The arterial duct provides the necessary shortcut for blood to bypass the immature lungs during embryogenesis. After birth, the lungs become functional for exchange of gases, and the duct closes within a few days. Ductal constriction is initiated by an increase in the concentrations of oxygen in the blood, along with lower levels of the hormone prostaglandin. In a subsequent phase of remodelling, medial smooth muscle cells proliferate and migrate towards the intima, which eventually leads to complete ductal occlusion and its subsequent obliteration.Reference Blalock and Taussig1, Reference Levin, McCurnin and Seidner2 Clinical situations requiring intervention to maintain or close the duct include cardiac malformations with systemic or pulmonary hypoperfusion, but also preterm neonates in whom the duct fails to close spontaneously.Reference Hermes-DeSantis and Clyman3 Postnatal physiological closure can now be prevented in certain patients with cardiac malformations in whom continued patency is advantageous. In the short term, patency is maintained in most cases by infusion of prostaglandins, and in the longer term by the insertion of stents.Reference Alwi, Choo, Latiff, Kandavello, Samion and Mulyadi4 Closure has now been prevented by insertion of expandable stents for prolonged periods of up to 3 years.Reference Galantowicz and Cheatham5–Reference Schneider, Zartner, Sidiropoulos, Konertz and Hausdorf8 Disruption of the endothelial cell layer, or other injuries imposed during insertion of the stents, along with irritation of the tissues by the implanted stent, are known to initiate complex inflammatory processes. These responses stimulate the proliferation of smooth muscle cells, which constitute the major cell populating the medial layer of the vessel wall. The cellular proliferation is associated with an altered state of differentiation of the cells, and with migration of cells from the media towards the intimal layer in a process termed neointimal formation. Excessive neointimal formation narrows the lumen of the vessel, and eventually restricts the flow of blood. The consequence of this process, called in-stent restenosis, can be a life-threatening inadequate supply of blood to essential body organs.Reference Michel-Behnke, Akintuerk, Thul, Bauer, Hagel and Schranz9, Reference Gibbs, Uzun, Blackburn, Wren, Hamilton and Watterson10 The control of neointimal proliferation, therefore, is of vital importance. It is essential for the postnatal physiological closure of the arterial duct, yet is detrimental after insertion of vascular stents. Experimental approaches aimed at understanding the molecular details of neointimal formation by analysis of gene expression could contribute to the development of treatments with reduced side effects.Reference Adams, Geary, Li, Rossini and Schwartz11–Reference Zohlnhofer, Richter and Neumann15 So far, the best characterized animal model for study of the arterial duct is the sheep. The genomic sequence of the sheep, however, is not yet completed.
Detailed characterization of the molecular processes associated with the closure of the duct in humans would be advantageous, but only very rare samples of ductal tissue from newborns with cardiac malformations are currently available. In this study, therefore, we analysed the overall profile of genomic expression, seeking to define the gene regulation of the postnatal arterial duct when its constriction had been prevented by insertion of stents. To provide comparisons, we also included in our analysis samples from patients with stented pulmonary arteries. We obtained 2 distinct profiles of gene expression, which may be indicative of common regulatory processes occurring during vascular remodelling in postnatal patients with patent arterial ducts and in those with stented pulmonary arteries.
Materials and methods
Tissue samples
The clinical data of the patients is described in Table 1. An unstented duct had been maintained in the open state by infusion of prostaglandin E1 (Alprostadil—Minprog®, Pharmacia, Erlangen, Germany), and removed during surgery on the first day after birth from a neonate with critical aortic coarctation and totally anomalous pulmonary venous connection (Sample D). The ligamentous arterial duct (Sample L) had been resected at the age of 5 months from a patient with double aortic arch. Stainless steel stents (316-L; classification of the American Association of Industry and Steel) had been implanted in 2 patients, each with tetralogy of Fallot and pulmonary atresia with duct-dependent pulmonary circulation, at the age of 3 (D1) and 4 days (D2), respectively. Infusions of prostaglandin had been initiated shortly after birth, and discontinued immediately prior to the intervention. Due to an obstructive thrombus developing in patient D2 2 weeks after the intervention, the stent was redilated. Transcutaneous measurements showed that the saturation of oxygen remained above 75% until elective cardiac surgery was performed. Under general anaesthesia, median sternotomy was performed to allow access to the heart. Surgery in the patients from whom the samples D, D1, D2, D5, A3 and A4 were obtained was performed with cardio-pulmonary bypass with or without cardiac arrest (Table 1). Surgery in patient L was done without cardio-pulmonary bypass. Samples A3, A4 and D5 were from female patients, while all others were from male patients.
Table 1 Clinical characteristics of the patients from whom we obtained the vascular tissueFootnote *

* The clinical details of sample D5 were deemed of no relevance here, since the gene expression was not included in the analysis due to the poor quality of RNA for this sample.
The specimens, after removal, were stored transiently in a 0.9% solution of salt at 4°C. For analysis, explants were divided into 2 equal parts. The first half was transferred to 4% formaldehyde for histological analysis. For analysis of gene expression, the struts of the stents were removed from the second half, and the samples were shock-frozen in liquid nitrogen. They were then stored at minus 80°C until the RNA was extracted.
Isolation of ribonucleic acid (RNA)
Frozen samples were crushed in liquid nitrogen with mortar and pestle. Total RNA was isolated with a commercial kit (RNeasy Mini Kit No. 74104, Qiagen; Hilden, Germany), and treated with deoxyribonuclease according to the protocol supplied by the manufacturer. RNA was resuspended in water and analyzed using the RNA Nano Assay (Agilent 2100 Bioanalyzer, Agilent Technologies; Waldbronn, Germany).
Deoxyribonucleic acid (DNA) microarrays
RNA expression profiling was performed as previously described.Reference May, Hauser and Wirth16, Reference Mueller, May, Perz, Hauser and Peuster17 Probes were synthesized according to the manufacturer’s protocol (Affymetrix; Santa Clara, CA). For biotin-labeled target synthesis starting from 3 μg of total RNA, reactions were performed using standard protocols (Affymetrix; Santa Clara, CA). Briefly, 3 μg total RNA were converted to double stranded DNA using 100 pmol of a T7T23V primer (Eurogentec; Seraing, Belgium) containing a T7 promoter. The complementary-DNA (cDNA) was then used directly in an in-vitro transcription reaction in the presence of biotinylated nucleotides. The concentration of biotin labeled complementary-RNA (cRNA) was determined by ultraviolet light absorbance. In all cases, 12.5 μg of each biotinylated c-RNA preparation was fragmented and placed in a hybridization cocktail containing four biotinylated hybridization controls (BioB, BioC, BioD, and Cre). Samples were hybridized to an identical lot of Affymetrix GeneChip HG_U133_Plus_2 for 16 hours. After hybridization, the Chips were washed, stained with streptavidin-phycoerythrin (SA-PE) and read using an Affymetrix GeneChip fluidic station and scanner with Affymetrix standard software GCOS 1.2 GeneChip® Operating Software that controls the fluidics stations and scanner (Order Nr. 690036, Affymetrix; Santa Clara, CA). All experiments were scaled to a target intensity of 150 using the preset default values of GCOS 1.2. For the generation of the GO categories, the lists of regulated genes were imported into the program GOSurfer.Reference Zhong, Storch, Lipan, Kao, Weitz and Wong18 For the significance ranking of the GO categories either the up or the down regulated genes were copied into the program DAVID.Reference Hosack, Dennis, Sherman, Lane and Lempicki19 The probability score, a derivative of Fisher’s Exact probability test was used to determine the significance.Reference Hosack, Dennis, Sherman, Lane and Lempicki19 For gene-enrichment analysis the EASE Score, a modified Fisher Exact P-Value was calculated. It ranges from 0 to 1, whereby a p-value = 0 represents perfect enrichment. Clustering analysis was performed using the program Gene Cluster, the expression data was logarithmical transformed, genes and arrays median centered and then normalized for hierarchical average linkage clustering as suggested in the manual.Reference Eisen, Spellman, Brown and Botstein20 The resulting data was then transformed into a graphical representation using the associated program Tree View.
Histology and immunohistology
Ductal samples were prepared according to standard procedures. Briefly, the samples were fixed by soaking in cacodylate-buffered aqueous solution containing 4% paraformaldehyde, then dehydrated by a series of steps with increasing ethanol content, and finally degreased in xylene and embedded in Technovit 9100 (Heraeus Kulzer; Hanau, Germany). Serial sections were immuno-labeled with monoclonal antibodies against Ki-67 antigen (Clone 7B11 Zytomed; Berlin, Germany) using strepavidin-biotin labeling of antibodies (ZytoChem-Plus Kit, AEC Chromogen, Zytomed; Berlin, Germany) and toluidine blue counterstaining. Alternatively, sections were stained with haematoxylin and eosin.
Results
Intimal proliferation in stented ducts
In an effort to correlate the physiological postnatal state of patients with patent arterial ducts after implantation of stents with the effects on gene expression, explanted ducts were analyzed by various techniques. The optimal position of the stents was confirmed by diagnostic imaging (Fig. 1, a and b). Histological examination of the stented ducts D1 and D2 showed that the inside of the vessel was completely covered by an intact endothelial cell layer. In both specimens, the struts of the stent had been overgrown by neointimal tissue, reducing the luminal diameter (Fig. 1c and 1d). The stented duct D2 had a smaller luminal area, and a more pronounced neointimal formation within the stent (Table 2). Immune histochemical staining, nonetheless, identified only a few actively dividing cells in the section of the duct D2, while abundant proliferating cells were observed in the luminal tissue of the neointima of sample D1 (Fig. 1e and 1f, respectively). The stents, therefore, had maintained the patency of both ducts, but while neointimal proliferation had continued in the duct D1, proliferation had progressed further in the duct D2, but was reduced at the time of explantation.

Figure 1 Neointimal formation in stented postnatal arterial ducts. Angiography of the stented ducts D1 (a) and D2 (b) was performed 3 weeks post implantation. Two ducts were kept open each by implantation of AISI (American Iron and Steel Institute) 316-L stents. 7 months after stent implantation the tissue samples were removed and stained with haematoxylin and eosin as shown for sample D1 (c) and sample D2 (d). The struts in sample D2 (d) were removed as a consequence of the preparation. The pictures were taken at a 25-fold magnification. Ki-67 cell proliferation associated antigen positive luminal cell layers (deep purple, indicated with arrows) inside of the stent D1 (e) and absence of Ki-67 antigen specific staining cells in sample D2 (f). The pictures were taken at a 40-fold magnification.
Table 2 Morphometry of the neointimal layer of the stented arterial ducts

Characteristic signatures of gene expression in postnatal arterial ducts in the presence and absence of stents
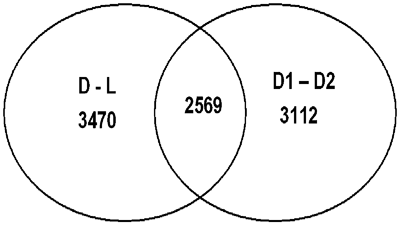
To identify biological processes that were regulated in the postnatal profile of gene expression, we compared samples taken from a patient with an open duct one day after birth, and the patient with a duct that had become ligamentous 5 months after birth. Over 10% of all sequences present on the gene array chip were differentially expressed in the sample from the patient with the open duct when compared to the patient with a ligament (Fig. 2, D to L). This indicates that closure of the duct is associated with a distinct alteration in the pattern of expression of genes. After implantation of the stent, mechanical hindrance of closure could either interfere with the postnatal programme of expression of genes, or alternatively, this programme could be executed irrespective of the presence of a stent. Interestingly, the analysis of the expression profiles from the two patients with stented ducts 7 months after birth revealed that the patterns were dissimilar (Fig. 2, D1, D2). Moreover, of the 54,684 sequences present on the chip, 5681 sequences were differently expressed in the samples taken from patients with stented ducts. A large fraction of these were also regulated in patients with ducts remaining unstented during postnatal development (Fig. 2, 2569). These analyses, therefore, may define a set of genes that are regulated during the physiological closure of the duct after birth. Furthermore, stents that prevented the physiological closure after birth could either prevent or delay the regulation of the genes for up to 7 months after birth.
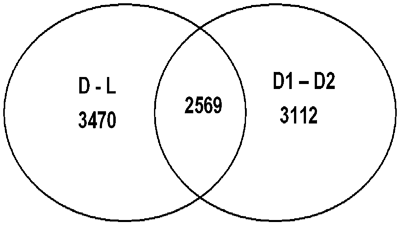
Figure 2 Common gene regulation in postnatal ducts with and without stents. Regulated genes were grouped together according to their expression in different tissue samples. 3470 sequences (probe sets) were differently expressed between native ductus (D) and ligament (L) tissues, 3112 sequences were differently expressed in stented ducts (D1 versus D2) and 2569 sequences were regulated in both native and stented tissue samples (center). The abbreviations for the tissue samples are indicated in Table 1.
Patients with stented pulmonary arteries and arterial ducts exhibit related patterns of gene regulation
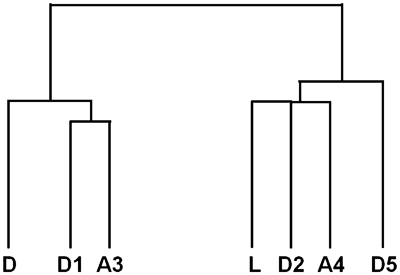
To decide whether the differential gene expression was specific for the postnatal closure of the arterial duct, we examined samples taken from 2 patients with stented pulmonary arteries. Again, the resulting profiles were dissimilar, albeit that a large fraction of the differentially expressed genes were also present among the genes regulated in the samples obtained from patients with patent ducts, suggesting that the regulatory processes could be replicated in blood vessels other than the postnatally patent arterial duct. The degree of similarity of the RNA profiles from the various tissue samples was calculated, and the graphical representation showed that the profiles of gene expression are grouped into two distinct clusters, a duct-related cluster and a ligament-related cluster (Fig. 3). The sample D5 has been included here solely to assess whether the RNA quality correlates with the patterns of gene expression. The grouping was independent of the tissue specificity, and could take place in the presence or absence of stents.
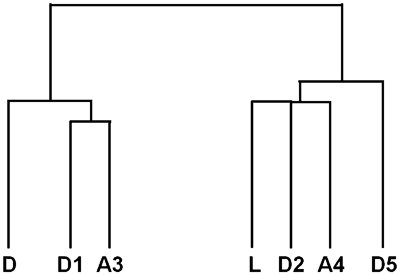
Figure 3 Two distinct profiles of gene expression from patients with patent arterial ducts and stented pulmonary arteries. We used Affymetrix gene expression to analyse the tissue samples using the program Cluster. The result is displayed as a graphical representation using the programme Tree View. Overall differences in gene expression are represented by the length of the vertical lines. The abbreviations for the tissue samples are indicated in Table 1.
The signature of gene expression is not related to the sample RNA quality
In clinical samples in particular, the quality of RNA is a known source of variability in profiles of gene expression.Reference Copois, Bibeau and Bascoul-Mollevi21 The possibility that the distinct patterns were solely due to the variability in the integrity of the RNA samples, therefore, needed to be excluded. To this end, we analyzed various parameters of quality. The intactness of the RNA was determined by the traditional ratio of the large ribosomal RNA versus the small ribosomal RNA, and by more recent elaborate methods. As previously reported, ranking of the sample RNA quality did depend on the method used for evaluation.Reference Imbeaud, Graudens and Boulanger22 Each single method correctly assigned the lowest quality to a sample with unequivocally poor RNA quality, which was included deliberately in this assay as a reference (Table 3, D5). The ribosomal RNA was degraded in this sample, and it could not subsequently be used for the quality ranking (Table 3, D5 28S:18S ribosomal RNA ratio and RIN). All of the methods, therefore, yielded similar overall results, even though individual samples varied somewhat in the position of their ranking. Only samples with a high RNA quality score were included in the analysis. None of these quality rankings correlated with the result for clustering of gene expressions (Table 3). The results lent no support to the notion that the quality of the RNA samples was a major determinant of the 2 distinct profiles observed. This conclusion was further strengthened by the finding that the observed gene regulation was highly specific, as outlined below.
Table 3 Quality of RNA related to selected clinical parameters.

*Due to the absence of rRNA peaks this parameter could not be determined.
Neointimal formation and muscle specific processes are regulated in the arterial duct and in stented pulmonary arteries
To identify the processes that were differentially regulated between the observed distinct profiles of gene expression, we subjected the data to statistical analysis. Commonly regulated genes in the three pair-wise comparisons, unstented ducts, stented ducts, and pulmonary arteries, respectively, were grouped into clusters according to their function. These clusters are groups of functionally related genes that are preset values in the programme we used for analysis. The highest significance score was assigned to the extracellular matrix synthesis cluster from genes that were differentially expressed in the patent duct (Table 4). Shortly after birth, while the duct was still open, the cells were apparently already preparing for closure by executing a transcriptional programme to form extracellular matrix, which is a key component of the ligament. A similar pattern was also detected in the stented duct, which contained actively proliferating cells, but not in the other stented duct, for which we observed no evidence of cellular proliferation (Fig. 1C compared to 1D). In the sample from the patient with an arterial ligament, a significant number of differentially expressed genes were functionally related to cardiac muscle (Table 5). During ligamentous formation, and in the stented blood vessels, therefore, a gene programme was executed that was indicative of neointimal formation.
Table 4 Genes related to the extracellular matrix compartment are overexpressed in the duct.Footnote *

* Genes that yielded at least two-fold higher signal in the open duct were assigned to Gene Ontology terms using the programme DAVID. The most significant term “Extracellular matrix” had a probability (P value) of 2.9 × 10−9.
Table 5 Genes related to the cardiac muscle are overexpressed in the ligamentFootnote *.

* Genes that yielded at least two-fold higher signal in the ligament were assigned to Gene Ontology terms using the program DAVID. The most significant term “Heart process” had a probability (P value) of 9.4 × 10.10
Discussion
In this study, we have investigated molecular processes in very rare clinical samples. As have others, we have used gene array analysis, the reliability of this method being generally accepted.Reference Heidecker, Kasper and Wittstein23, Reference Wang24 To detect regulated gene expression in response to insertion of stents, or during closure of the arterial duct, rather than tissue-specific differences, we made pairwise comparisons between the most highly related samples. The statistical limits used are the standard settings for the analysis of Affymetrix chips. We found 2 characteristic and reproducible distinct patterns of gene expression in multiple comparisons of samples of the closing postnatal arterial duct, in stented ducts 7 months after birth, and also in stented pulmonary arteries. In all pairwise comparisons, we observed a highly characteristic differential pattern of gene expression. These commonly regulated genes encompass a large fraction, up to 45% of all regulated genes, in each pairwise comparison (Fig. 2). When comparing each single profile with the others, all samples could unequivocally be assigned to either the ductal or the ligamentous type (Fig. 3). A search of the literature revealed that the processes that are active in the tissue obtained from patients with open ducts have also been found to be enhanced in coronary arteries, and in muscular arteries from the gastrointestinal tract.Reference Zohlnhofer, Richter and Neumann15 Thus, despite the present scarcity of samples, in our opinion the differential gene expression we have identified is reproducible and reliable. This finding, nonetheless, is unexpected. In an attempt to identify the process that caused the differential expression, we have correlated it with the data from other samples. After extraction from clinical samples, RNA quality is not always optimal. Therefore, we analysed the RNA carefully, dismissing the sample in which the quality was low (D5). When using multiple criterions, we were unable to detect any correlation between the quality of the RNA and the profiles of gene expression. Unexpectedly, the two samples from patients with stented pulmonary arteries varied widely, for reasons thus far undetermined. Also, we were unable to detect any relationship of the profiles of expression with the cardiac malformations observed in the patients themselves. With the exception of the role of the development of the duct, no common aspect could be identified in the other samples. To obtain possible clues from the expression of genes, we compared differentially regulated processes with known responses of the tissues to the insertion of stents. For this purpose, we used computer programmes that identify biological processes associated with the genes of interest. Extracellular matrix synthetic genes were expressed at significantly higher levels in the samples encompassing the patent duct (Table 5). This is characteristic for neointimal formation, whereby smooth muscle cells synthesize various collagens and other extracellular matrix proteins, in particular after vascular injury.Reference Ross and Klebanoff25 Among the over-expressed genes, collagen V and VI have been shown to be synthesized by homogeneous cultured smooth muscle cells.Reference Stepp, Kindy, Franzblau and Sonenshein26 It has been proposed that patterns of expression for contractile muscle, and matrix-specific patterns, are related to the state of proliferation and migration of smooth muscle cells.Reference Liau and Chan27–Reference Hao, Gabbiani and Bochaton-Piallat32 In this view, such a profile of expression could indicate the presence of active neointimal proliferation, but this remains to be confirmed.
In conclusion, by using very rare clinical samples, we have detected 2 characteristic patterns of gene regulation. In order now to identify the underlying mechanisms, we will need to analyse more samples, and collect more detailed clinical data.
Acknowledgements
We thank Robert Geffers for performing the array analysis.