Introduction
The Enemy Release Hypothesis (ERH) is a widely accepted mechanism by which to explain invasion success in exotic plant species; the ERH predicts that the loss of coevolved consumers or specialists when a species is moved beyond the native range will yield a competitive advantage over native plant species (with their own co-evolved herbivore systems) in its adventive range (Lake & Leishman, Reference Lake and Leishman2004; Lambert & Casagrande, Reference Lambert and Casagrande2006; Mukherjee et al., Reference Mukherjee, Jones, Cuda, Kiker and Overholt2012). The key assumptions of the ERH are that (a) herbivores can regulate plant populations; (b) specialist herbivores that are host specific to the plant are not present in the adventive range; (c) host-switching of native herbivores is rare; and (d) native plant species have more pressure from both specialist and generalist herbivores than do introduced species (Keane & Crawley, Reference Keane and Crawley2002; Cripps et al., Reference Cripps, Schwarzländer, McKenney, Hinz and Price2006). In a plant's native range there is a variety of selective pressures from specialist and generalist herbivores that shape populations (Orians & Ward, Reference Orians and Ward2010). When species are introduced to new areas, such dynamics are decoupled because specialist herbivores do not occur outside of their host plants’ ranges and native herbivores in the adventive range will not switch to new hosts (Orians & Ward, Reference Orians and Ward2010). The result is that exotic species can escape herbivory and have reduced herbivore abundance comprised mostly polyphagous generalists compared with native species which will have both specialist and generalist herbivore natural enemies (Frenzel & Brandl, Reference Frenzel and Brandl2003; van Lenteren et al., Reference van Lenteren, Babendreier, Bigler, Burgio, Hokkanen, Kuske, Loomans, Menzler-Hokkanen, Van Rijn, Thomas and Tommasini2003; Orians & Ward, Reference Orians and Ward2010).
The majority of studies related to the ERH have come from classical biological control studies, as the concept of enemy escape has important implications on the success of such programmes that rely on the role of herbivory in regulation of plant populations (Keane & Crawley, Reference Keane and Crawley2002; McClay & Balciunas, Reference McClay and Balciunas2005; Liu & Stiling, Reference Liu and Stiling2006; Prior et al., Reference Prior, Powell, Joseph and Hellmann2015). Since the ERH is a key assumption in biological control, it is important to understand the role of enemy release in a plant invasion before biological control is initiated. This can be achieved through the use of pre-introductory surveys, which involve conducting surveys of the invasive species in the adventive range for baseline information on a number of key components including establishing a list of herbivores associated with the target weed (Tewksbury et al., Reference Tewksbury, Casagrande, Blossey, Häfliger and Schwarzländer2002; Dudley et al., Reference Dudley, Lambert, Kirk, Hoddle and Johnson2006).
Such pre-emptive surveys have proven their worth in the biological control programme on the invasive alien species (IAS) Arundo donax (L.) in North America. Herbivore surveys on A. donax were able to identify that the reed was not enemy free in the adventive range and a specialist herbivore, Tetramesa romana Walker (Hymenoptera: Eurytomidae) had established from an earlier unknown introduction (Dudley et al., Reference Dudley, Lambert, Kirk, Hoddle and Johnson2006). From this, augmentative biological control could be initiated which involved increasing T. romana populations to heighten their impacts on the reed (Dudley et al., Reference Dudley, Lambert, Kirk, Tamagawa and Julien2008; Moran et al., Reference Moran, Vacek, Racelis, Pratt and Goolsby2017). There have been a number of similar examples of herbivore surveys uncovering the accidental introduction of native specialists on IAS and thus revealing that, unlike what the ERH predicts, these plants are no longer strictly enemy free in the adventive range (McClay, Reference McClay1988; Hoffmann & Moran, Reference Hoffmann and Moran1991).
In South Africa, the invasive reed, Arundo donax L. is currently being considered for biological control and thus would benefit from a pre-introductory survey. Arundo donax was deliberately introduced in the late 1700s, and was used for a wide range of purposes most notably for erosion control (Bell, Reference Bell, Brock, Wade, Pyšek and Green1997; Henderson, Reference Henderson2001). Arundo donax has since become one of the worst IAS in the country and is listed as a Category 1 species according to the National Environmental Management: Biodiversity Act (NEMBA) (Act No 10 of 2004) which prohibits it from being sold or planted and suggests that control efforts are required (Henderson, Reference Henderson2001; van Wilgen et al., Reference van Wilgen, Nel and Rouget2007). Despite the threat A. donax poses to South Africa's biological diversity, very few baseline data are available to guide management.
In this study, the role of enemy release was investigated by comparing the herbivore assemblages of A. donax and two native analogous tall-statured grasses, Phragmites australis (Cav.) Trin. ex. Steud. and Phragmites mauritianus Kunth. Biogeographical studies that look at herbivory in the native and introduced ranges of a particular IAS have generally found support for the ERH (Keane & Crawley, Reference Keane and Crawley2002). However, it has been found that community studies – studies that compare analogous native species and IAS co-occurring within the same community – are more equivocal than biogeographical studies (Colautti et al., Reference Colautti, Ricciardi, Grigorovich and MacIsaac2004). Such studies provide a clearer understanding of the role of herbivory within a specific region and provide insightful information on the validity of the ERH (Colautti et al., Reference Colautti, Ricciardi, Grigorovich and MacIsaac2004; Liu & Stiling, Reference Liu and Stiling2006).
The structural, genetic and mechanical attributes of a plant will influence the acceptability of plants to herbivores (Ehrlich & Raven, Reference Ehrlich and Raven1964). As such the two Phragmites spp. are expected to have a herbivore community similar to that of A. donax as they are phylogenetically related (same tribe), ecologically similar, sympatric, C3 perennials, and semi-aquatic species (Tracy & DeLoach, Reference Tracy, DeLoach and Bell1998; Lambert et al., Reference Lambert, Dudley and Saltonstall2010). Investigating the herbivores on the native Phragmites species will also play an important role in establishing baseline data for these tall-statured grasses in South Africa. To date, studies of insects associated with Phragmites spp. have largely focused on the species as wild hosts for important agricultural crops notably maize and sugarcane (Le Rü et al., Reference Le Rü, Ong'amo, Moyal, Ngala, Musyoka, Abdullah, Cugala, Defabachew, Haile, Matama and Lada2006a, Reference Le Rü, Ong'amo, Moyal, Muchugu, Ngala, Musyoka, Abdullah, Matama-Kauma, Lada, Pallangyo and Omwegab). In addition, the study could provide insight into how herbivory (including species richness, feeding guilds and diversity) is influenced by plant origin; P. mauritianus is endemic to Africa however P. australis is a cosmopolitan reed and as such the study will allow comparisons of herbivore communities on an endemic, cosmopolitan and introduced plant.
Exploration of the ERH on A. donax is predicted to reveal that the IAS will have a less diverse herbivore community than Phragmites spp., devoid of specialists and thus is expected to largely have escaped herbivory in South Africa. The native Phragmites species are expected to have a greater diversity of herbivores, with a higher number of both generalist and specialist species with a range of feeding guilds that help regulate reed populations.
Materials and methods
The study of the herbivores associated with the reed species was done in three parts: (1) a long-term study (hereafter referred to as LTS) in a localized area to determine patterns of herbivore assembly over time; (2) a nation-wide survey (hereafter referred to as NWS) across South Africa to record herbivores associated with the reeds across different biomes; and (3) a literature review to produce a list of all known herbivores recorded on A. donax, P. australis and P. mauritianus in sub-Saharan Africa.
Site selection – long-term study
The first part of the study evaluated herbivores associated with only A. donax and P. australis; P. mauritianus does not occur in the region where the LTS was conducted. Monthly surveys were carried out over a 2-year period on reed stands in the Free State Province, the centre of both reeds’ distributions in South Africa (fig. 1). Five study sites were chosen where A. donax and P. australis grow in close proximity (roughly within 5 km of each other). Sites were located in the Eastern Free State, a high-lying part of the province about 50 km from the Maloti Mountains in Lesotho. This area has wet, hot summers and dry, cold winters, with mean temperatures in summer ranging from 12 to 27°C and in winter ranging from −2 to 17°C (World Weather Online, 2016). Average annual rainfall is highly variable and ranges from 300 mm to over 900 mm (Moeletsi et al., Reference Moeletsi, Walker and Landman2011).
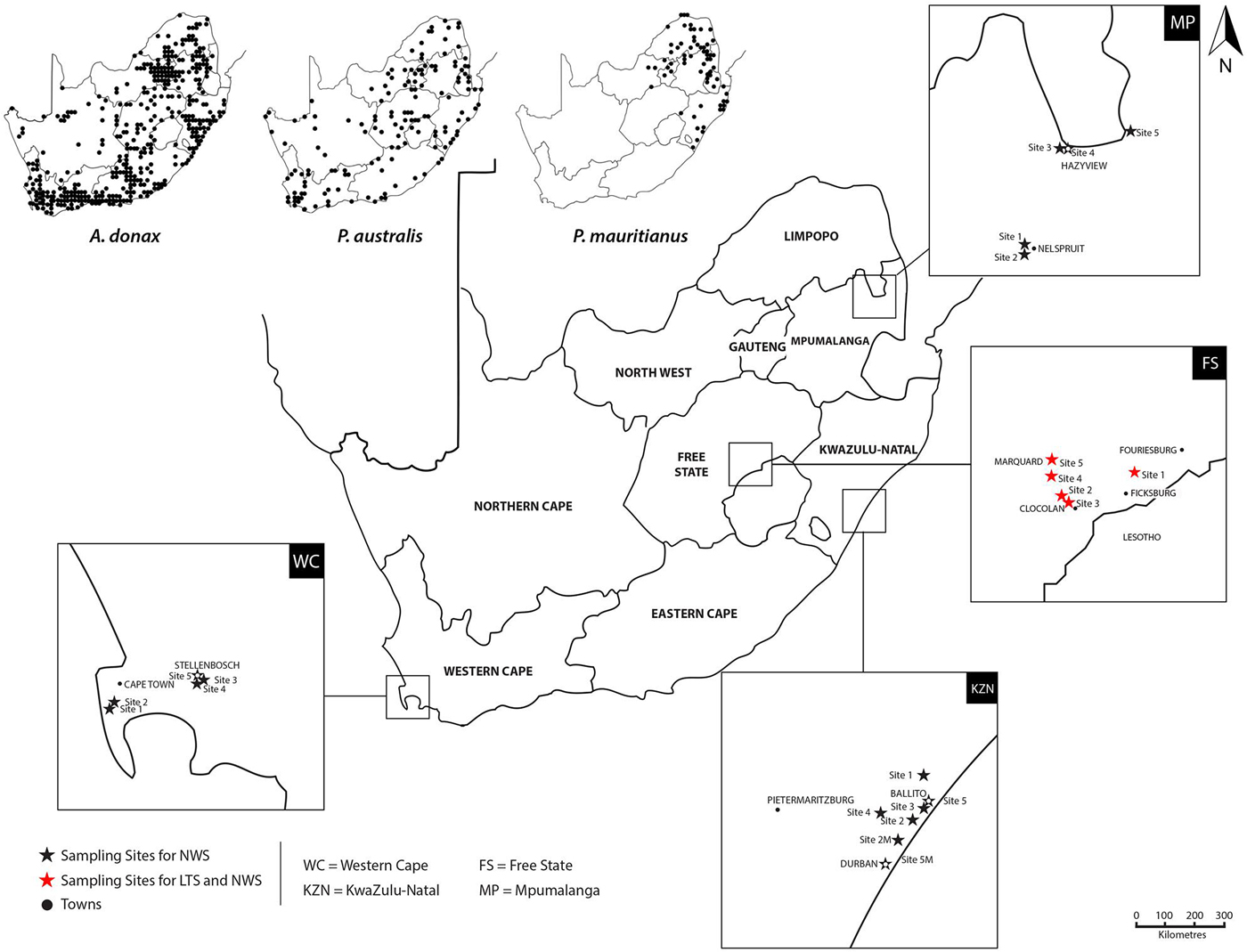
Fig. 1. Map of the sampling sites for the LTS and NWS (Supplementary Tables S1 and 2). The highlighted areas focusing in on the four sampled regions during the surveys. The distribution ranges for A. donax, P. australis, and P. mauritianus are shown in the top left of the map (Fish et al., Reference Fish, Mashau, Moeaha and Nembudani2015).
Site selection – NWS
The second part of the study involved a NWS across the distribution of all three reeds in South Africa. Sites were chosen in four provinces representing four of South Africa's nine biomes (Rutherford et al., Reference Rutherford, Mucina and Powrie2006); –(1) Fynbos, Western Cape Province, (2) Indian Ocean Coastal Belt, KwaZulu-Natal Province, (3) Highveld grassland, Free State Province, (4) Lowveld savanna, Mpumalanga Province (fig. 1). Sites were selected according to areas where all three reed species grew in close proximity (roughly within 5 km of each other); paired sites ensure that herbivore assemblages are comparable and also allows insight into the host range of the species. For P. mauritianus, sampling was only carried out in two regions, in the Mpumalanga and KwaZulu-Natal provinces, as the reed's distribution is restricted to the more tropical eastern parts of South Africa.
Data collection
Long-term study
Monthly sampling took place from June 2011 to March 2013. At each site, ten plants were selected haphazardly from both A. donax and P. australis stands. Reeds were first searched for ectophagous herbivores and these were then collected and immatures were reared to the adult stage. Reeds were then removed by digging up the rhizomes. Stem diameter, height, and the number of lateral shoots/branches were recorded. Stem diameter was taken at 0.5 m above roots to ensure consistency and also to take a measurement close to the first internode as stem diameter remains constant in a growth season at this point (van der Toorn & Mook, Reference van der Toorn and Mook1982). Reed density (number of stems) was recorded in 0.5 m-square quadrats at three random points every month.
Damage from the most abundant herbivores was recorded for A. donax and P. australis by observing presence or absence of injuries such as feeding scars, galls or emergence holes. Plant parts were separated into stems, lateral shoots, roots and flowers, and put into emergence boxes. Any herbivores that emerged were collected and identified. The feeding strategy of each herbivore was noted by recording feeding guild as gallers, stem borers, sap-suckers, pollen feeders, shoot borers or leaf miners. Voucher specimens were sent to taxonomists at the South Africa National Collection (Agricultural Research Council – Plant Protection Research Institute (ARC-PPRI)) for identification. Species found to be common (recorded in more than one sampling event) were deposited in the National Collection of Insects (ARC-PPRI), Pretoria.
Nation-wide survey
Sampling was carried out in the summer months of November 2014 until January 2015. At each site five whole plants were randomly selected for A. donax, P. australis and P. mauritianus stands. Reeds were first searched for ectophagous herbivores and these were collected. Plants were then dissected to record and collect endophagous species. Immature forms were reared to their adult stage. Lepidopteran stem borers were reared on an artificial diet according to Onyango (Reference Onyango1994). Larvae were kept separately in plastic vials until adult emergence. A search period of 30 min in each site for each reed species was also carried out. During this search, the reed stands were assessed for damage and any herbivores found associated were collected. For A. donax lateral shoots were examined following the protocol of the LTS. Unidentified herbivores were sent to the ARC-PPRI.
Literature review
A literature review was carried out to compile all known records of herbivores on A. donax, P. australis and P. mauritianus in sub-Saharan Africa. Research institutes involved with herbivore surveys on tall-statured grasses were consulted including the ARC-PPRI, the South African Sugarcane Research Institute (SASRI), the Integrated Pest Management program at North-West University, and the Institut de recherché pour le developpement (IRD) (Dr Le Rü, head of the noctuid stem borer diversity team). Relevant databases were searched in particular, the Centre for Agriculture and Bioscience International (CABI) and ScaleNET. Lastly, information on analogous agricultural tall-statured grasses as alternative hosts was obtained from Prinsloo & Uys (Reference Prinsloo, Uys, Prinsloo and Uys2015).
To further investigate the ERH, a literature review was carried out to record the number of herbivores found on A. donax, P. australis and P. mauritianus from Eurasia and North America where herbivore studies have taken place. This allowed for a comparison of species richness in native and introduced ranges.
Data analysis
The software programme Estimate S (Version 9.1.0) (Colwell, Reference Colwell2009) was used to calculate species diversity. The two indices used for this study were the Shannon Index and Simpson's Diversity Index. The Shannon Index is a nonparametric diversity descriptor that is used to assess the number of rare species across samples in terms of equitability (Peet, Reference Peet1974). The Simpson Index was used to compare the relative abundances of each herbivore species across samples to give an idea of the level of diversity of herbivores on each reed (Whittaker, Reference Whittaker1972; Hill Reference Hill1973; Peet, Reference Peet1974). The statistical interpretation of such indices can be controversial in diversity studies (Jost, Reference Jost2007), however, given that this study compares three analogous plants and performed sampling in paired sites within each geographical regions the diversity indices should provide comparable results on herbivore biodiversity.
Means calculated for A. donax, P. australis, and P. mauritianus diversity indices and herbivore damage recorded were compared for statistical significances using a student's t-test (two groups, data normally distributed) or by the Kruskal–Wallis one-way analysis of variance (ANOVA) on ranks with a Dunn post hoc test (three groups, data not normally distributed or uneven variance) using SigmaPlot software (Version 12.5). The significance level of acceptance for all tests was P < 0.01.
To test if adequate sampling was carried out and if all associated herbivores were collected, the Chao 2 and Incidence-Based Coverage (ICE) estimators were used (Chao et al., Reference Chao, Colwell, Lin and Gotelli2009). Data on species occurrence or incidence was used because not all species abundances could be accurately quantified. The ICE estimator is a modified version of Chao 2, which is used to ensure sampling does not overestimate species richness.
Results
List of herbivores on tall-statured grasses
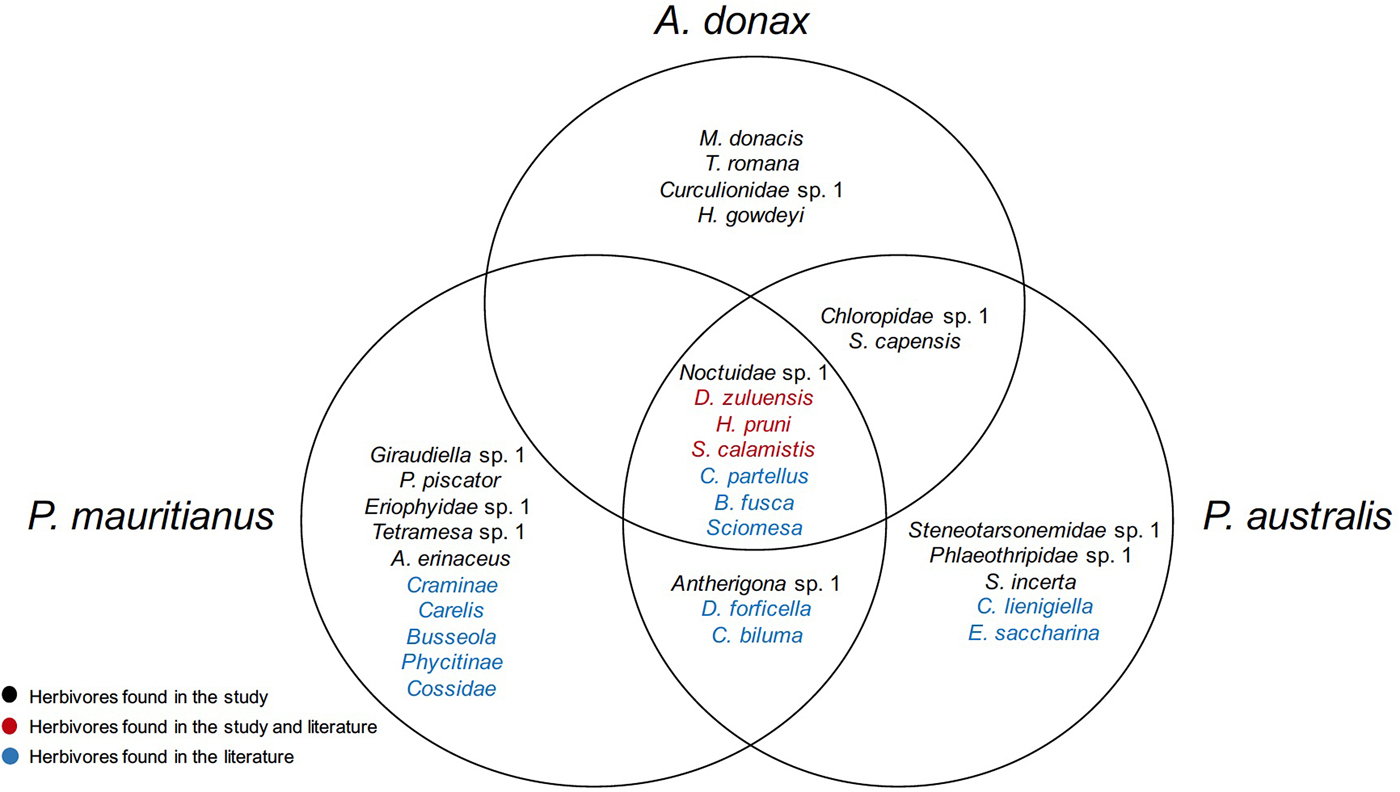
All herbivores recorded to date on A. donax (Table 1), P. australis (Table 2) and P. mauritianus (Table 3) are listed from the combined data from the LTS, NWS and literature review. In total, 13 herbivores were found on A. donax, 17 on P. australis and 20 on P. mauritianus. A number of herbivores are shared amongst the reeds; seven herbivores occurred on all three reeds, A. donax and P. australis shared two herbivores and P. australis and P. mauritianus shared three herbivores (fig. 2). Twelve herbivores were found in the literature review that were not found in the herbivore surveys (A. donax – 4, P. australis – 7, P. mauritianus – 10) (fig. 2).

Fig. 2. Venn diagram showing overlap of herbivores found on A. donax, P. australis, and P. mauritianus.
Table 1. Herbivores associated with A. donax excluding incidental visitors.

Species feeding habit is described by part of the plant damaged, feeding guild and host-specificity. Accessioned specimens are referred to by Rhodes University accession numbers (AcRH). *denotes that a species was present at a site. The incidence rate is calculated as the percentage of sampling events the herbivore was recorded in. NWS Site 1 = KwaZulu-Natal, Site 2 = Free State, Site 3 = Western Cape, site 4 = Mpumalanga.
Table 2. Herbivores associated with Phragmites australis excluding incidental visitors.

Species feeding habit is described by part of the plant damaged, feeding guild and host-specificity. Accessioned specimens are referred to by Rhodes University accession numbers (AcRH). * denotes that a species was present at a site. The incidence rate is calculated as the percentage of sampling events the herbivore was recorded in. NWS Site 1 = KwaZulu-Natal, Site 2 = Free State, Site 3 = Western Cape.
Table 3. Herbivores associated with Phragmites mauritianus excluding incidental visitors.

Species feeding habit is described by part of the plant damaged, feeding guild and host-specificity. Accessioned specimens are referred to by Rhodes University accession numbers (AcRH). * denotes that a species was present at a site. The incidence rate is calculated as the percentage of sampling events the herbivore was recorded in. NWS site 1 = KwaZulu-Natal, site 2 = Mpumalanga.
Species diversity
For the LTS, seven herbivore species were found associated with A. donax and six species with P. australis when excluding all incidentals (species recorded at only one sampling event). There was no significant difference found in the species diversity of these herbivores using the Shannon and Simpson's indices (Simpson's Index, t = 1.336, P = 0.218; Shannon's Index, t = 1.922, P = 0.091). For A. donax the Chao 2 and ICE estimators predicted that eight herbivores are likely to be associated with the plant but it is possible that this number could be as high as 20 (Chao 2 upper 95% CI) (Supplementary Fig. S1). For P. australis the Chao 2 and ICE estimators predicted that six herbivores are likely to be found in all five sites (Supplementary Fig. S1).
For the NWS sampling, A. donax had fewer associated herbivores than the LTS, with only five species and all of these species were encountered in the LTS. Both Phragmites species had significantly higher species diversity using Shannon and Simpson's indices (Simpson's Index, H = 18.95, P < 0.0001; Shannon's Index, H = 309.09, P < 0.0001). Phragmites australis sampling yielded three additional herbivores across South Africa with a total of nine herbivorous species being recorded (Table 2). Phragmites mauritianus was found to have ten herbivore species (Table 3). Coverage-based richness estimators found that the NWS was sufficient in sampling for herbivores on A. donax and P. australis however, there is a possibility of more herbivores on P. mauritianus. For A. donax the Chao 2 and ICE estimators predicted that five herbivores is the likely number of herbivores associated with A. donax with the possibility of only one more species (Chao 2 upper 95% CI) (Supplementary Fig. S2). For P. australis, the Chao 2 and ICE estimators predicted that nine herbivores are likely to be found across the sites but that there is the possibility for up to 15 more species to be found (Supplementary Fig. S2). For P. mauritianus the Chao 2 and ICE estimators predicted that 12 herbivores are likely to be found in all five sites with the possibility of up to 20 (Supplementary Fig. S2).
Comparison of plant origin and herbivores
For A. donax, more herbivores have been found in Africa (n = 13) compared with North America (n = 4) despite both areas being adventive ranges (Table 4). In North America A. donax has largely escaped herbivory with only four species being recorded thus far (with one of these species being intentionally introduced as a biological control agent), however, in Africa, a number of native species have had a behavioural expansion onto the reed (Table 1). For P. australis, fewer herbivores were found in Africa (n = 17) compared with Eurasia (n = 154) and North America (n = 26) (Table 4). In Africa, P. australis has had relatively few herbivores recorded with only one known monophagous species. In North America, 12 species were found to be monophagous however of these, only two are considered native to the region. In Europe and Asia, however, diverse herbivore communities have been recorded with a total of 154 herbivores recorded with 54 of these herbivores being monophagous. For P. mauritianus, a total of 20 species have so far been recorded across sub-Saharan Africa.
Table 4. Comparison of the number of herbivores associated with A. donax, P. australis, and P. mauritianus in Eurasia, North America, and sub-Saharan Africa.

For A. donax, the native range is found in Eurasia and the number of species is compared with the two introduced ranges in North America and South Africa. For P. australis, all areas included are considered the native range. For P. mauritianus, being endemic to Africa only includes this region's records.
1 Tracy & DeLoach (Reference Tracy, DeLoach and Bell1998), 2Tewksbury et al., (Reference Tewksbury, Casagrande, Blossey, Häfliger and Schwarzländer2002), 3Dudley et al., (Reference Dudley, Lambert, Kirk, Tamagawa and Julien2008), 4Goolsby et al., (Reference Goolsby, Kirk, Moran, Racelis, Adamczyk, Cortés, Marcos García, Martinez Jimenez, Summy, Ciomperlik and Sands2011).
Potential for Arundo donax biological control
A potential biological control agent for A. donax, the galling wasp, T. romana was documented. Tetramesa romana has been released as a biological control agent in North America (Goolsby & Moran, Reference Goolsby and Moran2009); however, as in South Africa, the insects were already broadly distributed in western North America (Dudley et al., Reference Dudley, Lambert, Kirk, Hoddle and Johnson2006). Tetramesa romana was found in this study to be the most widespread, damaging and abundant herbivore on A. donax in both the LTS (incidence rate – 0.4) and NWS (incidence rate – 0.65) (Table 1 and Supplementary Table 3).
Tetramesa romana damages A. donax by producing galls in the stems and lateral shoots. Damage (number of exit holes) was highly variable across sites, however, was overall relatively low (LTS – 19% and NWS – 26% had damage) compared with surveys in North America (Dudley et al., Reference Dudley, Lambert, Kirk, Tamagawa and Julien2008). Tetramesa romana populations were highly dependent on the season, being most abundant in the summer months of November and December. In both surveys, the presence of T. romana galls was correlated with plants being significantly taller (LTS: U = 8000, P < 0.001; NWS: U = 595, P < 0.001) and having significantly more lateral shoots (LTS: U = 29,881.5, P < 0.001; NWS: U = 404, P < 0.001), suggesting herbivory can result in secondary stem proliferation. Stem diameter was also greater with T. romana galling, however, this was only significant for the LTS (LTS: U = 46,146, P < 0.001; NWS: t = 1.82, P = 0.071).
Discussion
Exploring the ERH and comparing introduced plants to analogous species in their native range is important as it is relative to these populations that invaders gain and lose interactions with enemies, mutualists and competitors (Mitchell et al., Reference Mitchell, Agrawal, Bever, Gilbert, Hufbauer, Klironomos, Maron, Morris, Parker, Power and Seabloom2006). To investigate such a link between plant origin and herbivory, three reed species, A. donax, P. australis and P. mauritianus were assessed to determine if herbivore assemblages followed patterns predicted by the ERH. Overall, the herbivore assemblages found on A. donax in South Africa when compared with the native analogous Phragmites spp. species did not match the predictions of the ERH; A. donax has two specialist herbivores from its native range, shares native herbivores with the Phragmites spp. and is fed upon by the same trophic guilds. The study was also beneficial in determining a potential biological control agent T. romana had been unintentionally introduced at some earlier time and was common across all sampling sites.
Investigating the ERH on the tall-statured grasses involved assessing the overall number of species or species richness, diversity indices and determining the feeding guilds, as each measure together provides a clearer picture of herbivore pressure on the plant populations. Looking at species richness on the tall-statured grasses, a total of 13 herbivores have been recorded on A. donax (eight species from herbivore surveys), 17 on P. australis (ten species from herbivore surveys) and 20 on P. mauritianus (ten species from herbivore surveys). According to the Chao estimators, the overall sampling effort from the herbivore surveys was sufficient for all three reed species. However, from results in the NWS for both P. australis and P. mauritianus there was potential to find more herbivores with up to 15 and 20 more species respectively. As such, the inclusion of the data from literature reviews helps to improve our understanding of herbivory in that an additional 12 species that are known to feed on the tall-statured grasses were included.
To understand the species composition of the herbivores found, species diversity indices were used. Species richness alone does not provide a definitive picture of herbivory; employing the Shannon and Simpson's indices allows insight into levels of biodiversity based on species richness and evenness (species abundance) (Menhinick, Reference Menhinick1964; Peet, Reference Peet1974; Purvis & Hector, Reference Purvis and Hector2000; Spellerberg & Fedor, Reference Spellerberg and Fedor2003). In the LTS, A. donax had a slightly higher Shannon index and about the same level of diversity for the Simpson's index compared with P. australis. However, in the LTS A. donax had significantly lower species diversity compared with both P. australis and P. mauritianus. Overall the Phragmites spp. have higher herbivore diversity compared with A. donax, while the two Phragmites spp. have similar levels of species and abundances to one another.
All three reeds had herbivores that occupied the same feeding guilds – sap-suckers, stem borers and gall formers. Investigating the feeding habits of the herbivores we can infer the level of pressure on plant populations. Each feeding guild has a varying impact on a plant as they target different life stages and parts of the plants (Andrew & Hughes, Reference Andrew and Hughes2005). For example, younger plants generally support more external herbivory (sap-sucking and chewing) and more mature plants will generally support larger endophagous herbivores (miners and stem-borers) (Andrew & Hughes, Reference Andrew and Hughes2005). With the tall-statured grasses having similar guilds of insects it is likely that all three reeds will have similar modes of damage from herbivory, however, the resultant pressure to populations will vary according to a number of factors, especially herbivore abundance.
All three reed species had pressure from sap-sucking herbivores with aphid species associated with them. On P. australis and P. mauritianus the cosmopolitan aphid, Hyalopterus pruni was found in 25 and 23% of NWS sites, respectively. On A. donax, the introduced aphid, M. donacis native to southern France (Blackman & Eastop, Reference Blackman and Eastop2008) was found to be abundant and widespread being found in 75% of the NWS sites. Both aphid species were found to reach high population size during certain times, however, it is unlikely that they were causing any major impacts on the reeds. For M. donacis, previous studies in North America have found that even at high densities the aphids have a low impact on A. donax (Dudley & Lambert, Reference Dudley and Lambert2007).
Another sap-sucking herbivore found in the study was the Chinch bug, D. zuluensis. Dimorphopterus zuluensis has previously been recorded as restricted to feeding on Phragmites with its main hosts being P. australis and P. mauritianus (Slater & Wilcox, Reference Slater and Wilcox1973). Dimorphopterus zuluensis was found to have a behavioural expansion in the LTS sites to utilize A. donax, mainly during autumn months. Some Blissinae species are known to shift to a secondary host plant when the primary host plant dries up however these species are unlikely to persist on the secondary host plant (Slater, Reference Slater1976). This was confirmed during NWS with no D. zuluensis being found on any A. donax plants highlighting that Phragmites spp. are the preferred host and utilizing A. donax is relatively rare and opportunistic.
Stem borers were found on all three reed species and when present they likely inflicted considerable damage on the reeds as internal stem-feeding are known to cause substantial fitness costs (Cronin et al., Reference Cronin, Bhattarai, Allen and Meyerson2015). The lepidopteran stem borers found are native to Africa (with the exception of the cereal crop borer, C. partellus) and have co-evolved with native grasses and sedges; most are known pests of agricultural crops such as sorghum and maize (Getu et al., Reference Getu, Overholt and Kairu2001; Moolman et al., Reference Moolman, Van den Berg, Conlong, Cugala, Siebert and Le Rü2014). In this study, 17 lepidopteran stem borers were recorded on the three reeds and all are widespread throughout Africa (Ong'amo et al., Reference Ong'amo, Le Gall, Ndemah and Le Rü2014). However, despite widespread distribution a general low incidence rate on wild hosts of stem borers has been found; borers generally prefer cereal crop hosts and are relatively specialized to a few host species (Ong'amo et al., Reference Ong'amo, Rü, Dupas, Moyal, Muchugu, Calatayud and Silvain2006; Le Rü et al., Reference Le Rü, Ong'amo, Moyal, Ngala, Musyoka, Abdullah, Cugala, Defabachew, Haile, Matama and Lada2006a, Reference Le Rü, Ong'amo, Moyal, Muchugu, Ngala, Musyoka, Abdullah, Matama-Kauma, Lada, Pallangyo and Omwegab; Ong'amo et al., Reference Ong'amo, Le Gall, Ndemah and Le Rü2014). For example, S. calamistis was found only on P. australis in this study but is known to prefer wild sorghum, Sorghum verticilliflorum (Steud.) and Sorghum rigidifolium (Stapf) (Prinsloo & Uys, Reference Prinsloo, Uys, Prinsloo and Uys2015). However, expansion of host ranges to other tall-statured grasses such as A. donax, P. australis and P. mauritianus can occur if there has been habitat destruction and therefore utilizing new species is a common mechanism to survive non-cropping seasons (Ndemah et al., Reference Ndemah, Schulthess, Le Rü and Bame2007). This most likely explains the occurrence of stem borers on A. donax in sub-Saharan Africa found in this study and the literature despite the reed not being native to the region.
In addition to stem borers, all three reed species were attacked by shoot flies that were found to bore through the lateral shoots and stems of the reeds. Shoot flies are common herbivores on grasses as they thrive on the extensive vegetative parts (Nartshuk, Reference Nartshuk2014). Shoot flies can impact tall-statured grasses as they can reach large numbers and feed on the embryonic tissue in the apical cone and can thus influence plant growth parameters (Nartshuk, Reference Nartshuk2014). As with the stem borers, the shoot flies found are known pests of important agricultural crops (Prinsloo & Uys, Reference Prinsloo, Uys, Prinsloo and Uys2015). For example, the muscid, Atherigona sp. found on both P. australis and P. mauritianus is an important pest of sorghum which is the preferred host (Prinsloo & Uys, Reference Prinsloo, Uys, Prinsloo and Uys2015).
For all three tall-statured grasses, galling herbivores were found in all sites sampled and were found to reach high abundances. These galling herbivores could not be identified to species level, however, it is likely that they are monophagous on Phragmites spp., as gall inducing phytophagous species are generally specialists as they must be finely adapted to the internal environmental of the host (Quiring et al., Reference Quiring, Flaherty, Johns, Morrison, Ozaki, Yukawa, Ohgushi and Price2006). On P. australis the mite, Steneotarsonemus sp. was the most abundant herbivore in the LTS with a mean of 27.8% of plants sampled having galls present. Two galling herbivores were found on P. mauritianus, a galling Eriophyid mite and a Cecidomyiid in the Giraudiella genus. The Giraudiella sp. will most likely be closely related to the galling midge Giraudiella inclusa (Diptera: Cecidomyiidae) found on P. australis in Europe (Skuhravá & Skuhravý, Reference Skuhravá, Skuhravý, Shorthouse and Rohfritsch1992; Tscharntke, Reference Tscharntke2008). The discovery of these specialist species may have important implications to control efforts on invasive P. australis genotypes, particularly in North America. In the USA, biological control of European P. australis haplotypes is being considered as some herbivores have been found to be specific to plant genotype (Schwarzländer & Häfliger, Reference Schwarzländer and Häfliger2000; Tewksbury et al., Reference Tewksbury, Casagrande, Blossey, Häfliger and Schwarzländer2002; Häfliger et al., Reference Häfliger, Schwarzlaender and Blossey2005; Blossey, Reference Blossey2014; Casagrande et al., Reference Casagrande, Häfliger, Hinz, Tewksbury and Blossey2018). Further research on host specificity may determine their potential as biological control agents.
For A. donax, the galling wasp T. romana was the most widespread and abundant herbivore. Tetramesa romana was also found to be established in North America and after host specificity and impact testing was subsequently used for augmentative biological control to increase their field populations (Dudley et al., Reference Dudley, Lambert, Kirk, Hoddle and Johnson2006; Goolsby & Moran, Reference Goolsby and Moran2009). Such biological control efforts along the Rio Grande in North America have shown to have had success in reducing A. donax populations; Moran et al. (Reference Moran, Vacek, Racelis, Pratt and Goolsby2017) report that releases of wasps from 2007 to 2014 have resulted in a 22% decline in A. donax biomass. This study did not measure T. romana impact directly but found that galling resulted in increased plant height, diameter, and lateral shoot production. Previous studies on T. romana impact have found that wasps increase lateral shoot production and leaf area (Dudley & Lambert, Reference Dudley and Lambert2007; Spencer et al., Reference Spencer, Tan and Whitehand2010). Our findings may reflect such pressure on A. donax plant productivity. Further investigation is now needed to assess T. romana impacts and assess if augmentative biological control is likely to enhance such pressures on A. donax populations.
With the overall herbivore load determined on A. donax, P. australis and P. mauritianus it is possible to test if the predictions of the ERH are met for A. donax in South Africa. In this study A. donax did have a reduced number of herbivores compared with Europe, however, there were more herbivores compared to North America with a number of feeding guilds. This higher level of herbivory was unexpected, particularly, when compared with surveys in North America where the plant has also had a long period of establishment and had multiple introductions (Tarin et al., Reference Tarin, Pepper, Goolsby, Moran, Arquieta, Kirk and Manhart2013) and as such the factors behind having greater herbivory are unclear. It was also a complicating factor that herbivores were introduced along with the invasive plant, as even though herbivory may still be lower than in the introduced range, it does not represent enemy-release sensu stricto (Allen et al., Reference Allen, Young, Bhattarai, Croy, Lambert, Meyerson and Cronin2015). For example, the monophagous gall-forming flies, Lipara (Diptera: Chloropidae) were introduced along with invasive P. australis from Europe into North America and it was found that in some areas of the adventive range the invasive P. australis genotypes had higher attack rates from Lipara than in the ancestral range (Allen et al., Reference Allen, Young, Bhattarai, Croy, Lambert, Meyerson and Cronin2015). Lastly, it may be important to consider that for A. donax release from herbivory may not be the leading factor for its invasive success; some plants are instead largely controlled by ecological factors (Keane & Crawley, Reference Keane and Crawley2002; Colautti et al., Reference Colautti, Ricciardi, Grigorovich and MacIsaac2004; Prior et al., Reference Prior, Powell, Joseph and Hellmann2015; Mlynarek et al., Reference Mlynarek, Moffat, Edwards, Einfeldt, Heustis, Johns, MacDonnell, Pureswaran, Quiring, Shibel and Heard2017).
For both Phragmites spp., despite being considered native to South Africa a relatively low herbivore diversity was found. For P. australis only seventeen species were found to be associated with the reed compared with over 150 species associated with the reed in Europe (Tewksbury et al., Reference Tewksbury, Casagrande, Blossey, Häfliger and Schwarzländer2002). Such a result was unexpected because P. australis is native to South Africa and Europe, so would be expected to have a similar assemblage of herbivores. In North America, species richness of herbivores on P. australis was also low compared with Europe (Tewksbury et al., Reference Tewksbury, Casagrande, Blossey, Häfliger and Schwarzländer2002), however, this lack of specialist herbivores has been attributed to the cryptic invasion of genotypes from Europe (Schwarzländer & Häfliger, Reference Schwarzländer and Häfliger2000; Saltonstall, Reference Saltonstall2002). It was determined that a cryptic invasion has not occurred in South Africa so this lack of herbivory is unlikely to be linked to plant genotype (Canavan et al., Reference Canavan, Paterson, Lambertini and Hill2018). Furthermore, with the inclusion of the known endemic species P. mauritianus it was possible to compare herbivore assemblages and it was found that the two species share similar species richness and diversity. A study by Weyl (Reference Weyl2015) found a similar pattern of herbivory in Myriophyllum spicatum L. (Haloragceae) populations in South Africa; M. spicatum is believed to be native yet lacked herbivores. Such results raise important questions on the diversification of herbivores on tall-statured grasses in Africa and the plant's evolutionary history in the region.
Conclusion
This study investigated the ERH with site-specific research that included native analogous species and thus a clearer picture of the role of herbivory on tall-statured grasses in South Africa. The predictions of the ERH were for the most part not met in this study as although A. donax had reduced species richness and diversity compared with the Phragmites spp. there has still been some recovery of herbivores; our assumption of A. donax being introduced with no coadapted herbivores was not met. The study also revealed that when related plants are present in the native range there is a greater probability that the associated stenophagous herbivores can expand their host range to include the new host. Enemy release may play some role in the invasive success of A. donax in South Africa where there are far fewer natural enemies than in the native range, but the plant is not devoid of natural enemies and has a similar herbivore assemblage to analogous native species, so the ERH is only partially supported by this study.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0007485318000627.
Acknowledgements
Funding for this work was provided by the South African Research Chairs Initiative of the Department of Science and Technology and the National Research Foundation of South Africa. Any opinion, finding, conclusion or recommendation expressed in this material is that of the authors and the NRF does not accept any liability in this regard. The authors thank Dr Des Conlong and Denise Gillespie for assisting with information on field collections and providing the diet vials. Dr Bruno Pierre Le Rü, Dr Marcela Skuhravá, Dr Charnie Craemer are thanked for assistance with herbivore identification.