Introduction
One fundamental and common dogma in ecology is based on the competitive exclusion principle (Hardin, Reference Hardin1960): species exploiting very similar niches cannot share it for an extended period of time. In such a way, it predicts that the more efficient species will ‘competitively exclude’ the less efficient species. Hence, interspecific competition has received considerable interest especially in understanding how it contributes to community assemblages although its role is controversial (Wiens, Reference Wiens1977; Connell, Reference Connell1983; Schoener, Reference Schoener1983; Goldberg & Barton, Reference Goldberg and Barton1992; Gurevitch et al., Reference Gurevitch, Morrow, Wallace and Walsh1992; Niemelä, Reference Niemelä1993). Basically, interspecific competition is divided in two mechanisms: exploitation and interference (Reitz & Trumble, Reference Reitz and Trumble2002; Duyck et al., Reference Duyck, David and Quilici2004). Exploitation occurs when individuals from different species exploit the same resource. In this case, individuals do not compete directly contrarily to interference in which individuals compete by direct contact. To reduce interspecific competition, the less competitive individuals may avoid their higher competitors temporally or spatially for example (Razgour et al., Reference Razgour, Korine and Saltz2011; Ronconi & Burger, Reference Ronconi and Burger2011). In the most severe cases, interspecific competition may however conduct to the complete displacement (i.e., exclusion) of the less competitive species from the ecological niche (Reitz & Trumble, Reference Reitz and Trumble2002).
According to Reitz & Trumble (Reference Reitz and Trumble2002), 78% of the displacements of insect and arachnid species are due to the introduction of alien species and biological invasions are known to be one of the major threats to biodiversity (Vitousek et al., Reference Vitousek, Mooney, Lubchenco and Melillo1997; Chapin et al., Reference Chapin, Zavaleta, Eviner, Naylor, Vitousek, Reynolds, Hooper, Lavorel, Sala, Hobbie, Mack and Diaz2000; Clavero et al., Reference Clavero, Brotons, Pons and Sol2009). Especially, alien predators may impact the ecosystem at least at two levels: in preying upon native prey species and in interfering with native predators (Zaret & Paine, Reference Zaret and Paine1973; Bourdeau et al., Reference Bourdeau, Pangle and Peacor2011; Snyder & Evans, Reference Snyder and Evans2006). Competition represents an important threat for native species sometimes leading to their decline although its effect are less often acknowledged than the effect of predation on native prey species. An example of such effect is provided by the proliferation of the invasive Asian ladybird Harmonia axyridis, which threatens native European ladybird species (Brown et al., Reference Brown, Frost, Doberski, Sparks, Harrington and Roy2011; Roy et al., Reference Roy, Adriaens, Isaac, Kenis, Onkelinx, San Martin, Brown, Hautier, Poland, Roy, Comont, Eschen, Frost, Zindel, Van Vlaendersen, Nedvĕd, Ravn, Grégoire, de Biseau and Maes2012; Katsanis et al., Reference Katsanis, Babendreier, Nentwig and Kenis2013).
In Western Europe, a single species of hornet occurred, the European hornet (Vespa crabro), until the accidental introduction of the yellow-legged hornet (Vespa velutina) from eastern China (Monceau et al., Reference Monceau, Bonnard and Thiéry2014a ). Observed for the first time in France in 2004, V. velutina extended its range to several European countries (see Ibáñez-Justicia & Loomans, Reference Ibáñez-Justicia and Loomans2011; Villemant et al., Reference Villemant, Barbet-Massin, Perrard, Muller, Gargominy, Jiguet and Rome2011). Like other Vespinae, V. crabro and V. velutina prey on a wide range of arthropods (Spradbery, Reference Spradbery1973; Edwards, Reference Edwards1980; Matsuura & Yamane, Reference Matsuura and Yamane1990). Especially, they are well known by beekeepers to attack honeybees in apiaries (Matsuura & Yamane, Reference Matsuura and Yamane1990; Baracchi et al., Reference Baracchi, Cusseau, Pradella and Turillazzi2010; Monceau et al., Reference Monceau, Maher, Bonnard and Thiéry2013a , Reference Monceau, Arca, Leprêtre, Mougel, Bonnard, Silvain, Maher, Arnold and Thiéry b , Reference Monceau, Bonnard, Moreau and Thiéry2014b ). Indeed, beehives represent a valuable food source, getting proteins (larva and adult honeybees) for feeding hornet brood and carbohydrates (mainly honey storage) for adult hornets, the two kinds of resources used by vespine wasps (Raveret Richter, Reference Raveret Richter2000). Additionally, V. crabro and V. velutina exhibit similar annual colony life cycle which can be divided in three major phases: (i) the foundation of the colonies following the emergence of foundresses from overwintering, (ii) the increase of the colony size and (iii) the emergence of sexuals for mating and the death of the colony. These three phases in the hornet life cycle can be monitored through their food preference because the nutritional requirements differ between adults and larvae, adult vespids mainly feeding on carbohydrates and larvae on proteins (Raveret Richter, Reference Raveret Richter2000; Monceau et al., Reference Monceau, Bonnard and Thiéry2014a ). Although a part of the carbohydrate intakes can be obtained from the larvae (Spradbery, Reference Spradbery1973; Edwards, Reference Edwards1980; Matsuura & Yamane, Reference Matsuura and Yamane1990; Archer, Reference Archer2012), three main peaks of foraging activity on carbohydrate sources are thus supposed to occur, roughly in spring, summer and autumn, each corresponding to the three phases of the hornet life cycle. Additionally, gathering proteins mainly occurs through summer and autumn with the colony growth. Protein requirements are even more important during the rearing of sexuals because they require more food, especially for gynes, and should thus raise a maximal consumption before the mating period in autumn (Spradbery, Reference Spradbery1973; Edwards, Reference Edwards1980).
Although the introduction of V. velutina represents an important threat to honeybees and beekeeping, it may also threaten V. crabro, which is protected in some areas within its native range (e.g., in Germany since 1987). Indeed, these two species share common characteristics: they are closely related, live in the same environment, belong to the same guild (predators), exploit the same kind of food sources and exhibit a similar annual life cycle. Considering all these similarities, interspecific competition may occur if the two species exhibit similar seasonal phenologies. The goals of this study were thus to characterize V. crabro and V. velutina seasonal phenologies by following the trapping yields of the two species on their two food requirements (carbohydrate and protein baits) in the course of time. We then compared the seasonal phenologies of each species to evaluate the extent of their temporal overlap. Therefore, the degree of overlap between V. crabro and V. velutina may provide a first assessment of the occurrence of interspecific competition between these two hornet species.
Materials and methods
Experimental design
Vespa crabro and V. velutina populations were monitored at two apiaries in the vicinity of Bordeaux (invaded by V. velutina in 2005): an apiary belonging to a professional beekeeper located in Artigues-près-Bordeaux (GPS: N44°51′37.20″ W0°28′43.28″, thereafter noted ART) and an experimental apiary of our research institute located in Villenave d'Ornon (GPS: N44°47′27.05″ W0°34′38.35″, thereafter noted VIL). These two apiaries were previously monitored to study V. velutina population dynamics in 2008 (see Monceau et al., Reference Monceau, Maher, Bonnard and Thiéry2013a ).
Seasonal phenologies of adult hornets were monitored with funnel traps composed of a glass jar (Le Parfait®, 1500 g, Ø 11 cm) equipped with a washable stainless steel funnel on the top (Supplementary fig. 1). In each apiary, four traps were positioned in between the hives (at ca. 1 m distance) and placed 50 cm above the ground on supports with a roof to protect from rainfalls (Supplementary fig. 1). For each site, two devices were baited with carbohydrate-based bait (40% apple concentrate in 250 ml of pure water) and two with protein-based bait (60 g of blended fresh fillets of farmed brown trout sexuals, Salmo trutta, provided by INRA St Pée-sur-Nivelle, in 250 ml of pure water).
All traps of ART and VIL were monitored weekly, at the same date (±1 day) from the 25th/24th of March to the 18th/17th of November in 2009 and 2010, respectively. Traps and baits were changed every week and the number of captured hornets was noted and pooled per bait, site and date.
Statistics
Poisson generalized linear models (GLMs) corrected for overdispersion were used to describe the variation in the number of hornet caught (trapping yields) depending on baits, sites, years and dates for each species separately. All predictors were standardized (Gelman, Reference Gelman2008) for model selection procedure generating all possible combinations of variables, except those including models containing interactions without their respective main effects. Models were then ranked using Akaike Information Criterion scores corrected for small sample sizes (AICc). The models displaying less than 2ΔAICc of difference with the best models were kept for model averaging procedure to obtain an average model (see Supplementary table 1) and predictors estimates assorted with their unconditional standard errors (SE), 95% confidence interval (95% CI), z statistic and relative importance (RI) (Burnham & Anderson, Reference Burnham and Anderson2002). Vespa crabro and V. velutina densities based on trapping yields were compared within and between site and year using χ2 tests. Finally, the deviation from random pattern of temporal activity overlap (i.e., whether a temporal niche overlap/segregation exist or not) was investigated using TimeOverlap program (v. 1.0, available at: http://hydrodictyon.eeb.uconn.edu/people/willig/Research/activity%20pattern.html, see Castro-Arellano et al., Reference Castro-Arellano, Lacher, Willig and Rangel2010 for details) based on Rosario randomization algorithm to compute Pianka's and Czechanowski's indices (Pianka, Reference Pianka1973; Feinsinger et al., Reference Feinsinger, Spears and Poole1981). These indices range from 0 (no temporal overlap) to 1 (complete temporal overlap). Bilateral tests based on 10,000 randomizations were performed and P-values were calculated as twice the proportion of the lowest score of either (i) the number of randomizations that have an overlap that is equal to or greater than the observed overlap value or (ii) the number of randomizations that have an overlap that is equal to or less than the observed overlap value. This analysis was first performed on the trapping yields obtained for each bait by sites and years separately and then in pooling the yields by baits to obtain a global comparison.
All other statistics were calculated using R software (v. 3.0.1; R Development Core Team, 2013) implemented with the following packages: epicalc for overdispersion detection, dispmod for fitting overdispersed Poisson log-linear GLMs, arm for variable standardization and MuMIn for model selection.
Results
Seasonal phenologies
Vespa crabro
Capture yields for V. crabro varied between baits and sites (table 1, Supplementary table 1). There were more V. crabro caught (i) with carbohydrate than with protein bait (see table 2 for details) and (ii) in ART than in VIL (160 and 59, respectively). The numbers of V. crabro caught were similar in 2009 and 2010. The overall dynamics could be summarized in three series: (i) in April, (ii) from mid-June to late September and (iii) in October (fig. 1). This dynamics was similar for carbohydrate and protein baits (fig. 2).

Fig. 1. Overall trapping yields of V. crabro (grey bars, N VC = 219) and V. velutina (black bars, N VV = 6276), from March to November represented as a proportion of the total number of each hornet species (data pooled for 2009 and 2010, carbohydrate and protein baits, and ART and VIL).
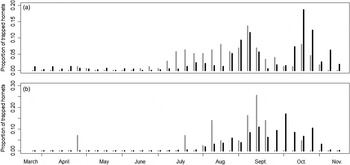
Fig. 2. Trapping yields of V. crabro (grey bars) and V. velutina (black bars) from March to November represented as a proportion of the total number of each hornet species caught with (a) carbohydrate bait (N VC = 176 and N VV = 818) and (b) protein bait (N VC = 43 and N VV = 5458) (data pooled for 2009 and 2010, and ART and VIL).
Table 1. Analysis of V. crabro capture yields according to the baits, sites, dates and years.

Each predictor selected in the best models are presented with its estimate (β) assorted with unconditional standard errors (SE), 95% confidence interval (95% CI), the z-test statistic (z) and associated P-value (P) and the relative importance of the parameter (RI). Significant predictors are in bold.
Table 2. Trapping yields for V. crabro and V. velutina per sites and years with the total number of both species.

CB, carbohydrate bait; PB, protein bait.
Vespa velutina
Capture yields for V. velutina differed between baits (table 3, Supplementary table 1): more hornets were caught with protein than with carbohydrate bait (see table 2 for details). Trapping yields also varied from March to November (fig. 1) with differences between baits and sites. This trend was consistent between years. For the carbohydrate bait, three series of captures could be observed: (i) from March to early June, (ii) from July to mid-September and (iii) from mid-September to November (fig. 2a). For the protein bait, a single wave was observed from mid-July to mid-November (fig. 2b). Additionally, dynamics differed between sites (table 3). The captures in spring ended later in ART than in VIL (ART: March to mid-June, VIL: March to April) and captures in VIL were more spread out than in ART during summer (Supplementary fig. 2).
Table 3. Analysis of Vespa velutina capture yields according to the baits, sites, dates and years.

Each predictor selected in the best models are presented with its estimate (β) assorted with unconditional standard errors (SE), 95% confidence interval (95% CI), the z-test statistic (z) and associated P-value (P) and the relative importance of the parameter (RI). Significant predictors are in bold.
Vespa crabro vs. Vespa velutina
Density
A general characteristic in both sites was the predominance of V. velutina in traps (table 2). In ART, the ratio between V. crabro and V. velutina was consistent over years (χ2-square test: χ2 1 = 3.00, P = 0.08) and was in average 1: 21 (table 2). In VIL, this ratio was not consistent over years (χ2 1 = 4.90, P = 0.03) with a larger proportion of V. crabro in 2010 than in 2009 (1:70 in 2009 vs. 1:37 in 2010, table 2). The average ratio in VIL was 1: 50 which is two times less than in ART (χ2 1 = 51.62, P < 0.0001, table 2).
Temporal niche overlap analyses
Temporal niche overlap analyses between V. crabro and V. velutina gives congruent results in using either Pianka's or Czechanowski's indices. Overall, these indices suggest that temporal pattern of activity in V. crabro and V. velutina partially overlapped (Pianka index range 0.53–0.86; Czechanowski index range: 0.41–0.70) but not more than expected by chance (all comparisons being non-significant, table 4).
Table 4. Summary of the tests for temporal niche overlap between V. crabro and V. velutina based on Pianka and Czechanowski indices and associated P-values (based on 10,000 randomizations).

ART, Artigues-près-Bordeaux; VIL, Villenave d'Ornon; CB, carbohydrate bait; PB, protein bait.
Discussion
Invasive alien species are one of the major threats to biodiversity (Vitousek et al., Reference Vitousek, Mooney, Lubchenco and Melillo1997; Chapin et al., Reference Chapin, Zavaleta, Eviner, Naylor, Vitousek, Reynolds, Hooper, Lavorel, Sala, Hobbie, Mack and Diaz2000; Clavero et al., Reference Clavero, Brotons, Pons and Sol2009). Especially, alien generalist predators often greatly impact the whole ecosystem by preying on native naïve prey, displacing and excluding intra-guild competitors and most of the time without any regulation by natural enemies (Zaret & Paine, Reference Zaret and Paine1973; Snyder & Evans, Reference Snyder and Evans2006; Bourdeau et al., Reference Bourdeau, Pangle and Peacor2011; Perdereau et al., Reference Perdereau, Dedeine, Christidès, Dupont and Bagnères2011; Haddaway et al., Reference Haddaway, Wilcox, Heptonstall, Griffiths, Mortimer, Christmas and Dunn2012; Roy et al., Reference Roy, Adriaens, Isaac, Kenis, Onkelinx, San Martin, Brown, Hautier, Poland, Roy, Comont, Eschen, Frost, Zindel, Van Vlaendersen, Nedvĕd, Ravn, Grégoire, de Biseau and Maes2012). To date, V. velutina is mainly known for its predation on honeybees but its effect on V. crabro, which belong to the same guild remains completely unknown (Monceau et al., Reference Monceau, Bonnard and Thiéry2014a ). Our study is thus the first relating a potential impact of V. velutina on its direct competitor V. crabro in Europe (see Choi et al., Reference Choi, Martin and Lee2012 in Korea) and it suggests that the two species show some degree of niche differentiation that potentially minimizes their interspecific competition.
In spring, V. velutina foundresses are the first to regain activity and their period of flight is longer than V. crabro (March to mid-June and late April to early May, respectively). This difference is expected to be the result of variation in overwintering duration, which is documented as a bet-hedging strategy to adapt to novel environments (Gourbière & Menu, Reference Gourbière and Menu2009). Contrarily to V. velutina (Monceau et al., Reference Monceau, Bonnard and Thiéry2012, Reference Monceau, Maher, Bonnard and Thiéry2013a ), the present data for V. crabro differs from previous observations in which the foundresses were found to fly from mid-April to early-July (Spradbery, Reference Spradbery1973; Edwards, Reference Edwards1980; Matsuura & Yamane, Reference Matsuura and Yamane1990). However, these data were acquired in a different area and consequently, such variability in flying period cannot be clearly attributed to the presence of V. velutina. During summer, foraging activity on carbohydrates begins earlier and is slightly longer in V. crabro than in V. velutina (from mid-June to late September and from early July to mid-September, respectively). Vespa velutina seasonal phenology has already been monitored in 2008 (the year before the present study), in the same locations using carbohydrate baits during summer and autumn (Monceau et al., Reference Monceau, Maher, Bonnard and Thiéry2013a ). We overall find common trends between these different surveys: a first slight increase in early-July to mid-August followed by a drastic increase of the foraging activity from mid-August to mid-September and then a slow down before the emergence of sexuals. Finally, in the last part of the cycle (when sexuals need carbohydrates before mating), the foraging activity on carbohydrates begins earlier and is longer in V. velutina than in V. crabro (from mid-September to late November and only during October, respectively).
Concerning the foraging activity on protein sources, the discrepancy between species is clearer. It begins earlier and is shorter in V. crabro than in V. velutina (mid-July to mid-November and early July to late November, respectively). Interestingly, this peak occurs between the two peaks of foraging activity for carbohydrates in V. crabro and V. velutina (mid- and late September, respectively). At the advent of gyne emergence, the ratio larva/worker is at its lowest and the productivity of the colony is at its maximum (Matsuura & Yamane, Reference Matsuura and Yamane1990). Although most of the foraging activity for proteins is concentrated in summer and autumn, a few individuals were also trapped in spring using protein bait (late April 2010 in ART: three V. crabro and three V. velutina in the same trap). In early spring, just at the exit from overwintering, foundresses are not supposed to feed on proteins. Thus, this single event could solely be an artefact.
Our data show that temporal patterns of activity of V. crabro and V. velutina overlap, which suggests competition, but most of the dynamics do not differ from expected in random events. This partial overlap probably results from the biological constraints of the Vespinae, hornet species exhibiting similar life-cycle timing (Spradbery, Reference Spradbery1973; Edwards, Reference Edwards1980; Matsuura & Yamane, Reference Matsuura and Yamane1990). The fact that the overlap is not total suggests that they do not probably compete directly for food sources at the same time. It does not however exclude indirect competition, especially in a ‘first-come, first-served’ fashion. Indeed, V. velutina foundresses emerge first from overwintering and are also more active, explorative and bolder than V. crabro (Monceau et al., in press). They can consequently monopolize the best food sources before V. crabro foundresses. After this phase, considered the most critical (Spradbery, Reference Spradbery1973), V. crabro takes advantage and seems to complete its life cycle quicker than V. velutina, suggesting that the ‘initial dominance’ of V. velutina is reversed. It should however be considered here that the populations of the two species greatly differ in number. The numerical dominance of V. velutina on V. crabro obviously depends on the number of nests in the vicinity of the experimental sites. Nonetheless, the localization of hornet colonies is often realized a posteriori, nests being usually cryptic. Most importantly, the difference in population size can be explained by their respective colony size. Indeed, V. crabro forms medium-sized colonies containing ca. 500–4500 cells (in average ca. 1100 adult produced), occupied by 300–400 workers at the maximum colony activity and produce in average 200 gynes and 350 males (Edwards, Reference Edwards1980; Matsuura & Yamane, Reference Matsuura and Yamane1990; Archer, Reference Archer1993; Hoffmann et al., Reference Hoffmann, Neumann and Schmolz2000). Vespa velutina can build larger nests containing more than 10,000 cells (in average ca. 6000 adults produced), occupied by more than one thousand workers at time and produce in average 350 gynes and 900 males (Martin, Reference Martin1995; Nakamura & Sonthichai, Reference Nakamura and Sonthichai2004; Monceau et al., Reference Monceau, Bonnard and Thiéry2014a ). The difference in sexual production is also visible with the last peak of foraging activity on carbohydrates, which is higher in V. velutina than in V. crabro in regards to the rest of the cycle.
Several Vespine wasps have already colonized different areas worldwide (see Beggs et al., Reference Beggs, Brockerhoff, Corley, Kenis, Masciocchi, Muller, Rome and Villemant2011 for review), including V. crabro in North America (Akre et al., Reference Akre, Greene, MacDonald, Landolt and Davis1980; Buck et al., Reference Buck, Marshall and Cheung2008; Kimsey & Carpenter, Reference Kimsey and Carpenter2012). In the present case, V. velutina largely surpasses V. crabro in term of colony size/productivity. Interestingly, the overall ratios between V. crabro and V. velutina are consistent between 2009 and 2010, although there were slightly more V. crabro relative to V. velutina in VIL in 2010. This consistency through two consecutive years suggests that less than 4 years after the colonization of this area (the first V. velutina was first observed in 2005 in this area), the co-occurrence of the native and the invading hornet species could be considered stabilized. To some extent, it seems like the native hornet species may take advantage from the presence of this alien species, although we cannot exclude that V. crabro population may have previously suffered from the introduction of V. velutina. These two hornet species are known to prey on domestic honeybees (Matsuura & Yamane, Reference Matsuura and Yamane1990; Baracchi et al., Reference Baracchi, Cusseau, Pradella and Turillazzi2010; Monceau et al., Reference Monceau, Bonnard and Thiéry2014a ). Some beekeepers in the invaded areas have observed an increase in the number of V. crabro in their apiaries since the introduction of V. velutina (Monceau et al., Reference Monceau, Bonnard and Thiéry2014a ). This suggests that V. crabro may benefit from the presence of V. velutina in apiaries. Indeed, V. velutina exerts a strong predation pressure and weakens honeybee colonies (Monceau et al., Reference Monceau, Arca, Leprêtre, Mougel, Bonnard, Silvain, Maher, Arnold and Thiéry2013b ). Vespa crabro may take advantage of the situation to chase on weakened colonies, which are less defended and/or scavenge on leftover dead bee bodies by V. velutina (N. Maher, personal observation). Predator facilitation (Charnov et al., Reference Charnov, Orians and Hyatt1976, also known as synergistic predation) may thus occur in this newly established association (Losey & Denno, Reference Losey and Denno1999). If so, this may be seriously considered because synergistic predation often results in an overall higher rate of prey consumption than if predator species would have occurred separately (Soluk & Collins, Reference Soluk and Collins1988; Kotler et al., Reference Kotler, Blaustein and Brown1992).
Conclusions and perspectives
The present data show that the seasonal phenologies of V. crabro and V. velutina partially overlap probably due to biological constraint common to Vespine wasps. It is impossible to conclude on the effect of V. velutina on V. crabro without any measurement of the seasonal phenology of the native species without its putative competitor (Colwell & Futuyma, Reference Colwell and Futuyma1971). However, in the present case of V. crabro, rather little population dynamics studies were done which do not allow comparisons before and after the introduction of V. velutina. Also, any removal of the alien hornet by eradicative methods is now impossible which do not allow any further study on V. crabro alone.
Although the invasive species is largely more numerous than the native one, a few years after the beginning of the invasion, these two species seem to be quite stable. Vespa crabro does not seem to be threatened by V. velutina, and we even suspect that the native could take advantage of the massive attack exerted by the alien hornet species on honeybee colonies. This would have however to be tested at a larger geographical scale in further studies.
Interspecific competition between V. crabro and V. velutina should also consider another dimension that is competition for nesting sites (Spradbery, Reference Spradbery1973; Edwards, Reference Edwards1980; Matsuura & Yamane, Reference Matsuura and Yamane1990; Matsuura, Reference Matsuura, Ross and Matthews1991; Gamboa et al., Reference Gamboa, Greig and Thom2002, Reference Gamboa, Noble, Thom, Togal, Srinivasan and Murphy2004; Liebert et al., Reference Liebert, Gamboa, Stamp, Curtis, Monnet, Turillazzi and Starks2006; Choi et al., Reference Choi, Martin and Lee2012). Even though the known cases of interspecific competition are rather rare or underreported in Vespids, Matsuura & Yamane (Reference Matsuura and Yamane1990) give the example of five Vespa species (Vespa analis, Vespa crabro, Vespa mandarinia, Vespa tropica and Vespa simillima) nesting in South-western Honshu (Japan). Although they all live in the same area, they either differ for their nesting habit or for their timing of nest initiation thus limiting to a certain extent potential competition. In the case of V. crabro and V. velutina, the timing of nest initiation could be problematic since V. velutina foundresses emerge from overwintering before V. crabro. Moreover, a recent study on the behaviour of foundresses shows that V. velutina is also more active, explorative and bolder than V. crabro potentially giving to the invasive hornet species an advantage when searching a convenient nesting site (Monceau et al., in press). Thus, it would be interesting to focus on this part of the hornet life cycle to fully understand the extent of V. velutina impact on V. crabro. Such information is crucial since it can help understand how V. velutina may have invaded and spread through Europe, foundresses being accountable for the invasion of new areas.
Supplementary Material
The supplementary material for this article can be found at http://www.journals.cambridge.org/BER
ACKNOWLEDGMENTS
This research was financially supported by France Agrimer # 797/2007–2010, a grant from Région Aquitaine and INRA. This research belongs to the Labex COTE ANR research programme. We are grateful to Mr. J. Martrenchar for allowing us to work in his apiary and INRA Research Centre of Bordeaux Aquitaine for installing our own experimental apiary.









