Introduction
Reward learning plays a central role in adaptive behavioural flexibility in all mammals, including humans. However, perturbations of reward learning and memory are centrally implicated in the aetiology of psychiatric disorders of ‘over-consumption’ such as drug and food addiction (Hyman, Reference Hyman2005; Volkow et al., Reference Volkow, Wise and Baler2017), binge eating (Avena and Bocarsly, Reference Avena and Bocarsly2012) and obesity (Wang et al., Reference Wang, Volkow and Fowler2002). Rates of the latter disorders and associated health conditions such as diabetes, heart disease, stroke and cancer have tripled in the last four decades (WHO, 2016) and will soon collectively present the largest global health burden (Tremmel et al., Reference Tremmel, Gerdtham, Nilsson and Saha2017).
This surge in binge eating and obesity is largely attributable to the widespread availability of cheap, highly processed, ‘highly palatable foods’ (HPFs). These are calorie-dense combinations of high-fat and sugar ingredients (Drewnowski, Reference Drewnowski2009) that are readily consumed in excess of homeostatic caloric demands (Erlanson-Albertsson, Reference Erlanson-Albertsson2005). The human reward systems governing food-seeking evolved largely under conditions of food scarcity and are highly responsive to the sensory qualities of foods indicating high-energy density. The macronutrient profiles of HPFs (high sugar, high fat and calorie dense) (Schulte et al., Reference Schulte, Avena and Gearhardt2015) and their sudden ubiquity in the modern food environment (Ulijaszek, Reference Ulijaszek2007) create a ‘perfect storm’ to hijack normally adaptive reward learning and motivational systems (Kelley and Berridge, Reference Kelley and Berridge2002). Sensory qualities of HPFs, such as their packaging, sight, smells, textures and tastes (Rolls, Reference Rolls2011) are readily associated with the rewarding effects of these foods, such that these ‘cue’ stimuli themselves become imbued with motivational, salience and reinforcing properties. These associations are stored as HPF-related maladaptive motivational memory (MMM) traces. MMMs underlie the cue-triggered craving, ‘hedonic hunger’ (Cameron et al., Reference Cameron, Chaput, Sjödin and Goldfield2017), highly-motivated seeking and overconsumption of certain foods (Volkow et al., Reference Volkow, Wise and Baler2017) that typifies obesity and binge eating. Activation of MMMs allows HPF sensory cues to override both top-down long-term goals (e.g. desire to lose weight) and homeostatic/interoceptive satiety signals, producing excessive eating (Hall et al., Reference Hall, Ayuketah, Brychta, Cai, Cassimatis, Chen, Chung, Costa, Courville, Darcey, Fletcher, Forde, Gharib, Guo, Howard, Joseph, McGehee, Ouwerkerk, Raisinger, Rozga, Stagliano, Walter, Walter, Yang and Zhou2019). Disorders of maladaptive overeating behaviour (overweight/obesity, binge-eating) can therefore be conceptualised as a direct consequence of dysregulated reward learning and MMMs (Stice et al., Reference Stice, Figlewicz, Gosnell, Levine and Pratt2013).
Given the centrality of MMMs to overeating, strategies for weakening or rewriting these memories are required for the effective management of overeating disorders. A promising area of neuroscientific research in this regard relates to memory reconsolidation, the process by which retrieved long-term memories (including MMMs) can destabilise in order to strengthen or incorporate new relevant information prior to restabilising (Przybyslawski and Sara, Reference Przybyslawski and Sara1997; Lee et al., Reference Lee, Nader and Schiller2017). The period of memory instability between destabilisation and restabilisation – the reconsolidation window – offers a unique opportunity to weaken MMMs by pharmacologically manipulating their restabilisation (Roesler, Reference Roesler2017).
Memory restabilisation has been repeatedly shown to require protein synthesis and consequent synaptic plasticity in key brain structures (basal ganglia, limbic system and cortex) (Suzuki et al., Reference Suzuki, Josselyn, Frankland, Masushige, Silva and Kida2004; Merlo et al., Reference Merlo, Bekinschtein, Jonkman and Medina2015). Blocking protein synthesis while a memory is unstable can therefore weaken the destabilised trace (Nader et al., Reference Nader, Schafe and Le Doux2000; Valjent et al., Reference Valjent, Corbillé, Bertran-Gonzalez, Hervé and Girault2006) by preventing synaptic re-scaffolding (Doyère et al., Reference Doyère, Dȩbiec, Monfils, Schafe and LeDoux2007). To the extent that MMMs govern over-eating behaviour, directly weakening these MMMs via reconsolidation blockade could produce long-term reductions in over-eating. Problematically, the majority of direct protein synthesis inhibitors known to block memory reconsolidation are too toxic to use in humans and alternative drug targets are required.
Cellular protein synthesis is subject to ‘master regulation’ by the serine/threonine kinase Mammalian Target of Rapamycin Complex 1 (mTORC1), the activity of which is necessary for synaptic plasticity (Parsons et al., Reference Parsons, Gafford and Helmstetter2006). Interfering with mTORC1 (‘mTOR’ hereafter) activity may therefore weaken destabilised memories by interfering with their restabilisation. Rapamycin, the eponymous inhibitor of mTOR, is a promising compound in this regard, as it is currently used in human medicine (e.g. in organ transplant rejection), blocks reconsolidation in experimental animal models of addiction and anxiety (Glover et al., Reference Glover, Ressler and Davis2010; Barak et al., Reference Barak, Liu, Hamida, Yowell, Neasta, Kharazia, Janak, Ron, Goltseker, Bolotin, Barak, Liu, Hamida, Yowell, Neasta, Kharazia, Janak and Ron2013) and has reconsolidation-independent craving–reducing properties in human opiate use disorders (Shi et al., Reference Shi, Jun, Zhao, Xue, Zhang, Kosten and Lu2009). However the ability to target mTOR to block reconsolidation of food reward memory remains untested in humans. If rapamycin could be shown to block food reward memory reconsolidation, there would be a strong rationale for its further investigation as a potential therapeutic tool in overeating disorders.
We therefore examined the possibility of weakening reward memories for HPFs in a sample of healthy participants with a self-reported propensity to periodically overconsume chocolate (a sub-clinical model of binge eating behaviour). Chocolate is a prototypical HPF and one of the most widely craved and overconsumed foods in western societies (Rozin et al., Reference Rozin, Levine and Stoess1991). We assessed the effects of 10 mg rapamycin (sirolimus) on established chocolate reward memory in combination with a retrieval procedure previously demonstrated to destabilise long-standing maladaptive reward (alcohol) memories by incorporating prediction error (PE) at retrieval (Das et al., Reference Das, Lawn and Kamboj2015; Hon et al., Reference Hon, Das and Kamboj2016; Das et al., Reference Das, Gale, Hennessy and Kamboj2018a; Reference Das, Walsh, Hannaford, Lazzarino and Kamboj2018b). We examined a range of validated indices of chocolate-reward memory strength and overeating behaviour (bingeing episodes). If the retrieval procedure successfully destabilise chocolate reward memories, and rapamycin sufficiently interferes with their restabilisation, reductions should be observed in outcome indices compared to rapamycin alone (without chocolate memory retrieval) or retrieval following placebo.
Methods
Participants and design
Participants were healthy adults with a self-reported propensity to periodically overconsume chocolate. This population was selected as (1) they have measurable pre-existing (naturalistic) motivational memories triggering the propensity to overeat and (2) they are at higher risk for further progression into binge eating and overweight/obesity.
Participants were recruited via online and locale advertising. Inclusion criteria were: ages 18–45; overconsuming chocolate (defined as a ‘Struggle to stop eating chocolate?’ and ‘Eating much more than planned or until uncomfortably full’) >3×/month; >20 lifetime chocolate overconsumption episodes; fluent spoken English; agreement to consume samples of chocolate and strawberry during the study; motivated to reduce chocolate consumption; blood pressure <145/90 and Food Cravings Questionnaire-Trait-chocolate [FCQ-TR-C (Hormes and Meule, Reference Hormes and Meule2016)] score >45. Exclusion criteria: Undergoing current treatment (psychological or pharmacological) for a diagnosed eating disorder or any other psychiatric condition; compensatory behaviours for bingeing (e.g. vomiting, using diuretics, thyroxin or slimming pills); drinking >30 UK units (240 g alcohol) per week; using recreational drugs >1×/week; body mass index (BMI) < 18.5 or >60; pregnancy or breastfeeding; highly restrictive dietary requirements (e.g. veganism, nut or lactose allergies) and any major health conditions including, medical contraindication to rapamycin.
Seventy-five participants were evenly and randomly allocated to one of three experimental groups, as typically used to infer reconsolidation effects: (1) chocolate reward memory retrieval + 10 mg rapamycin (RET + RAP), (2) chocolate reward memory retrieval + placebo (RET + PBO) and (3) control, non-chocolate memory retrieval + rapamycin (No RET + RAP). These manipulations allow us to differentiate retrieval-dependent drug effects from the simple effects of drug and retrieval per se.
Assessments and stimuli
Cue reactivity task
The task used 14 images taken from the FoodPics extended database (Blechert et al., Reference Blechert, Meule, Busch and Ohla2014). Nine were ‘HPF’ images of chocolate, for which the normative ratings of ‘urge to consume’ were highest. Five were ‘LPF’ images of vegetables for which the normative ‘urge to consume’ ratings were lowest.
Participants first selected their preferred 30 g bar of chocolate (chocolate UCS) from a ‘selection pack’ (Cadbury, Bourneville, UK) and were told they would consume this as for a ‘taste test’ after rating a set of pictures. All food images was then presented centrally on screen in a randomised order, and rated for (1) ‘pleasantness’ (‘liking’; −50 = ‘extremely unpleasant’, +50 = ‘extremely pleasant’), the image's effect on momentary ‘desire to eat’ the chocolate (‘wanting’; −50 = ‘greatly reduces desire to eat’, +50 = ‘greatly increases desire to eat’) and likelihood of bingeing on the depicted food (‘binge risk’ −50 = ‘extremely unlikely’, +50 = ‘extremely likely’). To aid interpretation of parameter estimates and plots, and plotting purposes, these scores were all re-scaled to a 0–100 scale prior to analysis.
Following image rating, participants' attention was directed to the chocolate UCS itself and they rated it for pleasantness, wanting and binge risk, on the same scales as above. They then received on-screen timed prompts (displayed sequentially for 6 s) instructing them to ‘pick up the chocolate’, ‘prepare to eat’ and ‘eat the chocolate now’. Participants consumed the chocolate accordingly, then rated the pleasantness of the chocolate and desire to eat more of the chocolate.
Chocolate reward memory retrieval (RET)
The preamble and set-up of this task was identical to the cue reactivity task above, in order to maximise expectancy of chocolate consumption such that robust PE could be provoked when it was withheld. Participants selected their preferred chocolate and were again told they would eat this after rating some images. Participants then rated six chocolate images, followed by the chocolate UCS itself, for liking, wanting and binge risk, recapitulating to Day 1. The subsequent on-screen prompts then instructed participants to ‘pick up the chocolate’, ‘prepare to eat’, as on Day 1. The final prompt, however read ‘Stop, do not eat!’ and participants were instructed to put the chocolate down, with the aim of generating a negative PE. Participants then rated their surprise at what had just happened, from −50(completely expected) to +50 (completely unexpected) and began a brief set of distractor tasks (not analysed here) to disengage working memory from the retrieval.
Non-chocolate memory reactivation (No RET)
This procedure was identical to the RET procedure, with the following substitutions: Instead of choosing, chocolate participants were given a non-binge food (low-palatability food (LPF): 30 g dried strawberry slices). They then rated six LPF, non-binge food images, followed by the strawberry itself from Day 1 for liking, wanting and binge risk as in the RET groups. The on-screen prompts then instructed them to ‘pick up the strawberry’, ‘prepare to eat’ and then ‘eat now’. Participants consumed the strawberry and then rated their enjoyment of the strawberry, urge to eat more and surprise, as above. This procedure thus paralleled the RET procedure in length, response demands and retrieval of food-related memories, but was specifically designed to not reactivate chocolate or bingeing memories.
Oculomotor bias
This visual probe task assessed attentional capture by chocolate images by pairing with non-binge food images. Image pairs were presented side-by side on screen and eye-movements to the image assessed recorded. The primary eye-tracking measures were summed fixation on each image in each trial (Dwell time), latency to first fixation on each image from trial start (fixation latency) and duration of this first fixation. See online Supplementary material for details.
Motivation to consume chocolate
This Progressive Ratio Task required sequentially increasing numbers of key presses in limited time to earn 3 g chocolate (one Cadbury's milk chocolate button, Bourneville, UK) or dried strawberries (one slice). Participants had to consume the food before continuing the task and rated the pleasantness of the food and their hunger level after each consumption. The primary extracted indices were (1) number of choices for chocolate v. strawberries, (2) the ‘break point’ in the number of required taps for the last trial participants decide to play for a food type and (3) an action-incentivisation index for each cue type calculated as (1/mean RT) × N choices (where mean RT = mean reaction time per press), which could account for the lack of motivation to consume where no choices for a particular food type were made. Full details are given in online Supplementary material.
Questionnaires
Chocolate consumption diary
An online diary was used to assess levels of naturalistic chocolate consumption in the week preceding (baseline) and following (post-manipulation) manipulation and at one month post-Day 1 (follow-up). The diary assessed peak chocolate craving, binge frequency and grams consumed. On Day 1 and Day 10, a Timeline Follow-Back calendar-based measure of chocolate consumption (in grams: TLFB-C) was used to ensure consumption data were available for the key peri-manipulation period. Full details are given in online Supplementary materials.
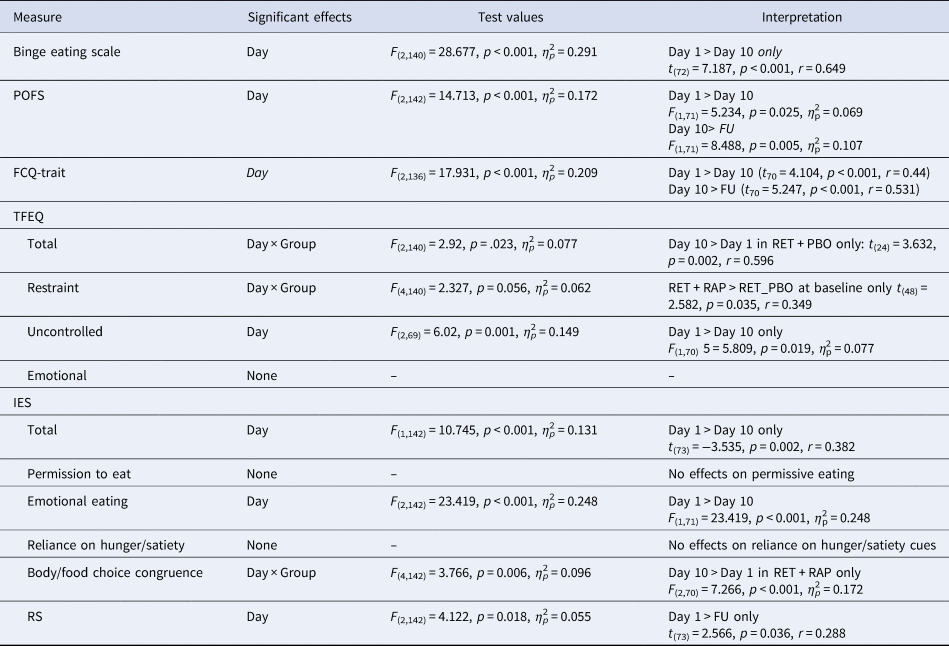
Subjective chocolate craving was measured Attitudes towards Chocolate questionnaire (ACQ) (Benton et al., Reference Benton, Greenfield and Morgan1998). General disordered eating behaviour was assessed using the Three Factor Eating Questionnaire (TFEQ) (Stunkard and Messick, Reference Stunkard and Messick1985), Power of Food Scale (POFS) (Lowe et al., Reference Lowe, Butryn, Didie, Annunziato, Thomas, Crerand, Ochner, Coletta, Bellace, Wallaert and Halford2009), Restraint Scale (RS) (van Strien et al., Reference van Strien, Peter Herman, Engels, Larsen and van Leeuwe2007) and Binge Eating Scale (BES) (Gormally et al., Reference Gormally, Black, Daston and Rardin1982). General food craving was assessed with the Food Craving Questionnaire State/Trait (FCQ-T/FCQ-S) (Cepeda-Benito et al., Reference Cepeda-Benito, Gleaves, Williams and Erath2001). Intuitive eating was assessed using the Intuitive Eating Scale (IES; Tylka and Van Diest, Reference Tylka and Van Diest2013)
The Beck Depression Inventory (BDI) (Beck et al., Reference Beck, Steer and Carbin1988); Spielberger Trait Anxiety Index (STAI-T) (Spielberger et al., Reference Spielberger, Gorsuch and Lushene1970) and Barratt Impulsiveness Scale (BIS) (Patton and Stanford, Reference Patton and Stanford1995) were completed to check baseline group equivalence on relevant mood and personality traits. On each testing day, the level of hunger was assessed by a 10-point visual ‘hunger ruler’ and blood glucose assessed on taken finger-prick glucose oxidase with an SDCheck monitor (Omron, UK). BMI, heart rate and blood pressure were also calculated to assess groups' biometric equivalence.
Drugs
Active drug was 10 mg enterically coated oral rapamycin tablets (Rapamune; Pfizer Limited). The dose was selected due to known tolerability in humans. Placebo was size-matched multi-vitamin tablets. See online Supplementary material for full details.
Procedure
Following telephone screening, eligible participants undertook three in-lab sessions as follows:
'Baseline' (Day 1): after providing informed consent, participants were randomised to a condition using a non-stratified code generated from random.org. They then completed the questionnaire measures in the following order: timeline follow-back for chocolate consumption TLFB-C; BES, RS, TFEQ, ACQ, BDI, STAI, POFS and FCQ-T. They then completed subjective hunger, fasting glucose, height, weight, heart rate and blood pressure measures followed by the chocolate cue reactivity task, progressive ratio task and attentional bias task. They were then briefed on completion of the chocolate diary and allowed to leave.
‘Manipulation Day’ (Day 1 + 48–72 h): Participants returned to the study centre having fasted for 4 h and were administered either rapamycin or placebo, as relevant to their group. They then immediately completed fasting glucose, heart rate and blood pressure measurements before completing the FCQ–state and subjective hunger measures. One hour post-drug administration, participants completed the RET or No RET procedure relevant to their condition and ACQ-state. Participants were medically monitored in-lab for 2 h following drug to monitor any acute adverse reactions.
‘Post-manipulation’: Day 10 (Day 1 + ~10 days). Participants re-completed all Day 1 measures, along with their guess on drug condition, and were asked to report any symptoms or adverse effects they had experienced over the previous week. They were then debriefed and reimbursed (£60). Follow-up (Day 1 + 1 month) participants completed the BES, IES, TFEQ, FCQ-T, food diary and TLFB-C measures remotely. Completion of follow-up was financially incentivised (£10). All procedures were approved by the UCL Research Ethics Committee and accorded with the Declaration of Helsinki (1975).
Analysis
Analyses were performed using IBM SPSS 25 and R for Windows. Primary measures (cue reactivity, attentional bias and progressive ratio task) were assessed using mixed ANOVA with a within-subjects factors of cue type (HPF v. LPF) and Time (Baseline v. post-manipulation). For questionnaire measures of chocolate craving and disordered food consumption the Time factor had three levels (Baseline post-manipulation, follow-up). All analyses included a between-subjects factor of Group (RET + PBO, RET + RAP, No RET + RAP). Where Pearson's correlations between PE ratings and key outcomes were significant, surprise was included as a covariate. All analyses were performed blind by RKD and the blinding code not broken until analysis was completed. The pre-registered analysis plan can be found on the Open Science Framework (https://osf.io/tqxdbDOI10.17605/OSF.IO/TQXDB). Full details are given in online Supplementary materials.
Results
Descriptive statistics for baseline variables of interest are displayed in Table 1. The groups only differed in the resting heart rate t (48) = 4.048, p < 0.001, r = 0.504 (RET + PBO > No RET + RAP). The mean BMI was on the healthy/overweight border and all groups reported high tonic chocolate craving. In all groups, there was a similar male/female split, representative of the prevalence of chocolate bingeing in the general population.
Table 1. Descriptive and inferential statistics for relevant trait, biometric and food consumption data at baseline (Day1)

Questionnaire abbreviations are as given in the ‘Assessments and Stimuli’ section. Significant of tests was assesses against the false discovery rate alpha using the Benjamini–Hochberg procedure. The p value for gender is for a χ2 test of gender by group. Descriptive data are mean ± s.d.
Chocolate cue reactivity
In the Day 1/Day 10 reactivity task, chocolate cue images were liked more than LPF cue images (F (1,72) = 95.125, p < 0.001, η 2p = 0.569). A Day × Group × Cue Type (HPF v. LPF) was also found (F (2,72) = 3.338, p = 0.041, η 2p = 0.085), indicating a decrease in liking of LPF images in No RET + RAP (F (1,72) = 4.797, p = 0.032, η 2p = 0.062), with no other changes in cue liking from baseline to post manipulation nor any between-group differences.
Greater urge to eat was observed for chocolate HPF cue images than LPF cue images overall (F (1,72) = 120.551, p < 0.001, η 2p = 0.626), with a Day × Image Type interaction (F (1,72) = 33.492, p < 0.001, η 2p = 0.317) indicating a decrease in urge to eat in response to chocolate cue images in all groups from Day 1 to Day 10 (F (1,72) = 36.39, p < 0.001, η 2p = 0.336) and no significant change in response to LPF cue images (F (1,72) = 1.109, p = 0.296, η 2p = 0.015). Binge risk in response to cue images was higher for chocolate than LPF cues F (1,72) = 173.259, p < 0.001, η 2p = 0.706, although a Day main effect indicated a general reduction in rated binge risk from Day 1 to Day 10 in all groups (F (1,72) = 10.008, p = 0.002, η 2p = 0.122).
Response to chocolate UCS.
During the sham ‘taste test’, there were no significant group differences on either day, nor Day 1 to Day 10 changes in anticipated enjoyment of the chocolate UCS (Day × Group: F (2,71) = 1.443, p = 0.243, η 2p = 0.039), actual enjoyment of the consumed chocolate (Day × Group: F (2,72) = 0.042, p = 0.959, η 2p = 0.001) or pre-consumption urge to eat the chocolate (Day × Group: F (2,71) = 1.193 p = 0.309, η 2p = 0.033). Post-consumption urge to eat more chocolate decreased in all groups from Day 1 to Day 10 (F (1,72) = 4.605, p = 0.035, η 2p = 0.06).
Surprise ratings during the retrieval manipulation correlated with rated binge risk for the chocolate UCS and were thus included as a covariate in assessing when assessing rated binge-risk. This yielded a borderline-significant main effect of Group (F (2,67) = 3.124, p = 0.05, η 2p = 0.085, and a significant Day × Group × Surprise interaction F (2,67) = 3.982, p = 0.023, η 2p = 0.106. To investigate the interaction, univariate models were assessed for Day 1 and Day 10 separately. As expected, no Group, Surprise or Group × Surprise effects were evident pre-manipulation on Day 1 (all Fs < 0.95, ps > 0.439, η 2p < 0.024). On Day 10, when co-varying for Day 1 ratings, Group (F (2,66) = 4.685, p = 0.013, η 2p = 0.124) and Group × Surprise (F (2,66) = 6.784, p = 0.002, η 2p = 0.275) effects were observed. Post-hoc tests showed that the groups did not differ significantly in their binge risk (all ps ⩾ 0.634).
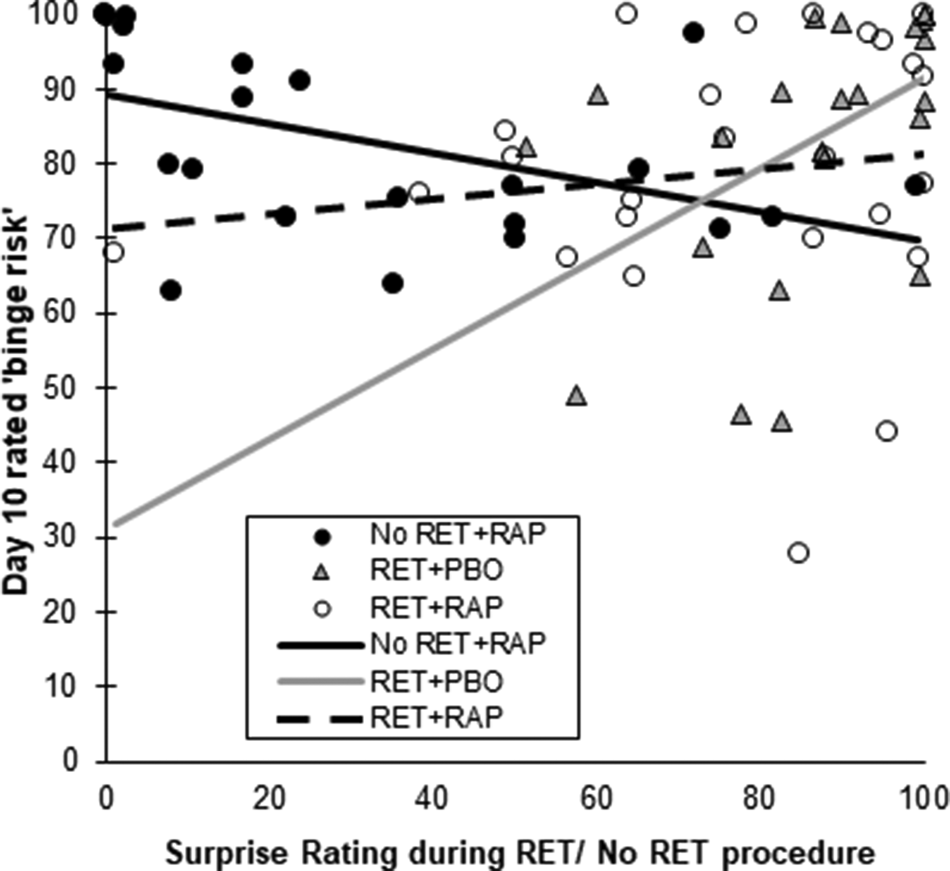
In RET + PBO, PE during retrieval was positively predictive of greater binge risk on Day 10 (R 2 = .25), representing a significantly greater slope for the Surprise effect than in RET + RAP (F (1,67) = 11.218, B = 0.802, p = 0.001, η 2p = 0.143). In No RET + RAP, greater surprise was predictive of lower chocolate binge risk on Day 10 (R 2 = 0.232), although this slope did not significantly differ from RET + RAP (F (1,67) = 3.189, B = 0.297, p = 0.079, η 2p = 0.045). There was no significant predictive effect of Surprise in RET + RAP (R 2 = 0.019), which was further reduced when two participants who rated their surprise as <40 (and therefore did not experience the intended PE) were excluded (R 2 = 0.003). This interaction suggests retrieval in the absence of rapamycin may strengthen MMMs proportional to the level of PE at retrieval and that rapamycin may abolish this effect. Scatterplots of this interaction are shown in Fig. 1. Including surprise ratings as a covariate in the ANOVAs assessing liking, wanting and binge risk in response to cue images did not substantially affect the findings.

Fig. 1. Relationship between surprise ratings during retrieval/non-retrieval procedures and subsequent perceived binge risk on chocolate UCS (30 g chocolate bar). The slope was positive in RET + PBO (light solid line), non-significant in RET + RAP (dashed line) and negative in No RET + RAP (solid black line). Both binge risk and surprise ratings have been scaled to range from 0–100.
Motivational salience of chocolate cues (attentional bias)
As expected, initial fixations were faster on chocolate images than LPF images (F (1,68) = 6.284, p = 0.015, η 2p = 0.085), demonstrating an extant attentional bias to chocolate. A Day × Image type interaction (F (1,68) = 10.263, p = 0.002, η 2p = 0.131) represented an increase in time to first fixations from Day 1 to Day 10 on LPF images only (F (1,68) = 8.622, p = 0.005, η 2p = 0.113), with no change in orienting to HPF images (p = 0.902). A Day × Group interaction showed a general increase in time to first fixation, (regardless of Image Type) in No RET + RAP only (F (2,67) = 3.539, p = 0.034, η 2p = 0.094).
Dwell times were also greater on chocolate than LPF images overall (F (1,68) = 62.169, p < 0.001, η 2p = 0.478). A Day × Image Type × Group interaction was also observed (F (2,68) = 3.433, p = 0.038, η 2p = 0.092). Examination of the simple effects of Day showed trend-level decreases in dwell time on HPFs in RET + PBO (F (1,68) = 3.452, p = 0.067, η 2p = 0.048) and trend-level decreases in dwell time on LPF images in No RET + RAP (F (1,68) = 3.755, p = 0.057, η 2p = 0.052). Durations of first fixations were longer on chocolate than LPF images (F (2,67) = 55.212, p < 0.0001, η 2p = 0.448), but no other significant effects were observed.
Motivation to earn chocolate reward: Progressive ratio task
Break point to earn chocolate (HPF) was significantly higher than for strawberries (LPF) (F (1,70) = 38.06, p < 0.001, η 2p = 0.352). Break points also decreased overall from Day 1 to Day 10, indicating lower general motivation to earn food on Day 10 (F (1,70) = 7.751, p = 0.007, η 2p = 0.1). A Reward Type × Group interaction was found, indicating a higher break point for earning strawberries in No RET + RAP than RET + RAP (t (47) = 3.042, p = 0.01, r = 0.406). This was further evident in a lack of a difference in the break point between chocolate and strawberries in No RET + RAP (F (1,70) = 1.64, p = 0.205, η 2p = 0.023). That is, No RET + RAP did not find chocolate more motivating than strawberries overall.
The action-incentivisation index (calculated as above to deal with the lack of reaction time data in subjects where a certain reward type was never selected) again showed greater motivation to earn chocolate than strawberries (F (1,70) = 41.151, p < 0.001, η 2p = 0.37), a general reduction in motivation to earn any reward from Day 1 to Day 10 (F (1,70) = 5.31, p = 0.024, η 2p = 0.071) and a Group × Reward type interaction (F (2,70) = 4.349, p = 0.017, η 2p = 0.111). The interaction was driven by lower action-incentivisation by chocolate in RET + RAP than No RET + RAP (t (47) = 3.069, p = 0.014, r = 0.409). Indeed, No RET + RAP showed no differential action incentivisation between chocolate and LPFs (F (1,70) = 2.768, p = 0.101, η 2p = 0.038). Liking ratings of consumed chocolate were higher than for strawberries (F (1,72) = 13.246, p = 0.001, η 2p = 0.155), commensurate with the greater motivation to earn chocolate rewards that would be expected in this population.
Food diaries
Mean daily chocolate consumption (in grams) reduced significantly in all groups (F (1.763,118.116) = 15.616, p < 0.001, η 2p = 0.189). Repeated contrasts showed that this reduction happened between Baseline and Day 10 (F (1,67) = 21.969, p < 0.001, η 2p = 0.247) with no further reduction Day 10 to follow-up (F (1,67) = 0.108, p = 0.744, η 2p = 0.002). Analysis of logged chocolate binges during each period (baseline, Day 10, follow-up) showed a reduction across Days (F (1.67,111.874) = 5.438, p = 0.009, η 2p = 0.075), with repeated contrasts showing that the significant reduction occurred between baseline and Day 10: (F (1,67) = 7.023, p = 0.01, η 2p = 0.095), with no further reduction from Day 10 to follow-up (F (1,67) = 0.097, p = 0.757, η 2p = 0.001). A main effect of Group was also found (F (2,67) = 4.674, p = 0.013, η 2p = 0.122), with greater bingeing in No RET + RAP than RET + RAP (t (44) = 3, p = 0.012, r = 0.412).
Departures from Sphericity and examination of mean/s.d. binge scores at each time point revealed a striking reduction in binge episodes in RET + RAP by Day 10 (see Fig. 2a), with the exception of the six group-level ‘outliers’. Examination of 95% confidence intervals (CIs) of the marginal means revealed a significant presence of chocolate binge behaviour (>0) in all groups at baseline, which was maintained in RET + PBO and No RET + RAP through Day 10 and follow up, but abolished in RET + RAP following manipulation (see Fig. 2b)

Fig. 2. Changes in food-diary rated chocolate binge frequency (a) box and whisker plot of daily bingeing on chocolate post manipulation. In RET + RAP, all but six participants did not binge at all in the post-manipulation period. (b) Estimated marginal means of chocolate binge frequency from baseline to follow up. All Bars = mean ±95% CI. *Significantly >0.
Likelihood of bingeing on other foods similarly decreased between baseline and test (F (1,69) = 13.982, p < 0.001, η 2p = 0.168) with no further reductions between test and follow-up (F (1,69) = 0.021, p = 0.886, η 2p < 0.001). Repeated contrasts showed No RET + RAP binged on other foods more frequently than the RET + RAP group (t (71) = 0.328, p = 0.041, r = 0.39), with exploratory analysis suggesting this difference was observed only at follow-up (t (71) = 2.471, p = 0.005, r = 0.281). Diary-rated hunger did not differ between groups on any day (F (2,69) = 2.024, p = 0.14, η 2p = 0.055), however hunger decreased from Day 1 to Day 10 (F (1,69) = 5.637, p = 0.02, η 2p = 0.076) and further from Day 10 to follow-up.
Questionnaire measures
Reductions in scores were seen on the BES, POFS FCQ-Trait and RS across the course of the study (see Table 2 for statistics), indicating generalised improvement in eating behaviour. An increase in the total TFEQ score was observed from Day 1 to Day 10 in RET + PBO only, with a Day × Group interaction on the RS reflecting greater eating restraint in RET + RAP than RET + PBO at baseline only (Day 1). A specific effect on the body-food choice congruence subscale of the IES was observed in RET + RAP only, reflecting an increase in ‘healthy’ food choices (commensurate with promoting health and maintaining a healthy body weight) in the group.
Table 2. Highest order significant effects from Day (Day 1, Day 10, Follow-Up) × Group mixed ANOVA on questionnaire measures of disordered eating

Where multivariate or corrected statistics were used in the case of non-sphericity, these are reflected in the test degrees of freedom.
Discussion
The mTOR kinase complex has been demonstrated to play a key role in the reconsolidation of memories (Barak et al., Reference Barak, Liu, Hamida, Yowell, Neasta, Kharazia, Janak, Ron, Goltseker, Bolotin, Barak, Liu, Hamida, Yowell, Neasta, Kharazia, Janak and Ron2013; Roesler, Reference Roesler2017), suggesting that mTOR inhibitors may be therapeutically employed as reconsolidation-blockers to selectively weaken maladaptive memories. The current investigation found minimal evidence for an effect of 10 mg oral rapamycin on the reconsolidation of chocolate reward memories. Putatively sensitive indices of MMM integrity (attentional bias, cue reactivity and break point) largely did not show differential responses to the manipulations. However, with the exception of six participants, food diaries showed complete abolition of chocolate binges and significant improvements in food choices in the ‘active’ group (RET + RAP). In the absence of coherent triangulation of the different measures employed, it is not possible to determine whether the latter self-reported effects are indicative of a reconsolidation blockade effect, as several alternative mnemonic and non-mnemonic interpretations of these effects exist, which will be discussed in turn.
Notably, RET + PBO displayed mild reduction in eye-tracking metrics of memory strength (dwell time on HPF images), but increases in some indices of disordered eating behaviour (TFEQ). The former could be interpreted as context-specific extinction learning produced by the cue reactivity task on Day 1 and Day 10 and the latter by strengthening of original MMM by the retrieval procedure in the absence of drug on Day 3, depending on which memory trace type was reactivated. Indeed, it has recently been shown that pure presentation of retrieval cues with PE strengthens memory via reconsolidation (Bavassi et al., Reference Bavassi, Forcato, Fernández, De Pino, Pedreira and Villarreal2019). Rapamycin may have abolished such a memory strengthening effect in RET + RAP. Since PE (depending on its level) elicits reconsolidation or the mutually exclusive process of new learning and extinction, inter-individual variation in trace dominance at could produce inconsistent effects. New learning putatively accrues proportional to PE, explaining why PE during the retrieval procedure was positively predictive of self-rated chocolate-cue induced ‘binge risk’ at test in RET + PBO, but not in RET + RAP. The reduction in liking of LPF images (which substituted for HPF ‘retrieval’ cues in No RET + RAP) may be taken as further support for this interpretation. While different synaptic mechanisms are implicated in new learning or extinction v. reconsolidation of an existing memory (de la Fuente et al., Reference de la Fuente, Freudenthal and Romano2011; Flavell et al., Reference Flavell, Lambert, Winters and Bredy2013; Li et al., Reference Li, Meloni, Carlezon, Milad, Pitman, Nader and Bolshakov2013; Merlo et al., Reference Merlo, Milton, Goozée, Theobald and Everitt2014), both processes may be regulated via mTOR complex (Glover et al., Reference Glover, Ressler and Davis2010) signalling and therefore disrupted by rapamycin, with potentially differing mnemonic and behavioural consequences, depending on which memories are reactivated, or what is being learned if new learning is elicited.
It is possible that the selected dose (10 mg) of rapamycin may have been too low to interfere with reconsolidation. Future research may assess higher doses of rapamycin, however, doses above 20 mg are likely to be poorly tolerated owing to the potent immunosuppressive effects. Indeed, it might be impossible to achieve the necessary central concentrations of rapamycin for reconsolidation blockade without unacceptable levels of immunosuppression. It will be prudent to focus on analogue drugs (rapalogues), which have greater specificity for mTORC1, a lower immunosuppressive profile and potential for post-retrieval intravenous administration.
Alternatively, there may have been a true (albeit limited) reconsolidation blockade in RET + RAP, explaining the isolated effects on chocolate binge occurrence and healthy food choices, although it is unclear why this would only be evident in the self-reported behavioural outcomes, with no apparent effect on putatively more sensitive in-lab measures.
Limitations
If mTOR is involved in destabilisation of memories, antagonising its activity prior to retrieval may paradoxically prevent destabilisation, preventing any interference effects. Due to the slow peak and long half-life of oral rapamycin, it was necessary to dose prior to memory retrieval so that drug would be active during the critical ‘reconsolidation window’ following destabilisation (Faliagkas et al., Reference Faliagkas, Rao-Ruiz and Kindt2018). A solution to this issue is intravenous administration immediately post-retrieval, however this is difficult to implement outside of a hospital setting, limiting the breadth of potential therapeutic application (assuming this route of administration was more effective than oral dosing).
The transition between different and exclusive memory states is a behaviourally ‘silent’ process, lacking a valid biomarker in humans. As such, interpretation of negative or mixed findings in human reconsolidation research continues to be confounded by the quandary of whether (or to what degree) reconsolidation processes were engaged by the retrieval procedure, or whether the drug intervention was simply ineffective. Regarding the current findings, we cannot be certain whether the retrieval procedure effectively destabilised chocolate reward memories or not, which would preclude observing an effect of rapamycin. This distinction is critical, since important reconsolidation-modifying therapeutics may be discounted through inadequate retrieval/destabilisation procedures and research into pharmacological ‘dead ends’ may continue on the basis of ambiguous findings, due to the possibility that target memories were not destabilised. Multiple non-retrieval related state variables may further interact to determine reconsolidation engagement, including the reward-specific satiety status of the individual, energy status (e.g. central glucose availability), arousal and stress levels, hormonal milieu (and menstrual cycle phase in females) and genetic/epigenetic factors determining neurotransmitter signalling and histone methylation/acetylation. We were unable to measure all of these factors here and much work needs remains to be done in determining the key organism-level arbiters of reconsolidation. The manipulation of reconsolidation as a therapeutic target requires it to be reliably engaged in the context of naturalistic maladaptive memories. Achieving this aim necessitates the development of biomarkers of the transition from retrieval to destabilisation and new learning and this should be considered the top priority for the field.
Importantly, participants in the current study did not display severe of ‘clinical’ levels of binge-eating behaviour, potentially limiting the scope for improvement in outcome measures. The availability and ubiquity of chocolate and chocolate advertising (in contrast illicit drugs for instance) may make chocolate bingeing a particularly difficult behaviour to target reconsolidation interference, as there is a real risk of rapid relearning of maladaptive associations. It is possible that the approach would have more chance of success in the context of disordered substance-using populations and should therefore be assessed in these populations. Indeed, we did not find evidence of the non-mnemonic craving–reducing effects of rapamycin shown by Shi et al. (Reference Shi, Jun, Zhao, Xue, Zhang, Kosten and Lu2009), suggesting that these effects may not extend to food reward.
Conclusion
We did not find convincing evidence of comprehensive MMM reconsolidation blockade by 10 mg oral rapamycin in sub-clinical chocolate over-eaters. Mild evidence of abolition of subsequent chocolate bingeing and a shift to healthier food choices was observed in the ‘active’ group, however replication will be required to determine whether this represents a reliable effect. Given the modest findings, its potential for immunosuppression and unfavourable pharmacokinetics for reconsolidation research, oral rapamycin may not be the optimal drug preparation to pursue as a reconsolidation-blocking pharmacotherapeutic in the context of binge eating.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S003329171900312X.
Financial support
This work was funded internally by a studentship awarded to Ms. Katie Walsh. This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflict of interest
None.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.