Introduction
The discovery of synthetic auxin herbicides during World War II provided a selective weed control option for many agricultural systems. Among the synthetic auxins, 2,4-D is used widely for broadleaf weed control in aquatics, cereal crops, corn (Zea mays L.), fruit, non-cropland, nuts, pastures, turf, and other crops (Peterson et al. Reference Peterson, McMaster, Riechers, Skelton and Stahlman2016). Use of 2,4-D on these sites helps to control plants that are poisonous or allergenic, obstruct traffic line of sight, and host crop pests (Bovey Reference Bovey1980). Additionally, 2,4-D use improves crop and forage yields by decreasing plant competition for light, water, and nutrients; aids in the establishment of desirable vegetation; allows for reduced tillage and soil conservation; increases land values and aesthetics; reduces labor requirements; decreases food costs; and improves farm stability and income (Bovey Reference Bovey1980; Burnside Reference Burnside1996). Applications of 2,4-D can also reduce mowing frequency by controlling weeds that grow more quickly than turf. Weeds potentially causing physical injury or discomfort in parks or athletic fields such as lawn burweed (Soliva sessilis Ruiz & Pavón) and puncturevine (Tribulus terrestris L.) are controlled by 2,4-D (Long Reference Long1974; McCarty et al. Reference McCarty, Everst, Hall, Murphy and Yelverton2008). A main drawback of 2,4-D use is the potential for non-target damage from particle or vapor drift both to desirable vegetation and crops lacking 2,4-D tolerance. Applications of 2,4-D increased from 416,000 to 24,040,395 kg annually from 1945 to 1964 because of its benefits, effectiveness, and low production cost (Peterson Reference Peterson1967). A recent estimate in 2011 put 2,4-D annual use at 11,611,964 kg (USDA 2014), but future annual use could increase to 79,832,257 kg by 2020 dependent on grower adoption of genetically engineered 2,4-D–resistant corn and soybean [Glycine max (L.) Merr.] varieties (USDA 2014).
Several environmental factors, including light, temperature, and humidity, influence 2,4-D uptake, with translocation occurring in the phloem following absorption (Monaco et al. Reference Monaco, Weller and Ashton2002; Peterson et al. Reference Peterson, McMaster, Riechers, Skelton and Stahlman2016). Selectivity between plants tolerant and sensitive to 2,4-D is influenced predominantly by translocation (Ashton Reference Ashton1958; Pillmoor and Gaunt Reference Pillmoor and Gaunt1981). Further, sensitive species commonly metabolize 2,4-D through direct conjugation resulting in phytotoxic metabolites, whereas tolerant species metabolize 2,4-D through ring hydroxylation resulting in nonphytotoxic metabolites (Peterson et al. Reference Peterson, McMaster, Riechers, Skelton and Stahlman2016). In sensitive dicots, a three-phase response to the application of synthetic auxin herbicides leads to plant death. First, plants are stimulated, resulting in abnormal growth (epinasty and tissue swelling) with subsequent stimulation of ATPase, ethylene biosynthesis, gene expression, enzyme synthesis, and abscisic acid (ABA) accumulation (Grossmann Reference Grossmann2009; Monaco et al. Reference Monaco, Weller and Ashton2002). Second, and within 24 h, plant growth is inhibited, stomates are closed, transpiration and carbon assimilation is reduced, and reactive oxygen species are overproduced (Grossmann Reference Grossmann2009). Finally, chloroplast and membrane damage and a disruption of the phloem leads to wilt, chlorosis, senescence, necrosis, and plant death in several days or weeks (Grossmann Reference Grossmann2009; Monaco et al. Reference Monaco, Weller and Ashton2002).
To date, the mechanism of action (MOA) for synthetic auxin herbicides is not completely understood, although substantial progress has recently been made (Christoffoleti et al. Reference Christoffoleti, de Figueirdo, Peres, Nissen and Gaines2015). Auxin-binding protein (ABP1), the auxin receptor SCFTIR1/AFB (SCF, Skp1, Cullin, F-box; TIR1, transport inhibitor response; AFB, auxin-signaling F-box), and the auxin receptor SCFSKP2A (SKP2A, S-phase kinase-associated protein 2A) are involved in auxin transport and reception and the mechanisms of auxin action (Christoffoleti et al. Reference Christoffoleti, de Figueirdo, Peres, Nissen and Gaines2015; Dharmasiri et al. Reference Dharmasiri, Dharmasiri and Estelle2005; Mithila et al. Reference Mithila, Hall, Johnson, Kelley and Riechers2011; Peterson et al. Reference Peterson, McMaster, Riechers, Skelton and Stahlman2016). As such, these are potential mechanisms for resistance development in weeds (Christoffoleti et al. Reference Christoffoleti, de Figueirdo, Peres, Nissen and Gaines2015; Dharmasiri et al. Reference Dharmasiri, Dharmasiri and Estelle2005; Mithila et al. Reference Mithila, Hall, Johnson, Kelley and Riechers2011). While resistance to synthetic auxin herbicides is relatively slow to develop compared with other MOAs (Cranston et al. Reference Cranston, Kern, Hackett, Miller, Maxwell and Dyer2001), the development of resistance to synthetic auxins is troublesome, because it substantially reduces the availability of selective herbicide options for weed control in several managed systems.
Twenty-eight dicotyledonous weed species, five in the United States, are confirmed to be resistant to synthetic auxin herbicides (WSSA Group 4) (Heap Reference Heap2017). The majority of the dicotyledonous weeds species confirmed resistant to synthetic auxin herbicides have an annual life cycle, but spotted knapweed (Centaurea stoebe L.), Canada thistle [Cirsium arvense (L.) Scop.], and tall buttercup (Ranunculus acris L.) are among the perennials reportedly resistant to synthetic auxin herbicides (Heap Reference Heap2017). The first report of resistance to synthetic auxin herbicides occurred in 1957 on the biennial wild carrot (Daucus carota L.) (Switzer Reference Switzer1957), with later reports arising in 1979 (Heap Reference Heap2017). Often, resistance to 2,4-D or MCPA was confirmed for these species, and biotypes have been suspected but not confirmed to be resistant to other synthetic auxin herbicides.
Buckhorn plantain, also called narrow-leaved or ribwort plantain, is a perennial broadleaf weed often found in turf, pastures, meadows, and roadsides (Cavers et al. Reference Cavers, Bassett and Crompton1980; Uva et al. Reference Uva, Neal and DiTomaso1997). Buckhorn plantain tolerates close mowing and is commonly found in dry, unirrigated sites (Cavers et al. Reference Cavers, Bassett and Crompton1980; Uva et al. Reference Uva, Neal and DiTomaso1997) but is also found in unmown or irregularly mown pastures, meadows, and roadsides (Cavers et al. Reference Cavers, Bassett and Crompton1980). Among the synthetic auxins: 2,4-D and premixtures of 2,4-D with other synthetic auxins, clopyralid + triclopyr, and MCPA + mecoprop + dicamba typically provide excellent control (>90%); fluroxypyr and triclopyr alone provide intermediate control (~50%); and dicamba provides poor control (<50%) (Branham Reference Branham1990; Hall Reference Hall1976; McCullough et al. Reference McCullough, Johnston, Reed and Yu2015; Patton et al. Reference Patton, Weisenberger, Kao-Kniffin, Branham, Voigt, Christians, Thoms, Hoyle, Munshaw, Hathaway, Nikolai, Bauer, Fresenburg, Xiong, Kreuser, Thompson, Gardner, Soldat and Koch2017; Stier and Newman Reference Stier and Newman1999; Watschke Reference Watschke1983). The phenoxycarboxylic acid herbicides (2,4-D, dichlorprop, MCPA, mecoprop) typically control buckhorn plantain, with a variable response among pyridinecarboxylic acid herbicides (clopyralid, fluroxypyr, triclopyr), and the benzoic acid dicamba does not control buckhorn plantain.
A biotype of buckhorn plantain with suspected resistance to 2,4-D was identified in 2014 in a cemetery in central Indiana. The site had received repeated annual applications of 2,4-D + mecoprop + dicamba for approximately 30 yr. As the deregulation of genetically engineered crops with 2,4-D tolerance occurs, there will be an increased risk of resistant weed biotypes spreading through selection pressure from greater use of the herbicide. The objectives of this study were (1) to confirm and quantify the level of the resistance in the buckhorn plantain population to 2,4-D and triclopyr using dose–response experiments and (2) to find alternative herbicides that could be used to control this 2,4-D–resistant buckhorn plantain population in managed turf.
Materials and Methods
Case History
A failure to control buckhorn plantain at a cemetery in Greenwood, IN (39.616°N, 86.118°W) was reported following the application of 2,4-D + mecoprop + dicamba at 1.3 + 0.4 + 0.1 kg ae ha−1. The cemetery grounds had been managed by the same lawn care company for approximately 30 yr. Few to none of the typical synthetic auxin herbicide epinasty symptoms, including bending, twisting, curling, and cupping, were visible after the application. The site contained buckhorn plantain covering 10% to 40% of the ground across the 40-acre property, and no other perennial broadleaf weeds were present (Figure 1). Further, the Kentucky bluegrass (Poa pratensis L.) and perennial ryegrass (Lolium perenne L.) turf at the site was thin due to a suboptimal cutting height (<5 cm) that favored competition from the buckhorn plantain. Eight buckhorn plantain plants were collected in late August from this location and also from a 2,4-D–susceptible population in West Lafayette, IN, for a preliminary herbicide-dose evaluation. The suspected resistant (R) population and the susceptible (S) population were sprayed with 2,4-D (Weedar® 64, Nufarm, Burr Ridge, IL 60527) at 1.7, 3.4, and 6.7 kg ae ha−1 and compared with a nontreated control. Results from this preliminary dose–response screening affirmed the concern of potential 2,4-D resistance, and subsequent field and greenhouse experiments were initiated.

Figure 1 (A) An abundance of buckhorn plantain remained at a cemetery in Greenwood, IN, following applications of 2,4-D + mecoprop + dicamba. (B) Treated plants had few of the classical synthetic auxin herbicide epinasty symptoms visible, including bending, twisting, curling, and cupping, and no chlorosis or necrosis of leaf tissues after herbicide applications.
Greenhouse Studies Confirming Resistance
Greenhouse experiments were initiated in the fall of 2014 at Purdue University (West Lafayette, IN) to quantify the response of the buckhorn plantain biotypes to two synthetic auxin herbicides from different chemical families. A second collection of buckhorn plantain plants from Greenwood, IN, suspected to be resistant, and a known susceptible biotype from West Lafayette, IN, were collected in September 2014 and transplanted into 11 by 11 cm square pots (Hummert International, Earth City, MO 63045) filled with a Whitaker silt loam soil (fine-loamy, mixed, active, mesic Aeric Endoaqualf) with a pH of 6.8 and an organic matter content of 3.1%. After being transplanted, plants were watered as needed and fertilized every other week with two water-soluble fertilizers (3:1 mixture of 15 N–2.2 P–12.5 K and 21 N–2.2 P–16.6 K, respectively; Scotts, Marysville, OH 43040) to provide the following (in mg L−1): 200 N, 26 P, 163 K, 50 Ca, 20 Mg, 1.0 Fe, 0.5 Mn and Zn, 0.24 Cu and B, and 0.1 Mo. Seventy-six percent of the nitrogen provided was in the nitrate form. After 8 wk of establishment in the greenhouse, dose–response experiments were initiated.
The experiments were conducted in the greenhouse starting in November 2014 and were repeated February 2015. Each experiment was arranged as a randomized complete block design within herbicide (either triclopyr or 2,4-D) with five blocks. For each biotype, 2,4-D dimethylamine (Weedar® 64, Nufarm, Burr Ridge, IL 60527) doses of 0, 0.001, 0.0168, 0.168, 0.42, 0.84, 1.68, 3.36, 6.72, 13.4, 26.9, 53.8, and 107.5 kg ae ha−1 were tested, with 1.68 kg ae ha−1 as the standard application rate. Additionally, each population was tested for response to triclopyr (Turflon® Ester Ultra, Dow AgroSciences, Indianapolis, IN 46268) doses of 0, 0.001, 0.0112, 0.056, 0.14, 0.28, 0.56, 1.12, 2.24, 4.48, 8.96, 17.9, and 35.8 kg ae ha−1, with 1.12 kg ae ha−1 as the label application rate. Treatments were applied using compressed air in a track spray chamber (Generation III Research Sprayer, DeVries Manufacturing, Hollandale, MN 56045) calibrated to deliver a volume of 140 L ha−1 using a TeeJet® 8002EVS nozzle (TeeJet Technologies, Spraying Systems, Wheaton, IL 60187) at 275 kPa.
Following the herbicide application, day/night temperatures and photosynthetically active radiation were measured continuously (hourly) with a mini-weather station (WatchDog 2475 Plant Growth Station, Spectrum Technologies, Plainfield, IL 60585). Temperatures averaged 24.5 C with an average daily light integral of 10.3 mol m−2 d−1 during the first experiment and 24.0 C and a daily light integral of 11.6 mol m−2 d−1 when repeated.
Weed epinasty was visually assessed at 1 wk after application (WAA) on a 0% to 100% scale, where 0% was no epinasty and 100% represented complete epinasty with all leaves exhibiting symptoms, including twisting or bending of stems and curling of leaves. Chlorophyll content of the leaves was assessed at 3 WAA using a CCM-200 Chlorophyll Content Meter (Opti-Sciences, Hudson, NH 03051) to provide a chlorophyll content index. One measurement was taken per plant on a fully expanded leaf not among the newest or oldest leaves.
Digital images (1.92 megapixels at 180 dpi resolution) were taken of the individual buckhorn plantain plants at 4 WAA using a camera (Canon PowerShot SX260 HS, Canon USA, Melville, NY 11747) and light box similar to that described by Ghali et al. (Reference Ghali, Miller, Grabow and Huffman2012). Images were collected with the light box using camera settings of F-stop equal to f/3.5, 1/25-s exposure, and ISO speed equal to 100. Images were analyzed for percent green pixels with ImageJ (v. 1.48v, National Institutes of Health, Bethesda, MD 20892) (Schneider et al. Reference Schneider, Rasband and Eliceiri2012) using color threshold settings of hue=37 to 107, saturation=83 to 255, and brightness=47 to 255 in a modified macro (Soldat et al. Reference Soldat, Obear, DeBels and Barak2012). Images were taken of a green calibration disk, and data were converted from selected green pixels to plant area (cm2). At 4 WAA, plants were harvested approximately 1 to 2 cm above the soil surface to obtain a leaf fresh weight and also a leaf dry weight after 3 d of drying in a forced-air dryer at 60 C. The root zone and caudex of the plants was kept intact during harvest, and plants were allowed to regrow for an additional 3 wk after harvest. Following 3 wk of regrowth, a second leaf harvest was made at 7 WAA, and the dry weight was determined after 3 d of drying. A nontreated control for both herbicides was included in the experiment.
ANOVA was conducted with PROC GLM using SAS v. 9.4 (SAS Institute, Cary, NC 27513) and code provided by Seefeldt et al. (Reference Seefeldt, Jensen and Fuerst1995). There were no significant experiment by dose by population interactions, so data were combined across experiments. The dose required to reduce shoot weight by 50% relative to the untreated plants was calculated using a log-logistic dose–response curve (Equation 1) for each herbicide and each biotype (Seefeldt et al. Reference Seefeldt, Jensen and Fuerst1995):
where y is the buckhorn plantain response (i.e., epinasty, chlorophyll content index, fresh weight, dry weight, leaf area, or regrowth), GR50 is the herbicide dose giving a 50% response, b is the relative slope around GR50, D is the upper limit of the model, C is the lower limit of the model, and x is the rate of 2,4-D or triclopyr (kg ae ha−1) (Table 1). All regression analyses were conducted using Prism software (Prism 6 for Windows, GraphPad Software, La Jolla, CA 92037), and populations were deemed to have a similar herbicide response when the 95% confidence interval for GR50 values overlapped.
Table 1 Regression analysis parameters for data presented in figures using Equation 1.

a Resistance factor (RF) is a ratio of the resistant population GR50 and the susceptible population GR50 that quantifies the level of resistance to the herbicide.
Results and Discussion
Greenhouse Studies Confirming Resistance
ANOVA for 2,4-D–treated buckhorn plantain biotypes revealed a dose by biotype interaction for five of the six evaluations or measurements. Across dose, epinasty was higher at 1 WAA for the S biotype than the R biotype (41% vs. 13%, respectively). Chlorophyll content index at 3 WAA was lower for the S biotype than the R biotype (31% vs. 41%, respectively) following the 2,4-D application. Regression analysis of dose by biotype interaction of chlorophyll content index at 3 WAA determined that the 2,4-D GR50 values for the R and S biotypes were 35.9 and 1.9 kg ae ha−1, respectively (Table 1; Figure 2).
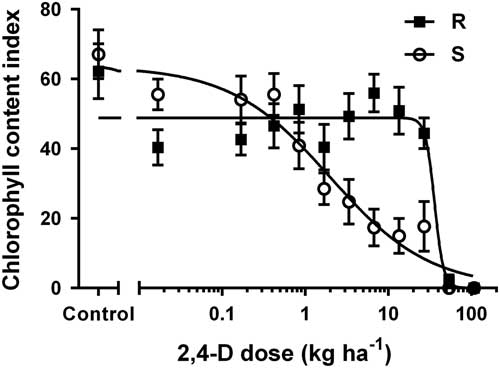
Figure 2 Chlorophyll content index of resistant (R) and susceptible (S) populations of buckhorn plantain at 3 wk after application of 2,4-D at various doses. Data for each biotype are combined over two experiments. Sigmoidal, nonlinear regression is shown with the standard error of the mean.
ANOVA of harvest data for 2,4-D–treated plants revealed a dose by biotype interaction for fresh weight, green leaf area, and plant regrowth, but not dry weight. While there was no dose by biotype interaction for dry weight, regression analysis and GR50 95% confidence intervals for dry weight indicated that the R and S biotype responses to 2,4-D were different (Table 1). Each measurement type yielded different GR50 and resistance factor (RF) values, but all support the same conclusion, namely, that the R biotype was ≥6.2-fold resistant to 2,4-D compared with the S biotype. The 2,4-D dose giving a 50% response (GR50) in the R biotype was 31.3, 29.5, 13.7, and 20.0 kg ae ha−1 for fresh weight, dry weight, green leaf area, and regrowth, respectively (Table 1; Figure 3). Comparatively, the 2,4-D dose giving a 50% response (GR50) for the S biotype was 4.6, 4.7, 0.45, and 2.3 kg ae ha−1 for fresh weight, dry weight, green leaf area, and regrowth, respectively (Table 1; Figure 3). The GR50 dose for the S biotype was near or above the maximum 2,4-D label rate of 1.68 kg ae ha−1. This is consistent with the authors’ anecdotal observations that S biotypes of buckhorn plantain are more difficult to control with 2,4-D than broadleaf plantain (Plantago major L.). Further, because buckhorn plantain is more difficult to control with 2,4-D than other species, it may help explain its ability to escape an application and develop herbicide resistance.

Figure 3 Fresh weight (A), dry weight (B), and leaf area estimates (C) of resistant (R) and susceptible (S) populations of buckhorn plantain at 4 wk after application (WAA) of 2,4-D at various doses. Plants were allowed to regrow for an additional 3 wk following the harvest at 4 WAA. Regrowth dry weights of the R and S biotypes of buckhorn plantain are shown (D). Data for each biotype are combined over two experiments. Sigmoidal, nonlinear regression is shown with the standard error of the mean.
The 2,4-D RFs for the R biotype of buckhorn plantain were 18, 6.8, 6.2, 30, and 8.8 for chlorophyll content index, fresh weight, dry weight, green leaf area, and regrowth, respectively (Table 1). This is considered moderate (RF=6 to 10) to high (RF=10 to 100) resistance (Beckie and Tardif Reference Beckie and Tardif2012), depending on which resistance factor or measurement is considered. Resistance to 2,4-D in annual broadleaf weeds ranges from species with low levels of resistance (RF=2 to 5), such as in yellow starthistle (Centaurea solstitialis L.) and wild radish (Raphanus raphanistrum L.) (Miller et al. Reference Miller, Shinn and Thill2001; Walsh et al. Reference Walsh, Powles, Beard, Parkin and Porter2004), to high levels in corn poppy (Papaver rhoeas L.), oriental mustard (Sisymbrium orientale Torn.), prickly lettuce (Lactuca serriola L.), and wild mustard (Sinapis arvensis L.) (Burke et al. Reference Burke, Yenish, Pittmann and Gallagher2009; Herbicide Resistance Action Committee 2016; Preston et al. Reference Preston, Dolman and Boutsalis2013; Rey-Caballero et al. Reference Rey-Caballero, Menéndez, Giné-Bordonaba, Salas, Alcántara and Torra2016). This range of 2,4-D resistance levels in annual broadleaf weeds is consistent with this experiment demonstrating moderate to high 2,4-D resistance in a perennial broadleaf weed.
ANOVA for triclopyr-treated buckhorn plantain biotypes revealed a dose by biotype interaction for only one of the six ratings or measurements. Across dose, epinasty was higher at 1 WAA for the S biotype than the R biotype (46% vs. 32%, respectively), but there was no dose by biotype interaction of epinasty at 1 WAA, indicating that both biotypes responded similarly to the dose screening. A lack of dose by biotype interaction for chlorophyll content index, fresh weight, dry weight, and green leaf area indicated a similar response to triclopyr among the R and S biotypes (Table 1; Figure 4). Calculated GR50 values for R and S biotypes ranged from 1.2 to 1.4 for S biotypes and 1.1 to 1.9 for R biotypes across fresh and dry weight and green leaf area data, which is near the maximum triclopyr label application rate of 1.12 kg ae ha−1. The RF for triclopyr ranged from 0.8 to 1.5 and, the R biotype was therefore determined to not be resistant to triclopyr. The ANOVA of regrowth data for triclopyr-treated plants revealed a dose by biotype interaction, but the triclopyr dose giving a 50% response (GR50) was 0.29 kg ae ha−1 for regrowth of the R biotype and 0.1 kg ae ha−1 for regrowth of the S biotype (Table 1; Figure 4). Because these GR50 values fell below the triclopyr label rate, the RF factor of 3.1 for regrowth was not considered as evidence of resistance to triclopyr.

Figure 4 Fresh weight (A), dry weight (B), and leaf area estimates (C) of resistant (R) and susceptible (S) populations of buckhorn plantain at 4 wk after application (WAA) of triclopyr at various doses. Plants were allowed to regrow for an additional 3 wk following the harvest at 4 WAA. Regrowth dry weights of the R and S biotypes of buckhorn plantain are shown (D). Data for each biotype are combined over two experiments. Sigmoidal, nonlinear regression is shown with the standard error of the mean.
Field Experiment Evaluating Alternative Control Options
A field experiment was initiated October 2014 at the cemetery in Greenwood, IN, to evaluate alternative herbicides to control the R biotype of buckhorn plantain and restore cemetery aesthetics. Unfortunately, we were unable to repeat the field experiment, as the caretaker treated the entire site with clopyralid at 0.56 kg ae ha−1 following the conclusion of our field experiment to restore cemetery aesthetics. Buckhorn plantain control in the field was ≤21% for 2,4-D, dichlorprop, MCPA, and mecoprop when rated at 32 WAA on June 22, 2015, which confirmed that these phenoxycarboxylic acid herbicides (WSSA Group 4) failed to provide acceptable control of the R biotype.
Clopyralid, a pyridinecarboxylic acid herbicide (WSSA Group 4), applied at 0.56 kg ae ha−1 provided 96% control of the R biotype of buckhorn plantain at 32 WAA in the field. Clopyralid was reported previously to control buckhorn plantain populations sensitive to 2,4-D, but this is the first test to confirm the effectiveness of clopyralid on a 2,4-D R biotype (Branham Reference Branham1990; Watschke Reference Watschke1983).
A premixture of 2,4-D + fluroxypyr + halauxifen-methyl provided 99% control of the buckhorn plantain R biotype at 32 WAA. Because neither 2,4-D or fluroxypyr alone provided >34% control of the R biotype of buckhorn plantain in our testing (unpublished data), the 99% control observed in field plots was attributed to the halauxifen-methyl (applied at 9 g ae ha−1) in the mixture. Halauxifen-methyl is a new arylpicolinate herbicide (WSSA Group 4) trademarked as Arylex™ (Epp et al. Reference Epp, Alexander, Balko, Buysee, Brewster, Bryan, Daeuble, Fields, Gast, Green, Irvine, Lo, Lowe, Renga, Richburg, Ruiz, Satchivi, Schmitzer, Siddall, Webster, Weimer, Whiteker and Yerkes2016).
Implications for Weed Scientists
While cross-resistance is reported among WSSA Group 4 benzoic acid, phenoxycarboxylic acid, and pyridinecarboxylic acid synthetic auxin herbicide families (WSSA Group 4) in catchweed bedstraw (Galium aparine L.), kochia [Kochia scoparia (L.) Schrad.], wild mustard, and yellow starthistle (Beckie and Tardif Reference Beckie and Tardif2012; Miller et al. Reference Miller, Shinn and Thill2001), resistance that is specific to herbicides within a chemical family may also occur. For example, Mangin and Hall (Reference Mangin and Hall2016) reported that spotted knapweed was highly resistant to clopyralid and picloram but not cross-resistant to 2,4-D. Resistance in this buckhorn plantain R biotype was limited to within a chemical family (phenoxycarboxylic acid) and not across all WSSA Group 4 synthetic auxin herbicides, as the pyridinecarboxylic acid herbicides clopyralid and triclopyr and the arylpicolinate herbicide halauxifen-methyl provided control in field or greenhouse experiments. This lack of cross-resistance is possibly attributable to pyridinecarboxylic acid herbicides having higher than average binding activity or a preferred site of action for AFB5, whereas AFB1, AFB2, and AFB3 are thought to preferentially bind to 2,4-D (Dharmasiri et al. Reference Dharmasiri, Dharmasiri and Estelle2005; Lee et al. Reference Lee, Sundaram, Armitage, Evans, Hawkes, Kepinski, Ferro and Napier2013; Walsh et al. Reference Walsh, Neal, Merlo, Honma, Hicks, Wolff, Matsumura and Davies2006). Similar to pyridinecarboxylic acids, the arylpicolinate herbicides have been shown to have higher than average binding activity or a preferred site of action for AFB5 (Walsh et al. Reference Walsh, Neal, Merlo, Honma, Hicks, Wolff, Matsumura and Davies2006).
The practical implication of this is that weeds developing resistance to WSSA Group 4 herbicides may or may not be cross-resistant to other Group 4 herbicides. Researchers should evaluate the potential cross-resistance to other synthetic auxin chemical families when resistance to a synthetic auxin herbicide is confirmed or suspected. Additionally, extension weed specialists should consider advising not just herbicides outside the Group 4 MOA as potential control alternatives when resistance is confirmed but also other families of Group 4 herbicides. In some crops, such as cool-season turfgrasses, there are few options outside Group 4 herbicides that provide effective control of troublesome broadleaf weeds. This is not to say that rotating herbicide MOA is not still a key factor in integrated weed management but that in some crops there may be few effective herbicides to rotate to without risking crop injury.
Consistent with Beckie and Tardif (Reference Beckie and Tardif2012), resistance developed in this case only after 30 yr of annual 2,4-D applications, because synthetic auxins are herbicides with a low risk for resistance development. Despite frequent use of synthetic auxin chemistry in turfgrass systems, this is the first report in the United States of a weed found in turf that is resistant to a synthetic auxin herbicide. Previously, a population of lawn burweed resistant to pyridinecarboxylic acid herbicides was discovered on a golf course in New Zealand (Harrington et al. Reference Harrington, Ward and Wells2001). Mithila et al. (Reference Mithila, Hall, Johnson, Kelley and Riechers2011) addressed the lack of resistance development in turf being attributable to several factors, including (1) using herbicide mixtures with two or more synthetic auxin herbicides comprising multiple families, (2) using grass species known to create dense swards to compete with germinating weeds, (3) fertilizing to promote increased density and reduced weed competition, (4) applying herbicides in high spray volumes to optimize spray coverage, and (5) removing tough to control weeds mechanically (hand weeding). Additional factors responsible for the lack of resistance development in turfgrass systems include (1) mowing to decrease weed seed production (Warwick and Briggs 1979), (2) harvesting grass clippings and inflorescences to reduce the weed seedbank (Gaussoin and Branham Reference Gaussoin and Branham1989), (3) reducing soil cultivation to minimize turf injury and recruitment from buried seed populations (Eggens Reference Eggens1980), and (4) applying PRE herbicides that prevent the emergence of weed seed (Proctor et al. Reference Proctor, Sousek, Patton, Weisenberger and Reicher2012).
Unfortunately, the discovery of a buckhorn plantain population resistant to 2,4-D on 16 ha of cemetery grounds in Greenwood, IN, is not unique. Additional populations of 2,4-D–resistant buckhorn plantain were subsequently confirmed in 2015 in Muncie, IN (AJP, unpublished data) and in 2014 in Salem, VA (SD Askew, personal communication). Practitioners typically control buckhorn plantain S biotypes successfully in turf with a premixture of 2,4-D + mecoprop + dicamba at a cost of $32 ha−1. However, this cemetery was forced to use clopyralid at the high label rate at a cost of $277 ha−1 to control their buckhorn plantain R biotype. As such, there is an economic incentive to use integrated weed management to prevent herbicide resistance development.
This is the first report of synthetic auxin herbicide resistance in a turf system in the United States. This report should serve as a notice for managers to implement resistance prevention strategies not already in place. It is uncommon to monitor sites for weed resistance in turf (Mithila et al. Reference Mithila, Hall, Johnson, Kelley and Riechers2011), because relatively few herbicide-resistant weeds have been documented in turf until recently (Brosnan and Breeden Reference Brosnan and Breeden2013; Cross et al. Reference Cross, Bridges, McCarty and McElroy2015). Turf managers will need to proactively educate themselves and their staffs about weed management techniques and herbicide resistance; scout for weed escapes following applications; rotate herbicide MOAs when possible; use tank mixtures with multiple MOAs when possible; and continue to use mechanical and cultural weed controls.
Acknowledgments
The authors thank the Midwest Regional Turf Foundation for partial financial support of this project. The authors also thank John Armes, William Cragen, Forrest Hayworth, Phil Lavanchy, and Bret Rush for their assistance with this project and Bryan Young for his helpful review of this paper.







