INTRODUCTION
Contributions to knowledge of Syllidae in Arctic and sub-Arctic regions are scarce; the first paper in which some species of Syllidae were described is that of Malmgren (Reference Malmgren1867), who erected three new genera and described 12 new species from the material collected in Spitzbergen, Greenland Island and Scandinavia (although several of these new taxa are not valid). Later, Fauvel (Reference Fauvel1911) found 5 species in the Kara Sea. Wesenberg-Lund (Reference Wesenberg-Lund1947, Reference Wesenberg-Lund1950a, Reference Wesenberg-Lundb, Reference Wesenberg-Lund1951, Reference Wesenberg-Lund1953) published several papers about polychaetes from the Arctic and north-west Atlantic Ocean and reported 15 syllid species in total. Ushakov (Reference Ushakov1955) reported about 30 syllid species in the Russian and North Atlantic sub-Arctic regions. Chamberlin (Reference Chamberlin1920), Grainger (Reference Grainger1954) and Pettibone (Reference Pettibone1954) also made significant contributions to the knowledge of Canadian Arctic and sub-Arctic Syllidae. Other researchers who contributed to the knowledge of sub-Arctic syllids were Saemundsson (Reference Saemundsson1918), Annenkova (Reference Annenkova1934) and Buzhinskaja (Reference Buzhinskaja1980).
In spite of the huge geographical area, only about 40 species of Syllidae have been recorded in the whole Arctic and sub-Arctic areas.
The purpose of the present study is to find out the diversity of Syllidae in the Arctic and sub-Arctic regions.
MATERIALS AND METHODS
The syllid specimens were collected from 50 samples obtained at 26 different stations, during the years 1999, 2005 and 2006. Stations are located in the Barents Sea, some Norwegian fiords and the Norwegian Sea. The specimens were collected between 19 and 340 m depth. They were fixed in formalin and posteriorly preserved in ethanol 70%. Data of these localities are shown in Table 1.
Table 1. Location, date, number, coordinates, depth and notes of the stations. -, unknown.

The benthic materials were collected by Akvaplan-Niva AS, which provides consultancy, research and laboratory services to companies, authorities, and other clients worldwide (http://www.akvaplan.niva.no/default.asp). The material was transferred to the Laboratorio de Biología Marina e Invertebrados de la Universidad Autónoma de Madrid. A part of the collection, including the types of the new species, were deposited at the Museo Nacional de Ciencias Naturales de Madrid (MNCNM); other selected specimens of all species found, except the new ones, were deposited in the Aqvaplan collections.
Examinations were made using a binocular compound microscope Nikon XN, a light microscope Olympus CH-2 as well as an optic microscope with interference contrast optics (Nomarsky). Drawings were made using a camera lucida drawing tube. Scanning electron microscope (SEM) observations and photographs were made in SIDI (Servicio Interdepartamental de Investigación) of the Universidad Autónoma de Madrid, Spain.
RESULTS
In this paper, 16 species of Syllidae are reported; 2 new species for the science are described and 3 species are new reports from the Arctic Ocean.
MATERIAL EXAMINED
Two specimens (MNCNM 16.01/11218, 11219), Station No. SK4, 1 specimen, Station No. 14-05, 1 specimen, Station No. 20-05, Station No. 11-05.
DISTRIBUTION
Arctic and sub-Arctic; North Atlantic and North Pacific; and Mediterranean Sea.
HABITAT
Common in cold waters and scarce in temperate seas.
MATERIAL EXAMINED
Two specimens, Station No. 11-05.
DISTRIBUTION
Arctic (Spitzbergen, Alaska), Bering Sea.
HABITAT
Muddy sediments in low water to 145 m depth.
Pionosyllis lamelligera Saint-Joseph (Reference Saint-Joseph de1887): 163, pl. 8, figures 30–38; Fauvel (Reference Fauvel1923): 288, figure 110 a–g; San Martín (Reference San Martín, Ramos, Alba, Bellés, Gosálbez, Guerra, Macpherson, Martín, Serrano and Templado2003): 79, figures 30 & 31.
Synmerosyllis lamellligera San Martín et al. (Reference San Martín, López and Aguado2009): 37.
MATERIAL EXAMINED
Two specimens, Station No. GFA-7 (1999) (MNCNM 16.01/11239), 1 specimen, Station No. GFLM-12 (MNCNM 16.01/11237), 1 specimen, HU-07, 1 specimen, HU-13, 2 specimens, Station No. SFEK-8 (MNCNM 16.01/11235, 11236), 1 specimen, Station No. VGPT2-15, 1 specimen, VGPT1-19 (MNCNM 1601/11238).
DISTRIBUTION
Atlantic European coasts, from France to Cape Verde Islands, Mediterranean Sea, Cuba; Arctic and sub-Arctic.
HABITAT
Algae, kelps, among ascidians, Posidonia oceanica beds, calcareous concretions; intertidal to more than 100 m depth.
TYPE SPECIES
Opisthodonta pterochaeta Southern, 1914.
DIAGNOSIS
Body long, slender, tapered anteriorly and posteriorly, with numerous segments. Prostomium pentagonal to triangular, with 4 eyes and 2 anterior eyespots. Three antennae. Palps short, fused basally, triangular in shape. Nuchal organs as 2 ciliated grooves between peristomium and prostomium. Two pairs of tentacular cirri. Antennae, tentacular and dorsal cirri elongated, smooth, distally tapered. Ventral cirri triangular. Compound chaetae falcigers and spiniger-like chaetae or only with falcigers. Dorsal simple chaetae present. Ventral simple chaetae present or absent. Aciculae of several anterior parapodia distinctly enlarged. Pharynx and proventricle long, pharyngeal tooth located distally of anterior rim of pharynx to posteriorly. Reproduction by epigamy.
REMARKS
The diagnosis above is a modification of the original in San Martín & Hutchings (Reference San Martín and Hutchings2006).

Fig. 1. Streptodonta exsulis sp. nov. (A) Anterior part, dorsal view (holotype); (B) anterior end, dorsal view (paratype); (C) anterior end, ventral view (paratype). Scale: A: 0.375 mm, B, C: 0.18 mm.

Fig. 2. Streptodonta exsulis sp. nov. (A) Aciculae, anterior parapodium; (B) aciculae, posterior parapodium; (C) compound chaetae, anterior parapodium; (D) ventral chaeta; (E) compound chaetae, posterior parapodium. Scale: 20 µ.

Fig. 3. Streptodonta exsulis sp. nov. (A) Anterior part, dorsal view; (B) anterior end, dorsal view; (C) anterior pseudospiniger compound chaeta; (D) anterior falciger compound chaeta; (E) posterior falciger compound chaeta and simple chaeta; (F) anterior chaetigers.

Fig. 4. Streptodonta exsulis sp.nov. (A) Ciliated chaetigers; (B) ciliated areas between chaetigers.
MATERIAL EXAMINED
Holotype (MNCNM 16.01/11248) and 1 paratype (MNCNM 16.01/11252), Station No. GFB-9, 1 paratype (MNCNM 16.01/11251), Station No. GFA-7 (1999), 1 specimen (MNCNM 16.01/11249), Station No. GFA-7 (2005), 1 paratype, Station No. GFB-2, 1 paratype (MNCNM 16.01/11250), Station No. GFB-4.
DESCRIPTION
Body slender, with numerous segments (Figures 1A, 3A). Holotype incomplete, 4.5 mm long, 1 mm wide, with 27 chaetigers Prostomium pentagonal to triangular, with 2 pairs of eyes arranged in close trapezoidal pattern. Median antenna inserted in middle of prostomium, lateral antennae in front of anterior eyes (Figure 1B); median antenna detached on all specimens, missing on holotype (Figure 1A), lateral antennae longer than prostomium and palps together. Palps small, triangular, basally fused, shorter than prostomium. Peristomium slightly shorter than following segments; nuchal organs well developed (Figures 1B, 3B). Dorsal tentacular cirri longer than lateral antennae, ventral tentacular cirri shorter than dorsal ones. Dorsal cirri similar in shape to antennae and tentacular cirri, smooth, filiform. Ventral cirri triangular, slightly shorter than parapodial lobes on anterior parapodia (Figure 1C), distinctly shorter than parapodial lobes in posterior region (Figure 4A). Ciliated areas present on dorso-lateral parts of each chaetigers, just above to parapodial lobes (Figure 4B). Compound chaetae with distinctly heterogomph articulation; two types of compound chaetae, falcigers and pseudospinigers; pseudospinigers numbering about 1–3 per parapodium (Figure 3F); blades indistinctly bidentated (Figures 2C, E, 3C), about 50–56 µm long (Figures 2C, E). Numerous falcigers on anterior parapodia, numbering 15–17 on middle and posterior parapodia; blades of falcigers short, bidentate, with proximal tooth longer than distal one, bending ventrally, especially in posterior segments, measuring about 20 µm on anterior parapodia (Figures 2C, 3D), about 12 µm on posterior parapodia (Figures 2E, 3E); blades of pseudospinigers and falcigers with short spines on cutting edge (Figures 2C, E, 3C–E). Ventral simple chaetae from midbody, bidentate, with proximal tooth longer than distal one and somewhat curved, smooth on margin (Figure 2D). Usually 4 aciculae on each anterior parapodium, thick, distally strongly knobbed, with terminal button (Figure 2A); from segment 24 backwards, acicula solitary, slender, expanded subdistally, somewhat rounded distally (Figure 2B). Pharynx long, through 19–20 segments; pharyngeal tooth small, located medially, relatively close to anterior rim of pharynx (located in about anterior 1/9 of pharyngeal length) (Figure 1A–C). Proventricle through 8–9 segments, with about 29 muscle cell rows (Figure 1A).
REMARKS
Streptodonta exsulis differs from Streptodonta pterochaeta (Southern, 1914), the only previously known species of this genus in terms of the morphology of chaetae, in having pseudospinigers (absent in S. pterochaeta) and falcigers that are different in anterior and posterior chaetigers (similar in S. pterochaeta). Streptodonta exsulis, sp. nov. has 4 aciculae in anterior chaetigers (2 in S. pterochaeta) and a pharyngeal tooth that is located medially, close to anterior rim of pharynx.
ETYMOLOGY
The specific name comes from the Latin exsulis (= exiled), refering to the pharyngeal tooth located differently than the other species of the genus Streptodonta.
DISTRIBUTION
Sub-Arctic east North Atlantic Ocean.
HABITAT
Fine sediments. In depths of 133–141 m.
MATERIAL EXAMINED
One specimen, Station No. MO4, 1 specimen, Station No. 11-05.
DISTRIBUTION
Sub-Arctic, North Atlantic, North Pacific, Mediterranean.
HABITAT
Clay, mud, 40–200 m depth.
Exogone naidina San Martín (Reference San Martín, Ramos, Alba, Bellés, Gosálbez, Guerra, Macpherson, Martín, Serrano and Templado2003): 262, figures 142 & 143.
MATERIAL EXAMINED
One specimen, Station No. GFB-3, 1 specimen, Station No. GFB-9.
DISTRIBUTION
Apparently cosmopolitan.
HABITAT
Any kind of substrate, especially abundant in algae with fine sediment in shallow waters.
Exogone verugera San Martín (Reference San Martín, Ramos, Alba, Bellés, Gosálbez, Guerra, Macpherson, Martín, Serrano and Templado2003): 271, figures 147 & 148.
MATERIAL EXAMINED
Two specimens, Station No. SFEK-8 (MNCNM 16.01/11243), 2 specimens, Station No. GFB-2, 2 specimens, Station No. GFB-3 (MNCNM 16.01/11240, 11246), 1 specimen, Station No. GFB-4, 5 specimens, Station No. GFB-9 (MNCNM/ 16.01/11245), 1 specimen, GFML-12 (MNCNM 16.01/1124), 2 specimens, Station No. SK4 (MNCNM 16.01/11241, 11242), 1 specimen, Station No. HU-07, 1 specimen, Station No. 12-05 (MNCNM 16.01/11244).
DISTRIBUTION
Sub-Arctic, North Atlantic, Mediterranean Sea.
HABITAT
Algae, seaweeds, sand, on bryozoans and gorgonians, calcareous concretions.
From intertidal to 1100 m depth.
Exogone (Parexogone) hebes Hartmann-Schröder (Reference Hartmann-Schröder1996): 173, figure 74; San Martín (2003): 236, figures 125 & 126.
MATERIAL EXAMINED
One specimen, Station No. GFB-4, 1 specimen, Station No. S5.
DISTRIBUTION
Sub-Arctic, North Atlantic, scarce in the Mediterranean Sea.
HABITAT
Sand, mud, intertidal to sublittoral.
Sphaerosyllis (Sphaerosyllis) bulbosa Hartmann-Schröder (Reference Hartmann-Schröder1996): 175.
Sphaerosyllis bulbosa San Martín (Reference San Martín, Ramos, Alba, Bellés, Gosálbez, Guerra, Macpherson, Martín, Serrano and Templado2003): 191, figures 98 & 99.
MATERIAL EXAMINED
One specimen, Station No. GFML-12.
DISTRIBUTION
Sub-Arctic, north-east Atlantic, Mediterranean.
HABITAT
Sand and other sediments, intertidal to 70 m.
Sphaerosyllis taylori San Martín (Reference San Martín, Ramos, Alba, Bellés, Gosálbez, Guerra, Macpherson, Martín, Serrano and Templado2003): 206, figure 108.
MATERIAL EXAMINED
One specimen, Station No. SFEK-8, 2 specimens, Station No. GFB-9, 1 specimen, Station No. GFLM-12, 1 specimen, Station No. HU-13 (MNCNM 16.01/11220, 11221, 11222).
DISTRIBUTION
North Atlantic; both North America and Europe. Mediterranean Sea.
HABITAT
Sand, especially coarse sand but also on muddy sand. Intertidal to sublittoral.
MATERIAL EXAMINED
Three specimens, Station No. 11-05.
DISTRIBUTION
Sub-Arctic, North Atlantic, North Pacific.
REMARKS
A re-description of this species is currently in preparation.
HABITAT
Algae, among hydrozoans and other sessile invertebrates, sand. Sublittoral.
Syllis (Typosyllis) armillaris Uschakov (1955): 161 (key), figure 51C–G.
Typosyllis (Typosyllis) armillaris Hartmann-Schröder (Reference Hartmann-Schröder1996): 152, figure 66.
Typosyllis armillaris Licher (Reference Licher1999): 189, figure 84.
Syllis armillaris San Martín (Reference San Martín, Ramos, Alba, Bellés, Gosálbez, Guerra, Macpherson, Martín, Serrano and Templado2003): 423, figures 232 & 233.
MATERIAL EXAMINED
One specimen, Station No. VGPT2-09, 2 specimens, Station No. SK4, 2 specimens, Station No. D2 (MNCNM 16.01/11223).
DISTRIBUTION
Apparently cosmopolitan.
HABITAT
Algae, seaweeds, sediments. Intertidal to sublittoral.
Syllis cornuta Rathke (Reference Rathke1843): 164, pl. 7, figure 12.
Typosyllis (Ehlersia) cornuta Hartmann-Schröder (Reference Hartmann-Schröder1996): 155.
Typosyllis cornuta Licher (Reference Licher1999) (in part): 57, figures 27 & 28.
MATERIAL EXAMINED
One specimen, Station No. 20-05.
DISTRIBUTION
Apparently cosmopolitan.
HABITAT
All kinds of habitats, intertidal to more than 1000 m depth.
Syllis fasciata Malmgren (Reference Malmgren1867): 161, pl. 8, figure 47, pl. 9, figure 52.
Syllis (Typosyllis) fasciata Uschakov (1955): 162 (key), figures 46B, 51A, B.
Typosyllis fasciata Licher (Reference Licher1999): 241, figure 102.
MATERIAL EXAMINED
One specimen, Station No. SK-4 (MNCNM 16.01/11230), 1 specimen, Station No. S5 (MNCNM 16.01/11232), 10 specimens, Station No. 11-05 (MNCNM 16.01/11231, 11233, 11234), 3 specimens, Station No. 20-05.
DISTRIBUTION
Arctic and sub-Arctic.
HABITAT
Sediment with many pebbles and stones. Sublittoral, at depths ranging from 28 to 178 m.
Syllis (Ehlersia) heterochaeta Moore (Reference Moore1909): 322, pl. 15, figures 1–4.
Syllis (Ehlersia) heterochaeta Uschakov (1955): 161 (key), figure 50B, C.
Typosyllis heterochaeta Licher (Reference Licher1999): 262, figure 109.
MATERIAL EXAMINED
One specimen, Station No. S5.
DISTRIBUTION
North Pacific, from California to sub-Arctic. Mediterranean Sea (Çinar & Ergen, Reference Çinar and Ergen2002). First report from sub-Arctic North Atlantic.
HABITAT
Sand, mud, among mussels, algae. Intertidal to about 620 m depth.
Chaetosyllis oerstedi Malmgren (Reference Malmgren1867): 45.
Syllis (Ehlersia) oerstedi Uschakov (1955): 161 (key), figure 50E.
MATERIAL EXAMINED
One specimen, Station No. SK4 (MNCNM 16.01/11224), 1 specimen, Station No. MO4 (MNCNM 16.01/11229), 2 specimens, Station No. S2 (MNCNM 16.01/11225, 11226), 2 specimens, Station No. S4, 2 specimens, Station No. S5 (MNCNM 16.01/11227), 2 specimens, Station No. D4 (MNCNM 16.01/11228).
REMARKS
Our specimens agree with the drawings of Syllis oerstedi by Uschakov (1955). However, Licher (Reference Licher1999) considered this species as nomina dubia. We consider that this species might be valid, but further detailed examinations on many more specimens are required for a final decision. A more detailed account and a re-description of this species are currently in preparation, including its habitat and distribution.
TYPE SPECIES
Syllis zebra Claparède, 1864.
DIAGNOSIS
Body of medium to large size, ribbon-like, strongly dorso-ventrally flattened, with numerous short segments. Dorsum provided with transversal bands of minute spinose papillae (at least some species; only distinct under SEM) or covering all the body (in one species). Prostomium with 4 eyes and 3 antennae. Palps free to each other. Two pairs of tentacular cirri. Antennae, tentacular and dorsal cirri distinctly articulated. Pharynx provided with a trepan, with or without mid-dorsal tooth. Compound chaetae with falcigerous blades; with simple dorsal and ventral capillary chaetae. Pygidium with 2 articulated anal cirri. Reproduction by means of Tetraglene stolons.
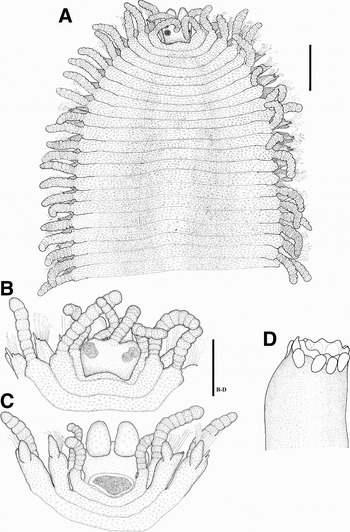
Fig. 5. Trypanosyllis troll sp.nov. (A) Anterior part, dorsal view (holotype); (B) anterior end, dorsal view (paratype); (C) anterior end, ventral view (paratype); (D) trepan (paratype). Scale: A: 0.375 mm; B,C,D: 0.18 mm.

Fig. 6. Trypanosyllis troll sp.nov. (A,B) Parapodia, lateral view; (C) aciculae, posterior parapodium; (D) compound chaetae, midbody. Scale: A,B: 92 µm; C,D: 20 µm.

Fig. 7. Trypanosyllis troll sp. nov. (A) Anterior part, dorsal view; (B) rows of papillae, dorsal view; (C) papillae; (D) anterior end, dorsal view; (E) dorsal cirri, midbody; (F) aciculae and falcigers, midbody.

Fig. 8. Trypanosyllis troll sp. nov. (A) Compound chaeta, anterior parapodium; (B) trepan.
MATERIAL EXAMINED
Holotype (MNCNM 16.01/11251), Station No. GFLM-13(MNCNM 16.01/11240, 11246), 1 paratype (MNCNM 16.01/11252), Station No. GFA-7 (2005), 1 paratype (MNCNM 16.01/11253), Station No. GFB-4.
DESCRIPTION
Body long and wide, dorso-ventrally flattened, ribbon-like (Figure 5A). Body covered with scattered minute papillae (Figures 5A–C, 6A, B, 7A–F), some of them arranged forming two rows of papillae on dorsum of each segment (Figure 7A, B, D); papillae densely present on antennae, dorsal cirri, parapodia and dorsum (Figure 7B, C). Largest specimen complete, 6 mm long, 2.5 mm wide, with 65 chaetigers. Prostomium oval, almost circular, with two dorsal lobes or cheeks around eyes, posteriorly bilobed; 4 eyes in close trapezoidal arrangement. Antennae inserted on anterior margin (Figure 5B), shorter than combined length of prostomium and palps; lateral antennae shorter than median antenna, with 9 rounded articles; median antenna with about 9–10 articles, inserted slightly in back to lateral ones. Palps oval, longer than prostomium, completely separated (Figure 5C). Peristomium dorsally reduced, shorter than following segments; dorsal tentacular cirri with 7–8 articles. Dorsal cirri thick, with 7–13 articles, shorter than body width, somewhat enlarged on middle segments, becoming tapered basally and distally (Figure 6A, B); alternating dorsal cirri long and thick with others shorter and slender (Figure 7E). Parapodial lobes distally bilobed. Ventral cirri digitiform, shorter than parapodial lobes (Figure 6A, B), inserted to parapodial lobes latero-posteriorly (Figure 6A, B). Compound chaetae numerous (Figure 7F), numbering about 17 on midbody parapodia, heterogomph, with almost smooth shafts and short blades; 20–23 µm long, distally acute and curved, bidentate, with proximal tooth minute, and margin with short spines (Figures 6D, 8A). Anterior parapodia with 2, occasionally 3, straight, acute aciculae, protruding beyond parapodial lobes, one larger and longer than others (Figures 6A–C, 7F). Dorsal and ventral simple chaetae not seen. Pharynx slender and short, through 8–9 segments, with trepan composed of 10 teeth (Figures 5D, 8B), without mid-dorsal tooth. Proventricle similar in size to pharynx, through 9–10 segments, with about 20 muscle cell rows.
REMARKS
This newly described species is similar to Trypanosyllis rosea (Grube, Reference Grube1863) described by Hartmann-Schröder (Reference Hartmann-Schröder1979), especially in the morphology of chaetae, the protruding of aciculae and the dense papillae covering body surface. Hartmann-Schröder (Reference Hartmann-Schröder1979) probably made some mistakes about this identification. The most important one is that Grube (Reference Grube1863) described a breeding stolon as Tetraglene rosea, which does not represent any characteristic features belonging to her specimens. Other authors considered that T. rosea corresponds to the reproductive stage of some other species, likely T. coeliaca. According to Çinar (Reference Çinar2007), no other species of Trypanosyllis has such a dense dermal papillation covering the body surface, although detailed observations under SEM of some species reveals transversal rows of minute papillae on dorsum of some segments (San Martín, Reference San Martín, Ramos, Alba, Bellés, Gosálbez, Guerra, Macpherson, Martín, Serrano and Templado2003). Likely, both the Hartmann-Schröder specimen from the Atlantic and ours from Norway could be the same species, but not Trypanosyllis rosea. Campoy (Reference Campoy1982) reported with doubts Trypanosyllis rosea from the Spanish Mediterranean, but he did not find the characteristic epidermal papillae, so this report is considered as doubtful, and probably referred to another species.
ETYMOLOGY
The specific name is given because the scattered minute papillae give it an ugly appearance that could remind us of the Trolls, strange beings of Viking mythology.
HABITAT
Fine sediments. In depths of 134–139 m.
DISTRIBUTION
East North Atlantic Ocean (Norway).
Myrianida inermis Nygren (Reference Nygren2004): 135, figures 65A–E.
MATERIAL EXAMINED
One specimen, Station No. GFB-9.
DISTRIBUTION
North-east Atlantic, north-east Pacific.
HABITAT
On hydroids, bryozoans and tunicates; sublittoral (Nygren, Reference Nygren2004).
Myrianida langerhansi Nygren (Reference Nygren2004): 140, figures 68A–E.
MATERIAL EXAMINED
Five specimens, Station No. 11-05.
DISTRIBUTION
North-east Atlantic, Mediterranean.
HABITAT
On hydroids, bryozoans and tunicates; from low intertidal to sublittoral (Nygren, Reference Nygren2004).
Proceraea cornuta Nygren (Reference Nygren2004): 47, figures 9A–C.
MATERIAL EXAMINED
Two specimens, Station No. 11-05.
DISTRIBUTION
North Atlantic and North Pacific.
HABITAT
Amongst algae with bryozoans; shallow waters (up to 20 m depth).
ACKNOWLEDGEMENTS
We would like to express our gratitude to the Akvaplan-niva field crew for collecting the samples and to the clients of Akvaplan-niva for permitting us to study them and for providing us with all the necessary information about the samples. Comments and suggestions of one anonymous referee greatly improved the quality of the paper. Finally, thanks to Celia Martín for her help with the English translation and her advice. This paper is a contribution to the project ‘Taxonomía y Sistemática de la Familia Syllidae (Polychaeta)’, funded by the Ministerio de Educación y Ciencia of the Spanish Government, Project number CGL2005-02442.











