INTRODUCTION
Seagrasses are widely distributed throughout shallow estuaries and coastal areas of temperate and tropical seas, providing vegetative habitat and shelter for various marine animals (Hemminga & Duarte, Reference Hemminga and Duarte2000). Many fish and invertebrate species use seagrass beds as feeding and/or nursery grounds, including many economically important species (Nelson, Reference Nelson1981; Edgar & Shaw, Reference Edgar and Shaw1995; Haywood et al., Reference Haywood, Vance and Loneragan1995; Huh & An, Reference Huh and An1997; Guidetti & Bussotti, Reference Guidetti and Bussotti2000). Seagrass habitats increase the number of microhabitats, sediment deposition and stabilization, food resources, and protection from predators, and reduce hydrodynamic forces (Lewis, Reference Lewis1984; Klumpp et al., Reference Klumpp, Howard, Pollard, Larkum, McComb and Shepherd1989; Connolly et al., Reference Connolly, Jenkins, Loneragan, Butler and Jernakoff1999; Hemminga & Duarte, Reference Hemminga and Duarte2000). However, despite the importance of these habitats in supporting regional biodiversity, seagrass habitats have been experiencing global declines in coverage, often related to the destruction of physical habitat, increased nutrient and sediment runoff, over-exploitation and decreased water quality due to anthropogenic activities (Dennison et al., Reference Dennison, Orth, Moore, Stevenson, Carter, Kollar, Bergstrom and Batiuk1993; Short & Wyllie-Echeverria, Reference Short and Wyllie-Echeverria1996; Erftemeijer & Lewis, Reference Erftemeijer and Lewis2006; Orth et al., Reference Orth, Carruthers, Dennison, Duarte, Fourqurean, Heck, Hughes, Kendrick, Kenworthy, Olyarnik, Short, Waycott and Williams2006; Burkholder et al., Reference Burkholder, Tomsko and Touchette2007; Cabaço et al., Reference Cabaço, Santos and Duarte2008).
Zostera marina (eelgrass) is the most common seagrass species in temperate coastal areas around the world. This species forms large eelgrass beds along the coastlines of southern Korea (Huh, Reference Huh1986; Huh & Kwak, Reference Huh and Kwak1997; Lee et al., Reference Lee, Moon, Hwang, Huh and Kim2000). Although many ecological studies of faunal assemblages in eelgrass beds have been conducted in Korean waters, they have primarily focused on fish species (e.g. Huh, Reference Huh1986; Go & Cho, Reference Go and Cho1997; Huh & Kwak, Reference Huh and Kwak1997; Kwak et al., Reference Kwak, Huh and Choi2006). Only a few studies in Kwangyang Bay have examined seasonal variation in the species composition and abundance of decapods in eelgrass beds of Korean waters (Huh & An, Reference Huh and An1997, Reference Huh and An1998). In contrast, many studies in other areas have described decapod assemblages in seagrass habitats (e.g. Holmquist et al., Reference Holmquist, Powell and Sogard1989; Kwak & Klumpp, Reference Kwak and Klumpp2004; De La Rosa et al., Reference De La Rosa, Rodríguez and Raso2006) and compared assemblage structure between vegetated and unvegetated habitats (e.g. Lazzari, Reference Lazzari2002; Nakamura & Sano, Reference Nakamura and Sano2005). Because invertebrate epifauna in seagrass meadows may have an essential role in the transfer of primary production to higher trophic level (Bauer, Reference Bauer1985), and in regulating densities of seagrass microbenthic taxa by their predations (Nelson, Reference Nelson1981), decapod crustaceans may potentially affect the structure of the seagrass community.
In many shallow seagrass habitats, vegetation patterns are important factors that control faunal assemblage structure (e.g. Guidetti, Reference Guidetti2000; Bloomfield & Gillanders, Reference Bloomfield and Gillanders2005; Kwak et al., Reference Kwak, Huh and Choi2006; Ribeiro et al., Reference Ribeiro, Carvalho, Gonçalves and Erzini2012), and variation in vegetation coverage may drive differences in habitat preferences among species (Guidetti, Reference Guidetti2000). For example, vegetated habitats increase the species richness and abundance of fish and decapod assemblages in South Australia (Bloomfield & Gillanders, Reference Bloomfield and Gillanders2005). Higher densities of juvenile fishes have been observed in Posidonia seagrass and rocky-algal reed habitats compared with unvegetated habitats in the Adriatic Sea (Guidetti, Reference Guidetti2000). In addition, vegetated habitat harbours large numbers of fish and crustaceans, and supports a greater diversity of faunal assemblages (Bell & Pollard, Reference Bell, Pollard, Larkum, McComb and Shepherd1989; West & King, Reference West and King1996). Due to the crucial role of seagrass beds and adjacent habitats in sustaining biodiversity, such studies are essential for future conservation efforts, as they elucidate the ecological mechanisms pertinent to the effective management of these areas (Bell & Pollard, Reference Bell, Pollard, Larkum, McComb and Shepherd1989; Ferrell & Bell, Reference Ferrell and Bell1991). Nonetheless, relatively few studies have compared decapod assemblages between vegetated and non-vegetated habitats (e.g. Lazzari, Reference Lazzari2002; Bloomfield & Gillanders, Reference Bloomfield and Gillanders2005; Nakamura & Sano, Reference Nakamura and Sano2005).
In the present study, we assessed spatial and temporal differences in decapod assemblages inhabiting eelgrass beds and unvegetated habitat in northern Jinhae Bay, Korea. Our objectives were (1) to assess differences in decapod assemblage structure among three types of habitats, and (2) to examine seasonal changes in diversities of decapod assemblages.
MATERIALS AND METHODS
Study area
Investigations were conducted in the northern part of Jinhae Bay located along the south-eastern coast of Korea (Figure 1). Jinhae Bay is semi-closed and connected by two inlets, the north-eastern and south-western inlets, to the open sea. Eelgrass beds are well-developed in the northern Jinhae Bay and provide habitat for a variety of invertebrates and small fish, which in turn serve as potential prey items for large fishes (Kwak et al., Reference Kwak, Park and Huh2014). The eelgrass bed within the study area forms subtidal bands in shallow waters (<7 m) and is distributed as patches along the shorelines. In the study area, seagrass beds are dominated by Zostera marina. The mean tidal ranges of the study area are 1.8 and 0.7 m at spring and neap tide, respectively. Three study sites were chosen in northern Jinhae Bay to examine differences in decapod assemblages among three types of habitat: eelgrass beds adjacent to tidal flats (ET) or rocky shore (ER), and unvegetated habitat (UV).

Fig. 1. Locations of the study sites of eelgrass beds adjacent to tidal flats (ET) and rocky shore (ER) as well as unvegetated habitat (UV) in northern Jinhae Bay, Korea.
Sampling
Decapod samples were collected using a 3 m beam trawl (1.9 cm mesh wing and body, 0.6 cm mesh liner). For each monthly sampling, four 6-min tows were conducted during the daytime at spring tide throughout 2002. The study area was ~180 m2 for each sampling event. The total area swept by 144 trawl hauls was 25,920 m2 (4 samplings × 12 months × 3 habitats × 180 m2). Although this method may be destructive to seagrass beds, beam trawl survey is also beneficial to estimate actual densities of benthic marine organisms in seagrass habitats (Coles, Reference Coles1986). Specimens were preserved immediately after capture in 5% formalin and later transferred to 70% isopropanol for storage. Samples were identified to the species level.
Surface water temperature and salinity were monitored monthly at each sampling using a thermometer and a salinometer, respectively. Eelgrass biomass was estimated by collecting all plants at the sea bottom from an area of 0.1 m2. The plants were dried at 80 °C for 24 h and then weighed to the nearest 0.1 g. Eelgrass biomass was expressed as dry weight per square metre (DW/m2). The sediments of each site were examined monthly throughout the year for both particle size and loss on ignition (LOI) analyses. The top 5 cm of sediments from the bottom were collected using three replicate 10 cm diameter cores. Sediment samples were first analysed to determine grain size and texture after drying at 80 °C for 24 h (Gee & Bauder, Reference Gee and Bauder1979). Sediment composition was based on four grain-size classes: gravel (>2.0 mm), sand (2.0–0.05 mm), silt (0.002–0.05 mm) and clay (< 0.002 mm). The proportion of each component size class was calculated for each sample. Next, because organic matter derived from microalgae (e.g. seagrass) may constitute the food source for deposit feeders such as decapods in coastal environments (Maclntyre et al., Reference Maclntyre, Geider and Miller1996), LOI was determined at 500–550 °C (4 h) to estimate organic content, following Heiri et al. (Reference Heiri, Lotter and Lemcke2001).
Data analysis
Differences in environmental variables in relation to habitat type were examined using univariate analyses. Student's t-test was used to determine eelgrass biomass between two eelgrass beds (ET and ER), and one-way analysis of variance (ANOVA) was applied to test spatial differences in LOI. One-way analysis of similarity (ANOSIM) was performed to test significant differences in sediment composition among three different habitats.
Diversity (H′) (Shannon & Weaver, Reference Shannon and Weaver1949) was calculated as: ![]() ${H}^{\prime} = - \sum {(n_i/N) \times \log \,(n_i/N)} $, where n is the number of individuals (abundance) of each species i in a sample, and N is the total number of individuals. Two-way ANOVAs were used to assess habitat and seasonal differences in abundance (number of individuals) and diversity of decapod assemblage. Habitat and season were considered fixed factors, and Tukey's test was used for post-hoc comparisons after ANOVA. The relationships between decapod abundance and environmental variables were analysed using linear regression. All species were considered in the analyses, and abundances were log (x + 1)-transformed. For each of six numerically abundant species which constituted >4% in total abundance, spatial and seasonal variations in mean abundance were analysed.
${H}^{\prime} = - \sum {(n_i/N) \times \log \,(n_i/N)} $, where n is the number of individuals (abundance) of each species i in a sample, and N is the total number of individuals. Two-way ANOVAs were used to assess habitat and seasonal differences in abundance (number of individuals) and diversity of decapod assemblage. Habitat and season were considered fixed factors, and Tukey's test was used for post-hoc comparisons after ANOVA. The relationships between decapod abundance and environmental variables were analysed using linear regression. All species were considered in the analyses, and abundances were log (x + 1)-transformed. For each of six numerically abundant species which constituted >4% in total abundance, spatial and seasonal variations in mean abundance were analysed.
To examine differences in decapod assemblages among habitats and among seasons, assemblage data were log (x + 1)-transformed, and Bray–Curtis similarity matrix was constructed (Clarke et al., Reference Clarke, Somerfield and Chapman2006) and then visualised using nMDS ordination. The matrix was then subjected to a series of two-way permutational multivariate analyses of variances (PERMANOVAs) to test for significant effects of habitat (three levels) and season (four levels) as well as their two-way interactions. PERMANOVA is a non-parametric distance-based analysis of variance that uses permutation procedures to test hypotheses. Two-way crossed ANOSIMs were used to test for significant differences in assemblage structure with respect to habitat or season based on the same factors used in the PERMANOVA (see above) and with the magnitude of the R-statistic indicating the relative importance of any such differences (Clarke et al., Reference Clarke, Gorley, Somerfield and Warwick2014). Global R-statistic values from the ANOSIM represent differences in the similarities (distance) within and among defined groups; R-values vary between 0 and 1. An R-value of zero indicates no differences in the average similarity among and within groups, and an R value of 1 indicates that the compositions of all samples within each group are more similar to each other than to any of the samples from any other group (Clarke et al., Reference Clarke, Gorley, Somerfield and Warwick2014). In cases in which ANOSIM detected a significant difference, pairwise ANOSIM comparisons were then used to determine which comparisons between habitats and between seasons were significantly different. To discriminate significant effects obtained by PERMANOVA and ANOSIM, canonical analyses of principal coordinates (CAPs) were used (Anderson et al., Reference Anderson, Gorley and Clarke2008). The relative contributions of species to the observed differences were assessed using correlation coefficients among each habitat or season and the canonical axis, and the correlations of individual species >0.50 with CAP axes 1 and 2 were plotted.
ANOVA and regression analyses were conducted using SYSTAT software (Systat version 18, SPSS Inc., Chicago), and multivariate analyses were performed using routines in the PRIMER v7 multivariate statistics package (www.primer-e.com) and the PERMANOVA + add-on module (Anderson et al., Reference Anderson, Gorley and Clarke2008; Clarke & Gorley, Reference Clarke and Gorley2015). Statistical differences were determined at the 0.05 significance level.
RESULTS
Environmental variables
Water temperature in the study area ranged from 7.4 to 27.7 °C, with a peak in July followed by a decline in October and reaching a minimum during winter. Salinity ranged from 25.4 to 34.2‰, with lower values in August and September when salinity dropped to about 25‰ (see also Kwak et al., Reference Kwak, Huh and Choi2006). Average eelgrass biomass ranged from 29.2 to 269.7 g DW m−2 at ET and from 38.7 to 278.5 g DW m−2 at ER (Figure 2), and the values varied greatly across the seasons in both eelgrass beds, although the average biomass did not significantly differ between the two eelgrass habitats (t-test, P > 0.05). Peak eelgrass biomass occurred during spring at both sites, followed by a sharp decline in winter (Figure 2). LOI also fluctuated seasonally, with a peak between spring and summer at all three study sites (Figure 2). Mean LOIs significantly differed among the three habitats (one-way ANOVA, P < 0.05). Tukey's post-hoc test revealed that mean LOIs were significantly higher in the two eelgrass habitats than in the unvegetated area, but values did not significantly differ between the eelgrass habitats. The proportion of sediment particle sizes significantly differed among habitats (one-way ANOSIM, P < 0.05; Table 1). The two eelgrass habitats contained relatively higher proportions of coarse particles (e.g. sand and silt), whereas the proportion of compact particles (e.g. clay) was higher in unvegetated habitat (Table 1).

Fig. 2. Seasonal variation in eelgrass biomass (DW m−2) and loss on ignition (LOI) in eelgrass beds adjacent to tidal flats (ET) and rocky shore (ER), and unvegetated habitat (UV) in northern Jinhae Bay, Korea. Error bars represent standard deviations.
Table 1. Mean proportions (± SE) of sediment grain size in eelgrass beds adjacent to tidal flats (ET) and rocky shore (ER), and unvegetated habitat (UV) in northern Jinhae Bay, Korea.

GENERAL DESCRIPTIONS OF DECAPOD ASSEMBLAGES
A total of 4195 decapods belonging to 31 species and five taxa (Penaeidae, Caridea, Anomura, Gebiidea and Brachyura) were collected during the study period, with caridean shrimps (13 species) and crabs (13 species) as the most widely represented taxa (Table 2). In terms of abundance, the dominant caridean shrimps were Palaemon macrodactylus (18.7%), Alpheus digitalis (4.2%) and Crangon uritai (3.5%), whereas Charybdis japonica (24.3%), Charybdis bimaculata (10.1%), and Hemigrapsus penicillatus (7.8%) were the most abundant crab species, and Trachysalambria curvirostris (2.7%) was the dominant prawn species. Only a species of hermit crab (Pagurus dubius) was caught during the study period.
Table 2. Species composition of decapod assemblages and total abundance of species (N) in eelgrass beds adjacent to tidal flats (ET) and rocky shore (ER), and unvegetated habitat (UV) in northern Jinhae Bay, Korea.

aDecapod species of commercial value.
The total number of decapod species was highest at the ET site (29 species), followed by ER (16 species) and UV (13 species). Only nine species were found at all three habitats, 15 occurred at both seagrass beds, but no species were exclusive of the UV. The highest abundance (2039 individuals) was also recorded at ET, whereas the lowest occurred at UV. In terms of taxa, the abundances of prawns, caridean shrimps and hermit crabs were highest at ET, but crabs exclusively occurred at ER. Among commercially important species, eight and four species were caught at ET and ER, respectively, but no commercial species were found in the unvegetated habitat. Overall, the number and abundance of decapod species (including commercial species) tended to be higher within seagrass beds than in unvegetated habitat in the study area. Only P. macrodactylus (caridean shrimps) and C. bimaculata (crabs) were numerically dominant in UV.
Spatial and seasonal variations in decapod abundance and diversity
Mean abundance and diversity were highest at ET, followed by ER and UV, during all seasons with the exception of autumn, when abundance was highest at ER (Figure 3). In terms of season, the two variables tended to be higher in spring, followed by winter and summer, and they were lowest during autumn (Figure 3). Two-way ANOVAs revealed that abundance and diversity of decapod assemblages differed significantly among habitats and among seasons (Table 3). The habitat × season interaction was not significant for abundance or diversity (Table 3). Tukey's post-hoc test indicated that eelgrass beds harboured significantly higher mean abundance and diversity compared with unvegetated areas, and values of all variables were highest during the spring (Table 3).

Fig. 3. Mean abundance and diversity of decapod species during different seasons in eelgrass beds adjacent to tidal flats (ET) or rocky shore (ER) or in unvegetated areas (UV) in northern Jinhae Bay, Korea. Error bars represent standard deviations.
Table 3. Results of two-way ANOVAs for abundance and diversity of decapods among eelgrass beds adjacent to tidal flats (ET) or rocky shore (ER) or in unvegetated areas (UV) over the four seasons in northern Jinhae Bay, Korea.

Tukey's test: P < 0.05. df = degrees of freedom; MS = mean square; Wi = winter; Sp = spring; Su = summer; Au = autumn.
Spatial and seasonal variations in abundances of dominant species
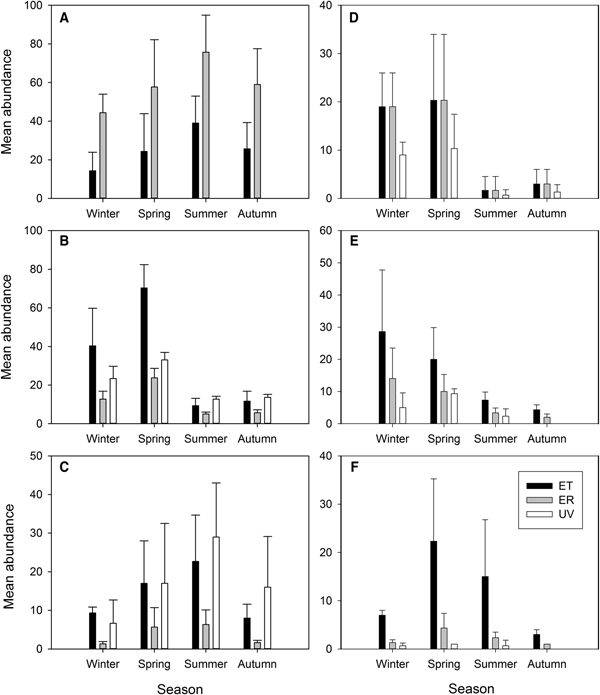
The mean abundances for each of the six dominant species varied with habitat and season (Figure 4). The mean abundance of C. japonica in ER was higher than in ET (Figure 4A), but did not differ seasonally. The mean abundance of P. macrodactylus and H. penicillatus differed between vegetated and unvegetated habitats, and between colder (winter and spring) and warmer (summer and autumn) seasons, with higher abundance during winter and spring at seagrass beds (Figure 4B and D). Charybdis bimaculata exhibited higher abundances in summer in both ER and UV compared with ET (Figure 4C), and those of P. dubius and A. digitalis differed among both habitats and seasons, with the highest values for both species occurring at ET, followed by ER and UV (Figure 4E and F).

Fig. 4. Mean abundance of six common decapod species during different seasons in eelgrass beds adjacent to tidal flats (ET) or rocky shore (ER) or in unvegetated areas (UV) in northern Jinhae Bay, Korea. Error bars represent standard deviations. (A) Charybdis japonica; (B) Palaemon macrodactylus; (C) Charybdis bimaculata; (D) Hemigrapsus penicillatus; (E) Pagurus dubius; and (F) Alpheus digitalis.
Decapod assemblage structure
Two-way PERMANOVAs revealed that the decapod assemblages differed significantly across habitats and seasons, with a higher F value for habitat compared with season (habitat: pseudo-F = 22.655, P = 0.001; season: pseudo-F = 13.331, P = 0.001). The habitat × season interaction was also significant (pseudo-F = 1.738, P = 0.020). Results of two-way crossed ANOSIM tests also indicated that the decapod assemblages differed significantly among habitats (global-R = 0.877, P = 0.001) and among seasons (global-R = 0.665, P = 0.001). Pairwise ANOSIM tests revealed that decapod assemblages differed significantly between all habitat and season comparisons (pairwise comparisons; P < 0.05), with the exception of the pairwise comparison between summer and autumn. The habitat differences were strongest between ER and UV (global-R = 0.963, P = 0.001).
The decapod assemblages plotted on the nMDS ordination axes exhibit discrete groups of samples according to both habitat and season (Figure 5). In terms of habitat, clear separation occurred between eelgrass beds and unvegetated habitat, whereas the sampling sites for the two eelgrass beds exhibited considerable overlap. In terms of season, two distinct seasonal groups, warmer (summer and autumn) or colder (spring and winter), were clearly separated on the ordination plot.

Fig. 5. nMDS ordination of decapod assemblages constructed from Bray–Curtis similarity matrix of the three habitats and four seasons. Wi = winter, Sp = spring, Su = summer, Au = autumn.
Because the PERMANOVA results indicated that both habitat and season significantly influenced decapod assemblage structure, CAP analyses were performed to discriminate among habitats and seasons (Figure 6). In the CAP plot for habitat, C. japonica and Sesarma pictum distinguished the ER from the other habitats, whereas multiple species characterized the decapod assemblages of the ET (Figure 6A). No species were significantly correlated with UV. Palaemon macrodactylus and C. bimaculata tended to be positively correlated with both ET and UV. In the CAP plot for season, each season exhibited clear separation, but some overlap occurred between summer and autumn samples (Figure 6B). Most species were highly correlated with spring samples, while C. bimaculata and H. penicillatus were correlated with summer and winter samples, respectively. However, no species were correlated with autumn samples (Figure 6B).

Fig. 6. Canonical analysis of principal coordinates (CAP) ordination plots of decapod assemblages to assess differences among habitats (A) and among seasons (B). Species correlations with each canonical axis are represented as vectors for species with correlations greater than 0.5. Vectors represent Pearson correlations, and the circle indicates a correlation of 1. Adi, Alpheus digitalis; Caf, Crangon affinis; Cbi, Charybdis bimaculata; Cha, Crangon hakodatei; Cja, Charybdis japonica; Cur, Crangon uritai; Ele, Eualus leptognathus; Hpa, Heptacarpus pandaloides; Hpe, Hemigrapsus penicillatus; Pdu, Pagurus dubius; Pma, Palaemon macrodactylus; Por, Palaemon ortmanni; Pqu, Pugettia quadridens; Ptr, Portunus trituberculatus; Spi, Sesarma pictum; Tcu, Trachysalambria curvirostris; Uma, Upogebia major.
Relationships between decapod abundance and environmental variables
Linear regression revealed that decapod abundances were significantly correlated with eelgrass biomass and the LOI of sediment throughout the study period (Figure 7; P < 0.05); however, neither water temperature nor salinity was significantly related to the abundance of decapods (P > 0.05).

Fig. 7. Relationships between decapod abundance and eelgrass biomass (DW m−2) and loss on ignition at three stations in northern Jinhae Bay, Korea. Black, grey and open symbols indicate eelgrass beds adjacent to tidal flats (ET), rocky shore (ER) and unvegetated areas (UV), respectively.
DISCUSSION
A total of 31 decapod species, primarily caridean shrimps and crabs, was collected from the study area. Palaemon macrodactylus, C. affinis and A. digitalis were the most abundant caridean shrimps, and C. japonica and C. bimaculata (Portunidae), H. penicillatus (Grapsoidea) and P. quadridens (Majoidea) were the dominant crab species. Similar to Jinhae Bay, those shrimps and crabs were also the dominant decapod species in eelgrass beds of Kwangyang Bay, Korea (Huh & An, Reference Huh and An1997, Reference Huh and An1998). In other unvegetated coastal habitats of southern Korea, however, those species were not common decapod species. For example, Trachysalambria curvirostris (prawn) and Crangon hakodatei (caridean shrimp) were dominant decapod species in southern Geojo Island (Cho et al., Reference Cho, Kim, Park and Cha2013), while C. hakodatei, Plesionika izumiae and C. bimaculata were common decapod species in Nakdong River estuary (Lee et al., Reference Lee, Kim, Lee, Shin and Chang2009). Thus, although abundant decapod species are often similar in seagrass beds, different dominances of these taxonomic groups were evident between vegetated and non-vegetated habitats indicating differences of habitat preferences of each decapod species.
In the present study, decapod assemblages exhibited clear differences in terms of species richness (i.e. number of species), abundance and diversity among habitats. A total of 29 and 16 species were found in the two seagrass habitats (ET and ER), respectively, whereas 13 species were caught in the unvegetated area. Decapod abundance and diversity were also significantly higher in seagrass vs unvegetated habitats, with the highest values occurring in eelgrass beds adjacent to tidal flats (two-way ANOVA post-hoc comparison, P < 0.05). Similarly, Bloomfield & Gillanders (Reference Bloomfield and Gillanders2005) documented significantly higher abundances of fishes and invertebrates in seagrass beds compared with non-vegetated habitats in southern Australia. Several studies comparing fish abundances between vegetated and non-vegetated habitats have reported similar results (e.g. Guidetti, Reference Guidetti2000; Kwak et al., Reference Kwak, Huh and Choi2006), as seagrass vegetation often supports higher densities and species richness of fish and decapod assemblages (Lazzari, Reference Lazzari2002).
The nMDS ordination and multivariate analyses confirmed that decapod assemblage structure differed significantly among habitats. These differences in assemblages were caused by variation in habitat use by individual decapod species. For example, C. japonica was limited to eelgrass beds, with its highest abundance in eelgrass beds adjacent to rocky shore. Penaeid and Crangonid shrimps (C. affinis and C. uritai) were also highly dependent on Zostera marina beds. All of the above decapod species are closely associated with seagrass habitats in Korean waters (e.g. Huh & An, Reference Huh and An1997, Reference Huh and An1998). Although the two eelgrass habitats exhibited similar patterns of species distributions, taxonomic composition did differ to some degree; for example, caridean shrimps were more strongly associated with tidal flats, whereas crabs were predominantly found in eelgrass near rocky shores. Portunid and grapsoid crabs tend to be associated with rocky habitats and are frequently found along rocky shores in shallow marine habitats (Hong, Reference Hong2006). Thus, the rocky shore habitat likely supports high abundances of crabs when adjacent to seagrass habitats. In unvegetated areas, P. macrodactylus (caridean shrimps) and C. bimaculata (crabs) were the most dominant species, although they also occurred in eelgrass beds. Charybdis bimaculata prefer to inhabit silt-clay bottoms in coastal areas (Narita et al., Reference Narita, Ganmanee and Sekiguchi2008) and were similarly abundant in other non-vegetated coastal habitats of Korea (e.g. Huh et al., Reference Huh, Park, Jeong and Baeck2010).
Although the overall sediment composition was similar among habitats, sediments of the unvegetated areas contained more clay compared with the eelgrass beds. Generally, seagrasses stabilize their underlying sediments by baffling tidal currents and dampening wave action (Orth, Reference Orth and Coull1977). In unvegetated environments, sediments are frequently resuspended and transported by wave and tidal currents, resulting in an unstable environment for many benthic invertebrates. However, due to a geographic feature protecting UV from wave action, our unvegetated study site could have more clay sediment than seagrass beds. In addition, fine sediments (e.g. clay-rich sediments) provide suitable environments for burrowing benthic invertebrates, whereas larger grain sizes provide more suitable habitat and/or shelter for larger crustaceans, i.e. decapods (Riddle, Reference Riddle1988). Consequently, in the present study area, the silt-clay dominant sediment may be suitable for the development of Zostera marina and its many resident decapod species.
Seasonal variation in both species composition and abundance tends to be considerable for decapod communities utilizing eelgrass beds. In the present study, the peak in decapod abundance corresponded closely to peak eelgrass biomass and LOI. When eelgrass biomass increased in spring, the number of decapod individuals also increased, followed by population declines with decreases in eelgrass biomass beginning in autumn. Because high seagrass density provides shelters and/or food resources for decapods (Hemminga & Duarte, Reference Hemminga and Duarte2000; March & Pringle, Reference March and Pringle2003), variations of decapod abundance corresponded to eelgrass biomass. A number of other studies have also demonstrated positive correlations between faunal richness or abundance and the above-ground biomass of seagrass beds (e.g. Leber, Reference Leber1985; Bell & Pollard, Reference Bell, Pollard, Larkum, McComb and Shepherd1989; Heck & Weinstein, Reference Heck and Weinstein1989; Huh & Kwak, Reference Huh and Kwak1997; Connolly et al., Reference Connolly, Jenkins, Loneragan, Butler and Jernakoff1999). Other studies of fish communities in eelgrass beds have reported similar patterns of variable faunal abundance in Korea. For example, fish abundance increased with increasing eelgrass biomass and water temperature in Angol Bay (Lee et al., Reference Lee, Moon, Hwang, Huh and Kim2000) and in Hamduck off of Cheju Island (Go & Cho, Reference Go and Cho1997). Kwak & Klumpp (Reference Kwak and Klumpp2004) demonstrated that seasonal variation of decapod abundance in Cockle Bay, Australia, was positively correlated with seagrass biomass. In addition, Ávila et al. (Reference Ávila, Yáñez and Vazquez-Maldonado2015) reported that seagrass shoot density and leaf length influenced the distribution of associated macrofaunal assemblages. The abundance of caridean shrimps was significantly associated with eelgrass biomass in Kwangyang Bay, Korea (Huh & An, Reference Huh and An1997). High seagrass densities provide a favourable habitat for many seagrass-associated decapods, whereas decapods common to unvegetated areas, such as C. bimaculata and P. macrodactylus, exhibited peak abundance in summer when water temperatures were highest. Although those species were also caught in all habitats, even abundant at seagrass beds, seasonal variations in their abundances seem to be related to water temperature, regardless of seagrass biomass.
This study provides important insights into the spatio-temporal variation in decapod assemblages in eelgrass beds and unvegetated areas of northern Jinhae Bay, Korea. Our findings document that decapod assemblage structure is significantly affected by both habitat type and season. These compositional differences were caused by variation in the abundances of the most common species. Moreover, decapod abundance was significantly higher in eelgrass beds compared with unvegetated habitat. Because the seagrass habitats supported high abundances of marine organisms, including commercially important species, these habitats play crucial roles, both ecologically and economically. In addition, studies of crustacean assemblages in seagrass habitats are meaningful in light of conservation and habitat monitoring efforts, because seagrass-associated crustaceans are less mobile and closely dependent on seagrass habitat, and thus, more easily influenced by environmental variations than most of the fishes.
ACKNOWLEDGEMENTS
We are grateful to Seong Oh Im (Korean Marine Environment Management Corporation) and Hyun Gi Choo (Korean Ocean & Fisheries Institute) for assistance with sampling and data analysis.
FINANCIAL SUPPORT
This work was supported by the Ministry of Maritime Affairs and Fisheries, Korea.