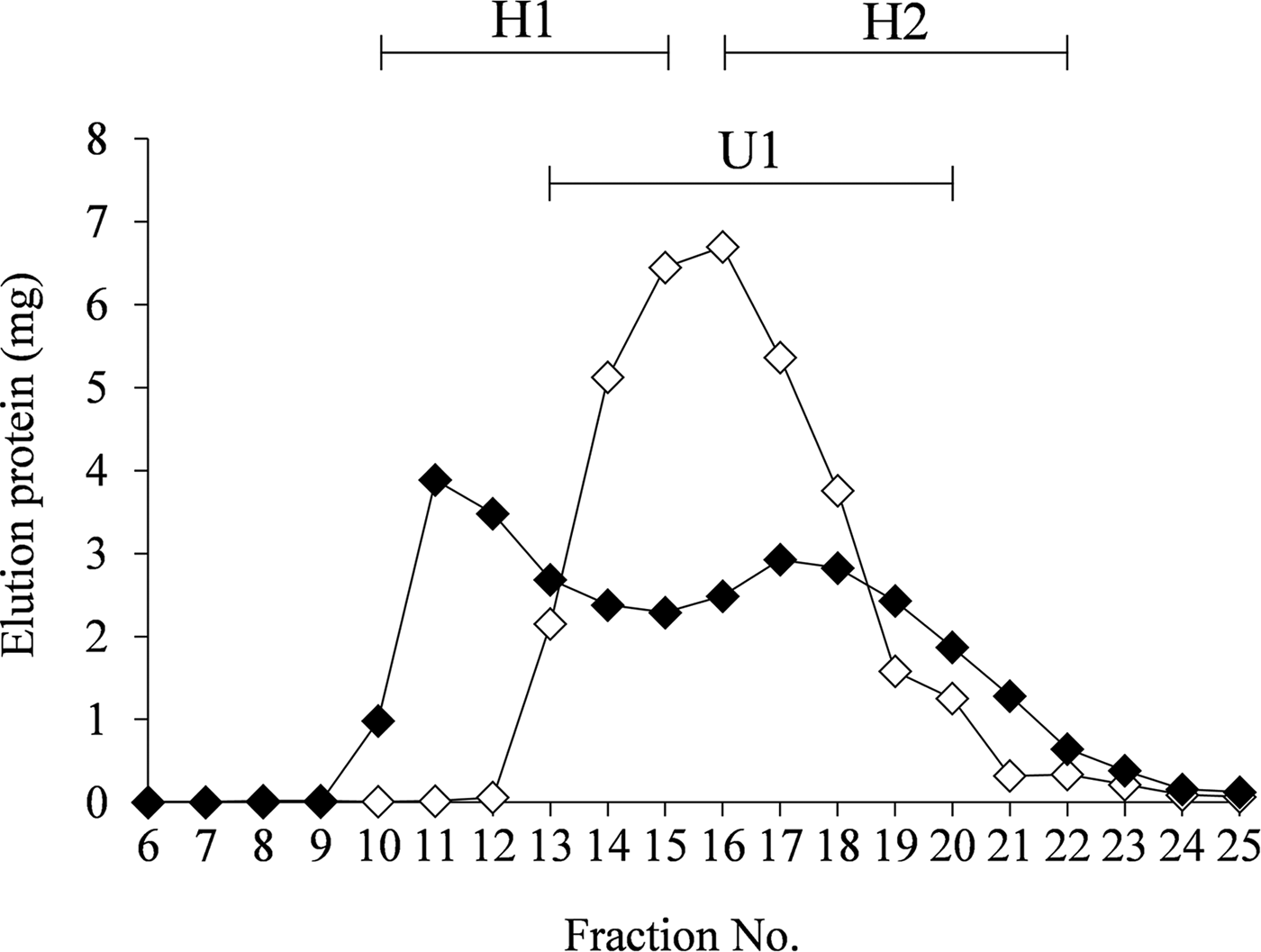
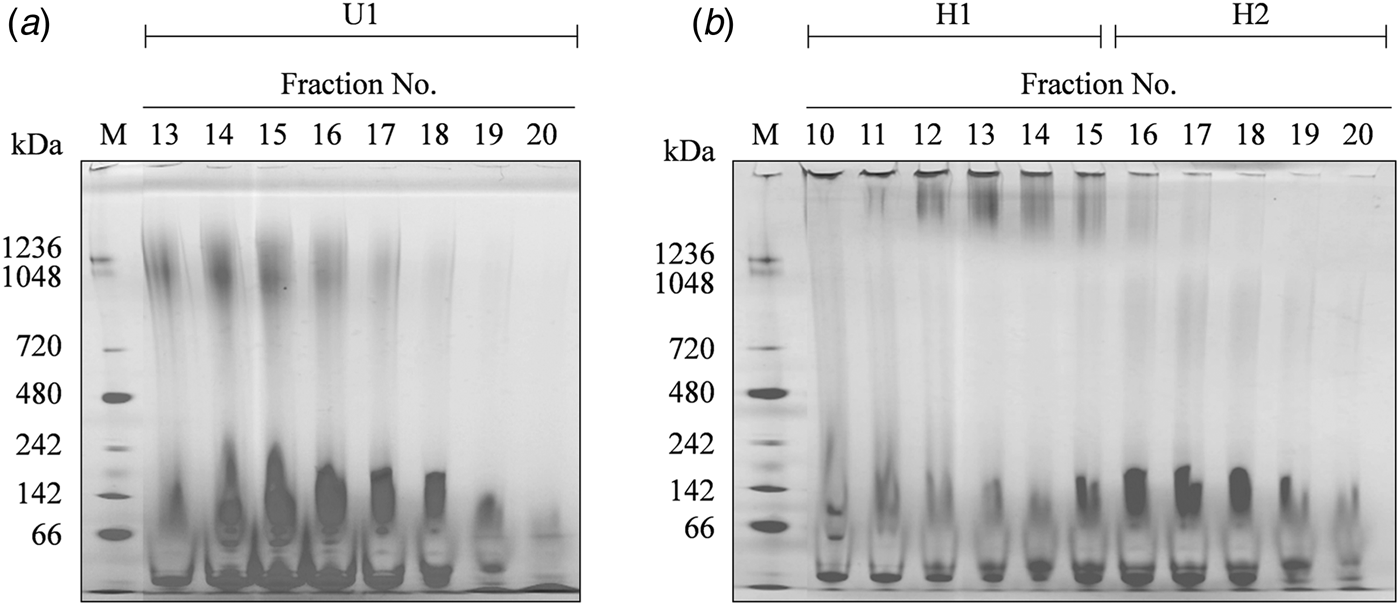
Casein micelles and β-Lg were mixed according to their relative ratios in unmodified defatted milk, and the unheated and heated (80 °C, 30 min) mixtures were subjected to gel filtration chromatography. The chromatographic patterns of both the samples were conspicuously different (Fig. 1). For the unheated sample, a large peak (U1) was obtained in fractions 13–20, whereas two peaks were obtained in fractions 10–15 (H1) and 16–22 (H2) for the heated sample. The fractions were analysed by Blue Native-PAGE (Fig. 2). A band at approximately 1200 kDa was observed for the U1 peak fraction of the unheated sample. On the other hand, for the heated sample, a band was detected at the top of the gel for the H1 fraction, and a weak smear band at approximately 900 kDa was observed for the H2 fraction.

Fig. 1. Elution profiles from gel filtration chromatography of the unheated (◇) and heated (◆) (80 °C, 30 min) mixtures of casein micelles and β-lactoglobulin. The protein concentrations in the mixtures were equivalent to those in the native defatted milk. The samples were then chromatographed using a Sephacryl S-500 column (GE Healthcare Japan, ϕ 1·2 cm × 27 cm). U1, peak fraction (13–20) of the unheated sample; H1, first peak fraction (10–15) of the heated sample; H2, second peak fraction (16–22) of the heated sample.

Fig. 2. Blue Native-PAGE (gradient gel: 3–12%) of the fractions obtained from gel filtration chromatography of the unheated (a) and heated (b) samples. U1, peak fraction (13–20) of the unheated sample; H1, first peak fraction (10–15) of the heated sample; H2, second peak fraction (16–22) of the heated sample; M, standard marker.
Next, the gel with maximum GFC peak fraction was cut out and isolated by SDS-PAGE after S–S bonds reduction by 2-mercaptoethanol. The bands corresponding to α-, β-, and κ-casein were detected in the profile of the approximately 1200 kDa band of fraction 15 of the U1 peak (Fig. 3). Unlike the theoretical ratio of casein, the κ-casein band was more strongly detected than α- or β-casein. SYPRO® Ruby, which was used as the detection reagent, is highly sensitive; however, the degree of dyeing varies according to the kind of the protein (Nock et al. Reference Nock, Ball, White, Skehel, Bill and Karuso2008). Moreover, the κ-casein band was more strongly detected in comparison with other caseins, when PAGE of the native defatted milk was dyed. Thus, it was suggested that the peak of U1 was a casein micelle. On the other hand, SDS-PAGE of fraction 12 of peak H1 showed the β-Lg and κ-casein bands, derived from the ultrahigh molecular weight proteins located at the top of the Blue Native-PAGE gel. Moreover, α-, β-, κ-casein and β-Lg were detected in the 900 kDa band of fraction 17 of peak H2; however, the intensity of the κ-casein band was found to be lower than that in the fraction 15 of peak U1. In addition, the casein bands of H2 were shifted toward the low molecular weight region as compared with those of U1, indicating that the micelles were smaller. These observations suggest that peak H1 is due to β-Lg/κ-casein complexes whereas peak H2 corresponds to casein micelles from which κ-casein is partially dissociated. Thus, U1 was defined as the native casein micelles and H2 as the κ-casein-dissociated micelles.

Fig. 3. 2D-PAGE (Blue-Native-PAGE/SDS-PAGE) of the fractions obtained from gel filtration chromatography of the unheated (a) and heated (b) samples. The first dimension gel, after analysis by Blue-Native-PAGE, was analysed by SDS-PAGE (15%). U1-15, maximum peak fraction (no. 15) of the unheated sample; H2-12, maximum peak fraction (no. 12) of the first peak of the heated sample; H2-17, maximum peak fraction (no. 17) of the second peak of the heated sample; M, standard marker; HLM, high molecular weight; LMT, low molecular weight.
The fractionated casein micelles and κ-casein-dissociated micelles were used in the following analyses.
The particle size and the zeta potential of casein micelles
To confirm the decrease in molecular weight of casein micelles due to heat treatment of milk, the average particle radius of casein micelles was measured by DLS. As shown in Table 1, the radius of native casein micelles was 138·6 ± 1·8 µm, and that of κ-casein-dissociated micelles was 121·4 ± 2·6 µm; thus, the casein micelle size decreased because of the dissociation of κ-casein.
Table 1. Characteristics of casein micelles in the fractions obtained from gel filtration chromatography

U1, native casein micelles; H2, κ-casein dissociated micelles. The zeta potential and average radius ware analysed using the zeta potential analyser Mobius (Wyatt Technology Corp.). The amount of sialic acid was measured using the fluorometric method described by Matsuno & Suzuki (Reference Matsuno and Suzuki2008) and Rao et al. (Reference Rao, Sharama and Rajput2012). The surface hydrophobicity was measured according to the method described by Hayakawa & Nakai (Reference Hayakawa and Nakai1985). Each value is the mean ± sd of three experiments. Asterisks indicate values significantly different (P < 0·05) from those of U1. Each value is the mean ± sd of three experiments. Asterisks indicate values significantly different (P < 0·05) from those of U1.
Moreover, the surface charge of the micelles, which affects the acidic coagulation of casein, was evaluated by measuring the zeta potential. The zeta potential value of native casein micelles was −19·43 ± 1·29 mV, and that of κ-casein-dissociated micelles was −16·02 ± 1·44 mV, demonstrating that the dissociation of κ-casein decreased the negative charge of micelles by approximately 18% (Table 1).
Therefore, the dissociation of κ-casein decreased the casein micellar negative charges. This decrease was suggested to inhibit the repulsion between micelles.
Sialic acid amount of casein micelles and surface hydrophobicity
The amount of sialic acid in κ-casein, which affects the electric charge of the casein micelles, was measured. The amount of sialic acid was 15·25 ± 1·06 µg/mg proteins in the native casein micelles and 12·71 ± 0·37 µg/mg proteins in the κ-casein-dissociated micelles (Table 1). Thus, the sialic acid content of casein micelles decreased by approximately 17% when κ-casein dissociated from the micelles. It has been reported that acid-milk gelation ability increases because of the increase in micellar surface hydrophobicity when neuraminidase degraded N-acetyl neuraminic acid of κ-casein (Cases et al. Reference Cases, Vidal and Cuq2003). Therefore, the degree of casein micellar surface hydrophobicity was measured. The surface hydrophobicity of native and κ-casein-dissociated casein micelles were 15·13 ± 0·48 F.I./mg and 16·83 ± 0·38 F.I./mg respectively. Thus, an increase of 10% was observed when κ-casein was dissociated from casein micelles It was suggested that the strength of acid milk gel increased when the hydrophobic interaction between the casein micelle increased because of the decrease in repulsion between them.
Comparison of the casein micellar isoelectric point
The isoelectric point of κ-casein dissociated casein micelles was evaluated to examine the influence of κ-casein dissociation on acid coagulability. The native casein micelles started precipitation at around pH 5·1, and were completely precipitated at pH 4·6. The κ-casein-dissociated casein micelles began to precipitate from around pH 5·3, and were completely precipitated at pH 5·0 (Fig. 4). Thus, the casein micellar isoelectric points increased after κ-casein dissociation; this was likely because the κ-casein-dissociated micelles were acid-coagulated at higher pH values. These results suggested that hard acid milk gel formation by heat treatment was induced by the increased micellar surface hydrophobicity and the decreased micellar surface electric charge. Thus, the κ-casein dissociated casein micelles directly affected the acid milk gel formation.
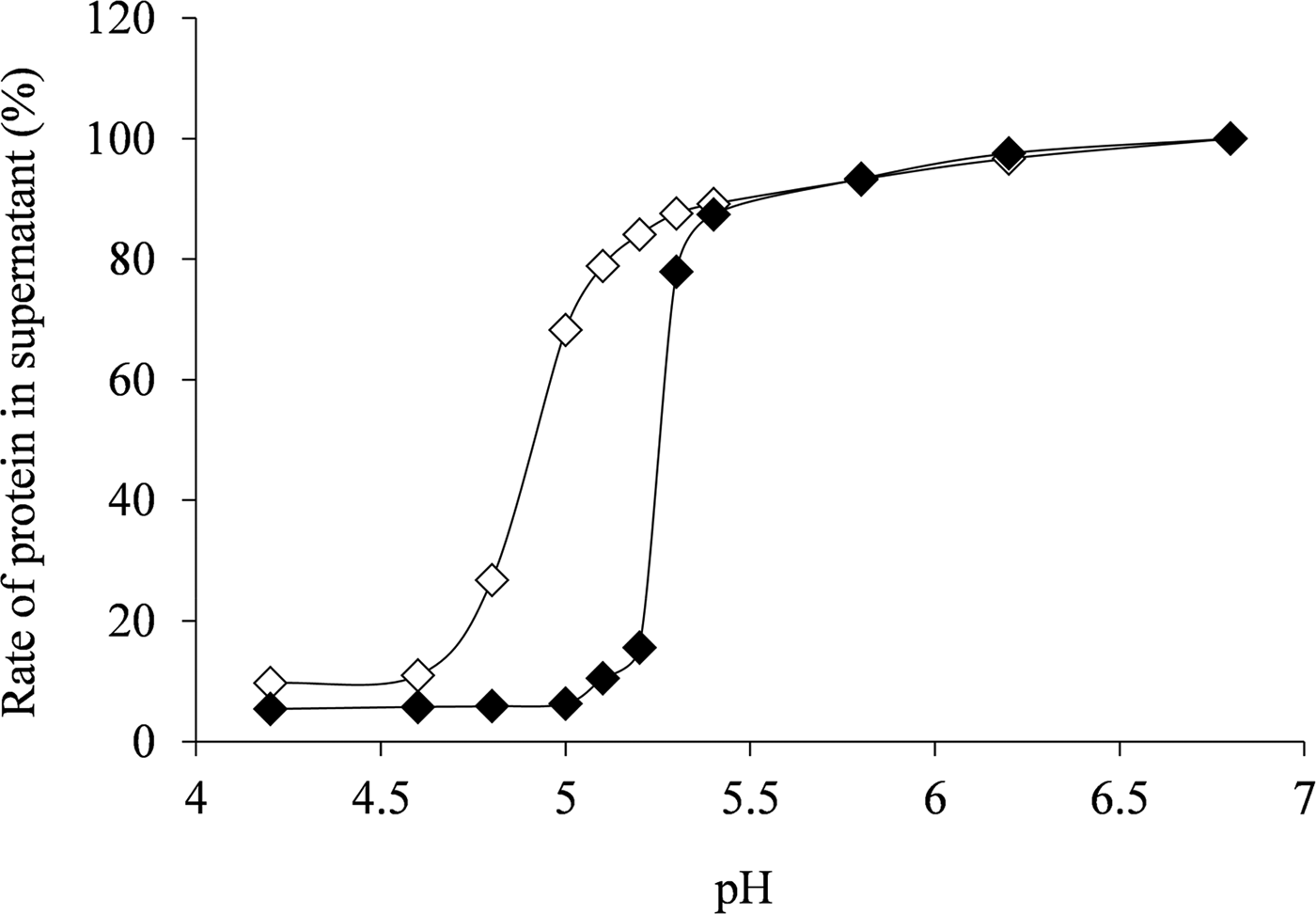
Fig. 4. Changes in supernatant proteins based on isoelectric point of native casein micelles (◇) and κ-casein-dissociated casein micelles (◆). All values were calculated in comparison to the amount of protein (100%) in the supernatant of lactic acid non-addition (pH 6·8).
Conclusions
This study evaluated the effect of κ-casein dissociation from micelles due to heat treatment of milk on the strength of yogurt curd. The sialic acid content and the negative charge of κ-casein-dissociated micelles were lower than those of native micelles. Furthermore, it was suggested that the hydrophobic interaction between micelles increased because of an increase in the micellar surface hydrophobicity, causing the micelles to cohere at the early stages of acidification, due to an increase in the isoelectric point. These results suggested that acid milk gel formation was induced by κ-casein dissociated casein micelles, which caused an increase in the hydrophobic interaction between the micelles and a decrease in the negative charges of the carbohydrate chains of κ-casein.
To our knowledge, this is the first report demonstrating the important effect of the structural properties of κ-casein-dissociated micelles on milk acid gel formation, thus providing useful information to improve the quality of dairy products. The authors wish to acknowledge Dr T. Tokai and K. Kurono, Shoko Scientific Co., Ltd., for measuring the zeta potential of casein micelles using Mobius (Wyatt).