Introduction
During the last few decades it has been shown that signalling pathways and genes expressed in oocytes and embryos are highly conserved among divergent species such as flies, worms, mice, cows and amphibians (Stanton & Green, Reference Stanton and Green2001; Vallée et al., Reference Vallée, Aiba, Piao, Palin, Ko and Sirard2008; Von Stetina & Orr-Weaver, Reference Von Stetina and Orr-Weaver2011). Thus, this supports the idea of using different organism models to understand fundamental biological processes during oogenesis that are common and extrapolated to other species.
Oogenesis in Xenopus laevis has been repeatedly used to study regulation of oocyte development and maturation. Their large size, relative abundance and clearly defined sequence of physical characteristics from oogonia to eggs make them ideal for studying oogenesis progression processes (Dascal, Reference Dascal1987; Rasar & Hammes, Reference Rasar and Hammes2006). This process can be divided into six stages (I–VI) according to the morphology of the developing oocyte. Stage I and II are both previtellogenic, stages III to V are vitellogenic and, finally, stage VI oocytes are considered mature (Dumont, Reference Dumont1972); nevertheless, oogenesis is a continuous and highly regulated process and no precise boundaries can be defined between stages.
Amphibian ovaries offer an appropriate model in order to study regulatory events during oogenesis due to the process not being synchronized and individual ovary lobes containing different stages of oogenesis (Dumont, Reference Dumont1972; Rasar & Hammes, Reference Rasar and Hammes2006). This means that despite oocytes at different stages of development being exposed to the same hormonal environment, there are other kinds of intra-ovarian regulators that bring about the progression from one stage to the next, which are crucial for the ongoing oogenetic process. In the present work we propose the vitellogenin uptake process as a proper framework to study such signalling events.
It is well known that ovarian folliculogenesis, oogenesis and ovulation are regulated by hormones (Wallace et al., Reference Wallace, Opresko, Wiley and Selman1983; Marilley et al., Reference Marilley, Robyr, Schild-Poulter and Wahli1998; Kidder & Vanderhyden, Reference Kidder and Vanderhyden2010). In more recent studies it has become apparent that this endocrine action on the ovary is mediated by intra-ovarian signals provided by paracrine factors (Edwards et al., Reference Edwards, Reader, Lun, Western, Lawrence, Mcnatty and Juengel2008; McGinnis et al., Reference McGinnis, Carroll and Kinsey2011) as well as by direct cell–cell communication via gap junctions (Villecco et al., Reference Villecco, Aybar, Genta, Sánchez and Sánchez Riera2000; Mónaco, et al. Reference Mónaco, Villecco and Sánchez2007; Luciano et al., Reference Luciano, Franciosi, Modina and Lodde2011; Luque et al., Reference Luque, Serrano, Mónaco, Villecco and Sánchez2013). These gap-junctional connections couple the developing follicle into a functional syncytium, allowing cells implicated in it to share metabolites and signalling molecules such as adenosine 3,5-cyclic monophosphate (cAMP), inositol 1,4,5-triphosphate (IP3), and calcium ions (de Boer & van der Heyden, Reference de Boer and van der Heyden2005; Pearson et al., Reference Pearson, Dale, Llaudet and Mobbs2005; Decrock et al., Reference Decrock, Vinken, Bol, D'Herde, Rogiers, Vandenabeele, Krysko, Bultynck and Leybaert2011; Luque et al., Reference Luque, Serrano, Mónaco, Villecco and Sánchez2013). Regarding this, we have demonstrated that direct gap-junctional communication is a requirement for the acquisition of endocytic competence by Xenopus laevis vitellogenic oocytes. Thus, oocytes with uncoupled or down-regulated gap junctions are unable to begin the vitellogenin uptake (Mónaco et al., Reference Mónaco, Villecco and Sánchez2007). This evidence suggested that a signal molecule small enough to pass through open gap junctions could be involved at the onset of vitellogenin incorporation. Consequently, we determined that using the stable cell-permeable cAMP analogue dbcAMP (N6,2′-O-dibutyryladenosine 3′-5′-cyclic monophosphate) in the incubation medium of oocytes in which gap junctions had been down-regulated with octanol, the follicle endocytic activity is rescued (Luque et al., Reference Luque, Serrano, Mónaco, Villecco and Sánchez2013) concluding that cAMP is the molecule that triggers the vitellogenin uptake by the oocytes. Despite our findings, the way in which the cAMP molecule triggers this vitellogenesis process remains unclear.
Cyclic AMP is a ubiquitous cellular messenger that regulates a multitude of cellular responses and exerts its modulator effect by adjusting the activity of other signalling pathways. These effects were initially attributed to the activation of the protein kinase A (PKA) and to the cyclic nucleotide gated channels. However, another target, the exchange protein directly activated by cAMP (Epac), is now known to be involved in mediating cAMP responses (Xiaodong et al., Reference Xiaodong, Zhenyu, Tamara and Fang2008; Gloerich & Bos, Reference Gloerich and Bos2010; Van Schouwen et al., Reference Van Schouwen, Selvaratnam, Fogolari and Melacini2011).
Epac acts as a molecular switch for sensing intracellular second messenger cAMP levels to activate the Ras superfamily small GTPases that leads to the initiation of the IP3/Ca2+ signalling pathway by activating a novel phospholipase C isoform (PLCε) (de Rooij et al., Reference de Rooij, Zwartkruis, Verheijen, Cool, Nijman, Wittinghofer and Bos1998; Kawasaki, Reference Kawasaki1998; Gloerich & Bos, Reference Gloerich and Bos2010). Epac protein has been shown to be involved in a large number of cellular functions such as cell division, differentiation, secretion and growth (Breckler et al., Reference Breckler, Berthouze, Laurent, Crozatier, Morel and Lezoualc'h2011). However, in spite of the diverse studies performed in this alternative cAMP pathway, not much information is known about its implications in the oogenetic regulation process.
A Xenopus laevis homologue of Epac, Xepac, was isolated and characterized as a novel gene expressing both maternally and zygotically with a specific spatial expression pattern restricted within the developing hatching gland (Lee & Han, Reference Lee and Han2005). Nevertheless, at present, there is no information about the different components of this non-canonical cAMP signalling pathway during Xenopus laevis oogenesis, and even less as to whether Xepac participates during vitellogenin uptake process.
In the present study, we investigated using different inhibitors, whether cAMP and/or Epac protein are involved in the vitellogenin uptake process through the activation of the IP3/Ca2+ signalling pathway.
Materials and methods
Biological material
Adult female Xenopus laevis specimens were kept in dechlorinated fresh water tanks at 18–20 °C on a 12 h light/dark cycle and fed three times weekly, alternating chopped heart meat with a balanced diet.
Frogs were anaesthetized on ice and ovarian lobes were removed via partial ovariectomy; subsequently, the incision was sutured and the animal was allowed to recover at room temperature.
The removed ovarian lobes were cut into small pieces and were rinsed several times with O-R2 solution and maintained at room temperature during the removal of ovarian follicles. The vitellogenic follicles (stages III–IV and V; Dumont, Reference Dumont1972) were dissected manually with the aid of a dissecting microscope and sharpened watchmaker's forceps and incubated at 20 °C in an O-R2 sterile solution.
RNA isolation and reverse transcription polymerase chain reaction (RT-PCR) analysis
In order to investigate the presence and temporary expression pattern of Xepac during Xenopus laevis oogenesis, we analysed total RNA isolated by RT-PCR from oocytes at different stages.
Total RNA was isolated from previtellogenic, vitellogenic and mature oocytes by the Illustra RNAspin Mini RNA Isolation Kit (GE Healthcare). cDNAs were synthesized by the M-MLV Reverse Transcriptase (Promega) and oligo(dT) primers.
The specific primers for Xepac including the 3′-untranslated region were 5′-AACTGATGGAGTTGTCCCGTAGC-3′ (upstream) and 5′-AGGTTCTACCTGAAGGCGCTTTAA-3′ (downstream) (Lee & Han, Reference Lee and Han2005), at an annealing temperature of 57 °C. For amplification of elongation factor 1-α (EF1α) the primers were 5′-AACCACCCAGGCCAGATTG-3′ (upstream) and 5′-GTAGTCAGAGAAGCTCTCCACG-3′ (downstream) (Agius et al., Reference Agius, Oelgeschläger, Wessely, Kemp and De Robertis2000) at an annealing temperature of 57 °C. EF1α primers sequences were obtained and modified from the website of Dr Edward M. de Robertis (http://www.hhmi.ucla.edu/derobertis/protocol_page/oligos.PDF). PCR amplifications were performed over 30 cycles and the PCR products were analysed on 1.5% agarose gels.
Preparation of vitellogenin-containing serum (VTG)
For the study of receptor-mediated oocyte endocytic activity it is essential that the receptor ligands (VTG) be present in the incubation medium. X. laevis females were injected with a dose of estrogen (4 mg estradiol-17β dissolved in 0.4 ml propylene glycol per 100 g body weight) in order to enhance liver vitellogenin (VTG) synthesis and its accumulation in the bloodstream. After 2–3 weeks, estrogen-treated animals were anaesthetized and bled exhaustively. The serum obtained was dark green and contained approximately 100–150 mg VTG/ml (R. A. Wallace et al., Reference Wallace, Misulovin and Wiley1980).
Down-regulation of gap junction
In order to down-regulate/uncouple gap-junctional communication between oocyte and the surrounding follicular epithelial cells, ovarian follicles that contain stage IV–V oocytes were incubated in octanol dissolved in dimethyl sulphoxide (DMSO) and diluted to 1 mM with sterile O-R2 (Luque et al., Reference Luque, Serrano, Mónaco, Villecco and Sánchez2013). To test the uncoupling gap junctions the absence of labelled yolk platelets was monitored through non-specific fluid-phase labelling with biotinylated albumin as an endocytic tracer (Luque et al., Reference Luque, Serrano, Mónaco, Villecco and Sánchez2013). Therefore, in octanol-treated follicles, the absence of labelled yolk platelets made it possible to confirm the uncoupling of gap junctions.
Endocytic tracer
To detect the pathway of vitellogenin endocytosis, biotinylated bovine serum albumin (b-BSA) was used as a tracer. BSA (Sigma Chemical Co., A 7906) was tagged with biotinyl-ε-aminocaproic acid N-hydroxysuccinimide ester (BNHS, Sigma Chemical Co., B 2643) by adding 10 μl of a 20 mg/ml solution (in dimethylformamide) for each 1mg of albumin present in the dialysis bag. After 1 h at room temperature, the reaction mixture was dialyzed at 4 °C overnight against an OR-2 solution. The biotinylated protein was stored at –20 °C until use.
Inhibition and rescue assays
In order to assess the participation of the IP3\Ca2+ signalling pathway during the vitellogenin uptake process, chemical inhibition and rescue assays were performed.
Batches of 15–20 vitellogenic follicles (coupled gap junctions) per well were cultured in 500 μl of sterile OR-2 solution that contained gentamicin (1 μg/ml) with or without the presence of different inhibitors (heparin, neomycin, ethylene diamine tetraacetic acid (EDTA), U-73122 y CdCl2). After 3 h of incubation in this medium VTG serum (100–150 mg/ml) and biotinylated BSA (1 mg/ml) were added to each culture and maintained for 24 h at 22 °C. Rescue experiments using the calcium ionophore A-23187 were performed.
Batches of 15–20 vitellogenic follicles treated with octanol (uncoupled gap junctions) per well were cultured in 500 μl of sterile OR-2 solution that contained gentamicin (1 μg/ml.). After 3 h of incubation in this medium, VTG serum (100–150 mg/ml) and biotinylated BSA (1 mg/ml) were added to each culture and maintained for 24 h at 22 °C with or without the presence of 8-pCPT-2′-O-Me-cAMP (8-CPT). The different chemical inhibitors and rescue drugs used are characterized in Table 1.
Table 1 Chemical inhibitors and rescue drugs

Follicle groups used in the experiments are as follows:
• Follicles with uncoupled gap junctions:
○ Group 1: In order to uncouple the gap junctions, the follicles were cultured in the presence of octanol (1 mM) at the beginning of the cultured period (negative control).
○ Group 2: In order to assess the implication of the Xepac protein during the vitellogenin uptake process, the follicles with uncoupled gap junctions were treated with 8-pCPT-2′-O-Me-cAMP (8-CPT). The 8-CPT is a specific and stable cell-permeable cAMP analogue that can selectively activate Epac pathway and it was added 3 h after the onset of the culture period to a final concentration of 10 μM.
• Follicles with coupled gap junctions:
○ Group 3: the follicles were cultured in OR-2 medium for 24 h containing antibiotic, VTG serum and endocytic tracer (positive control).
○ Group 4: In order to block the IP3 binding sites for Ca2+ release, follicular oocytes were microinjected with heparin (20 mg/ml) at the beginning of the cultured period. The microinjections were carried out using a Narishige IM300 Microinjector.
○ Group 5: to block the PIP2 hydrolysis, neomycin (10 mM) was added in the medium at beginning of the cultured period.
○ Group 6: In order to achieve the Ca2+ chelation, EDTA (1 mM) was added in the medium at the beginning of the cultured period.
○ Group 7: to inactivate the active sites of the enzyme PLC, U-73122 (10 μM) was added in the medium at the beginning of the culture period.
○ Group 8: In order to block the voltage-dependent calcium channels, CdCl2 (50 μM) was added in the medium at the beginning of the cultured period.
To confirm the participation of the IP3/Ca2+ signalling pathway as the possible cytoplasmic event that allows the vitellogenin uptake process to take place, rescue experiments using the calcium ionophore A-23187 (Bement & Capco, Reference Bement and Capco1990) were performed. The ionophore was added at the beginning of the culture period to three different groups of follicles treated with neomycin (10 mM), EDTA (1 mM) and U-73122 (10 μM) (Group 9, 10 and 11, respectively). The different groups are characterized and referenced in Table 2.
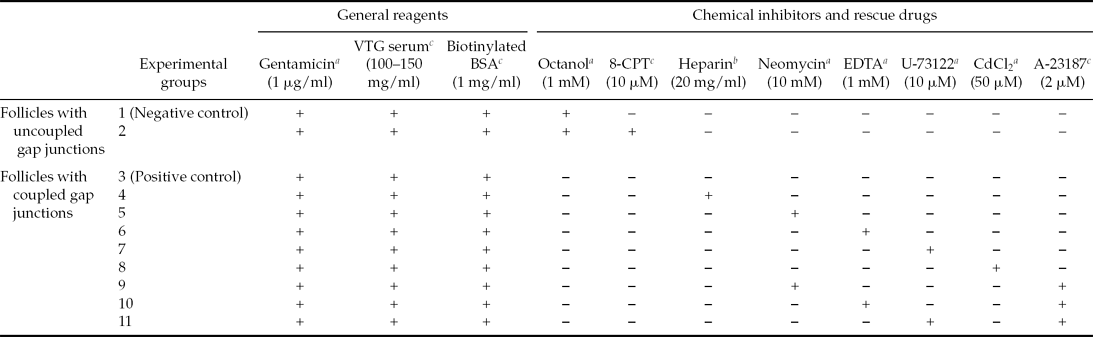
Table 2 Experimental groups

The final concentrations used (enclosed within brackets) were those reported in the literature as successfully used for similar tissues.
Different groups of oocytes were cultured in OR-2 medium for 24 h.
aAdded at the beginning of culture period.
bInjected into individual oocytes.
cAdded 3 h after the onset of the culture period.
Histological procedures and immunohistochemistry
The b-BSA was determined by immunohistochemistry. At the end of incubation (24 h), the collected follicles from incubation wells were fixed in 4% formol, dehydrated and embedded in paraffin-celloidin, as specified by Manes & Nieto (Reference Manes and Nieto1983). Sections of 7 μm thickness were serially obtained from blocks and mounted on poly-l-lysine-coated glass slides. Then they were deparaffinised, rinsed with phosphate-buffered saline (PBS, pH 7.4) and incubated with 3% bovine serum albumin, 0.02% Tween 20 in PBS (BSA/Tween 20/PBS) for 1 h at room temperature to prevent non-specific background staining (blocking solution).
In order to detect b-BSA, after blocking the slides were treated with a 1:3000 dilution of streptavidin-FITC conjugated (Sigma Chemical Co., E-2761) and incubated for 2 h at RT in a humid chamber. After extensive washing with PBS, an anti-FITC antibody conjugated with alkaline phosphatase was applied at a 1:1500 dilution overnight at 4 °C. The slides were then washed and alkaline phosphatase activity was detected by incubation with NBT/BCIP (Roche Biochemicals, 11 383 213 001/11 383 221 001) as a substrate. The reaction was stopped by rinsing the sections with distilled water. Then the slices were hydrated, mounted and observed with an OLYMPUS BX51 microscope.
Controls included incubated parallel and adjacent sections omitting the streptavidin-FITC conjugate. All images were captured in the same conditions with an OLYMPUS Q-Color5™ camera.
Results
Expression of the Xenopus exchange protein directly activated by cAMP (Xepac) during oogenesis
Semi-quantitative RT-PCR was used to investigate the presence and temporal expression pattern of Xepac during Xenopus laevis oogenesis, the results are shown in Fig. 1.

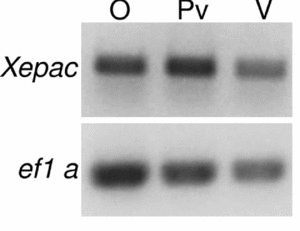
Figure 1 Expression of Xepac in whole ovary and in isolated oocytes of Xenopus laevis. cDNAs derived from X. laevis ovary (O), previtellogenic (Pv) and vitellogenic (V) oocytes were amplified using specific primer for Xepac and elongation factor 1 alpha (EF1α). Polymerase chain reaction (PCR) products were visualized with ethidium bromide and DNA bands were of the predicted size.
Xepac transcripts were detected at all stages of oogenesis (Fig. 1).
Involvement of Xepac in Xenopus laevis vitellogenesis
To assess the implication of the Xepac protein in the AMPc signalling pathway during the vitellogenin uptake process, experiments with octanol and a diffusible cAMP analogue (8-CPT) were performed.
Ovarian follicles with vitellogenin uptake inhibited due to the blockage of cAMP transfer from follicular cells to the oocyte (uncoupled gap junctions) were treated with 8-CPT (rescue drug), which specifically activates Epac.
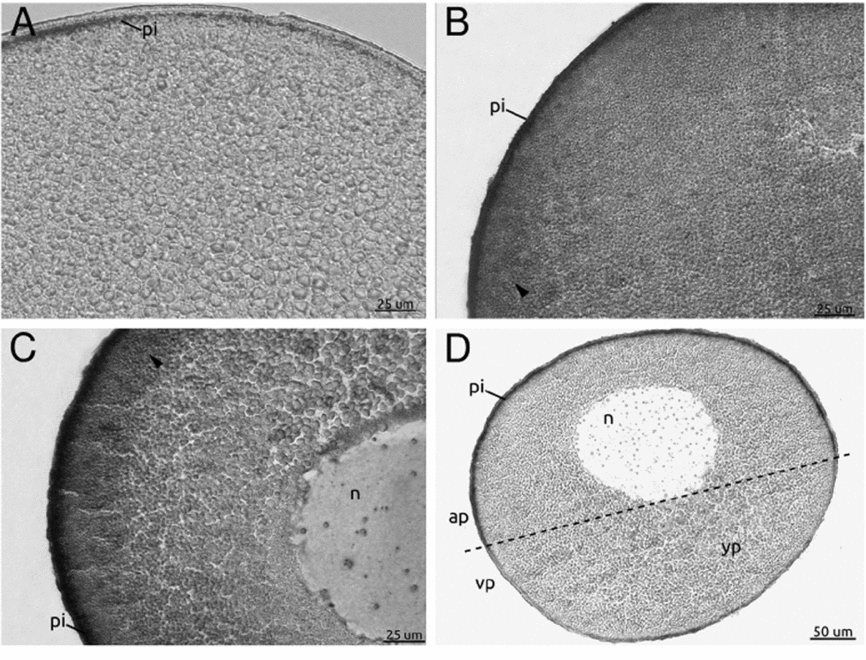
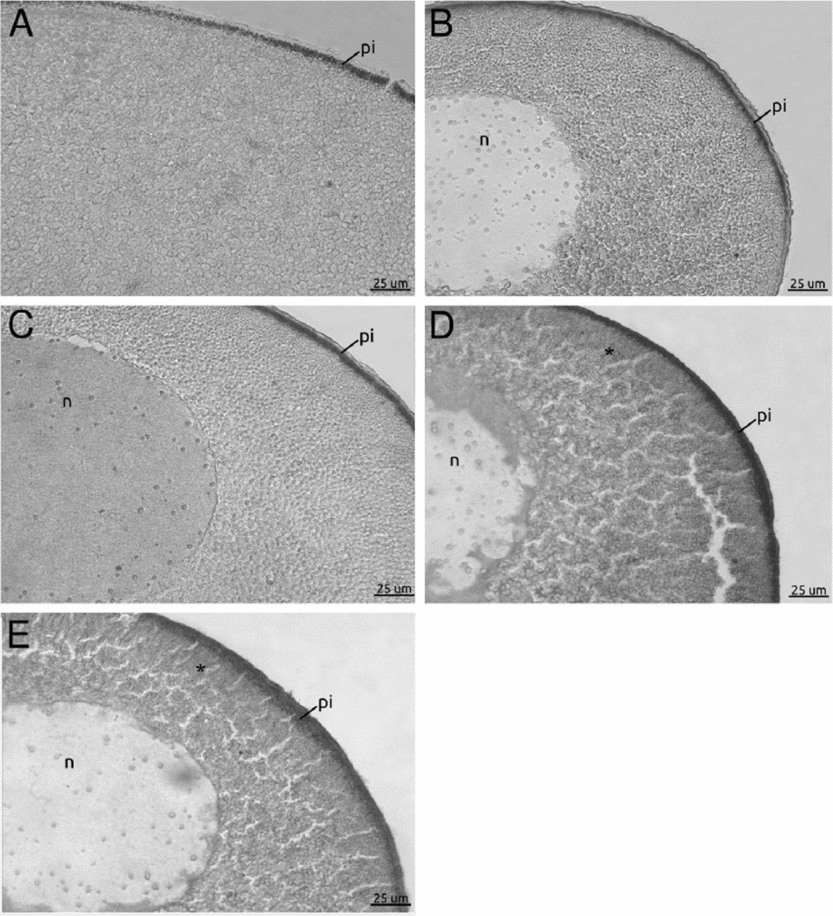
In Fig. 2A, a section from a follicle with uncoupled gap junctions showed inhibited vitellogenesis as evidenced by the absence of labelled yolk platelets (Fig. 2A). Figure 2B shows labelled yolk platelets seen in a section from a follicle with uncoupled gap junctions treated with 8-CPT. Therefore, this diffusible cAMP analogue rescues the endocytic activity in the follicles. The section from an untreated control follicle (coupled gap junctions) showed active endocytic activity evidenced by clearly labelled yolk platelets (Fig. 2C).

Figure 2 Rescue experiment to evaluate Xepac implication during Xenopus laevis vitellogenesis. (A–C) Stage IV oocytes treated with 1 mM octanol (A, B); 10 μM 8-CPT (B); and oocytes cultured without octanol treatment as a positive control (C). All the oocyte sections were revealed with alkaline phosphatase *biotinylated bovine serum albumin (b-BSA) detection. (A) No mark is observed in oocytes with uncoupled gap junctions. (B) 8-CPT completely restored the vitellogenic process (arrowhead). (C) Cortical mark (arrowhead) indicated VTG incorporation corresponding to oocytes with coupled gap junctions. (D) Schematic diagram of stage IV oocyte describing its main characteristics. ap, animal pole; n, nucleus; pi, pigment; vp, vegetal pole; yp, yolk platelets.
This result allows us to suggest that 8-CPT activates Epac protein triggering the IP3 signalling pathway resulting in increased intracellular Ca2+ levels and rescuing endocytic activity in follicles with uncoupled gap junctions. Therefore, once in the oocyte, the cAMP molecule (see Luque et al. Reference Luque, Serrano, Mónaco, Villecco and Sánchez2013 for more details) might bind to the Xepac protein and initiate a series of cytoplasmic signalling events that trigger the vitellogenin uptake process.
IP3/Ca2+ signalling pathway implication in Xenopus laevis vitellogenesis
The effect of inhibitors on IP3/Ca2+ signalling pathway in vitellogenic ovarian follicles is shown in Fig. 3.

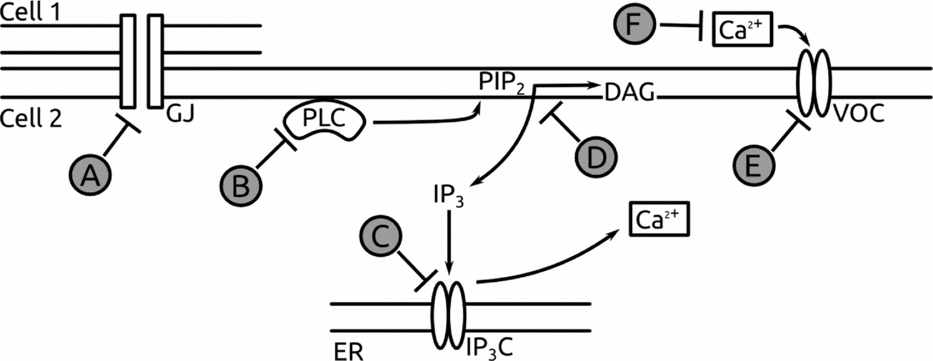
Figure 3 Inhibitors used in the experimental approach. The scheme shows the outline followed in order to inhibit the signalling pathways implicated in vitellogenesis at different stages. (A) octanol, (B) U-73122, (C) heparin, (D) neomycin, (E) CdCl2, (F) ethylene diamine tetraacetic acid (EDTA). ER, endoplasmic reticulum; GJ, gap junction; IP3C, IP3 channel; VOC, voltage-operated calcium channel.
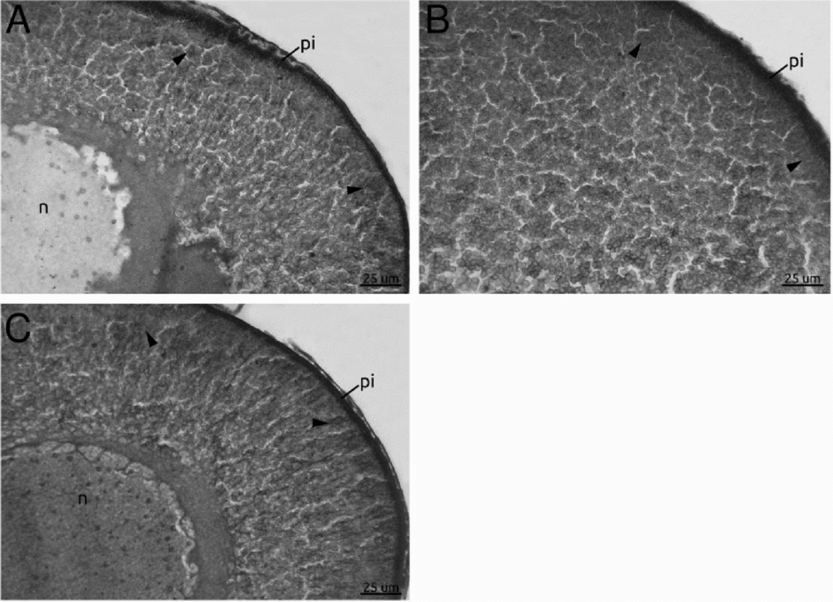
The pool of vitellogenic oocytes microinjected with heparin (Fig. 4A) or cultured with neomycin (Fig. 4B) have shown a inhibited endocytic activity because the labelled yolk platelets were not observed. Both inhibitors act at the level of IP3 which is known to stimulate Ca2+ release from the endoplasmic reticulum.

Figure 4 Inhibition experiments for the IP3/Ca2+ signalling pathway. (A–E) Oocyte sections revealed with alkaline phosphatase *biotinylated bovine serum albumin (b-BSA) detection. Oocytes cultured with heparin (A), neomycin (B), ethylene diamine tetraacetic acid (EDTA) (C), U-73122 (D), and ClCd2 (E). (A–C) Note the complete absence of vitellogenin incorporation due to the inhibition of the IP3/Ca2+ pathway. (D, E) Oocytes treated with U-73122 and ClCd2, showed a diminution but not a complete elimination of their endocytic capability. Observe the weak mark evidencing the incorporation of labelled BSA (asterisk). n, nucleus; pi, pigment.
Neomycin blocks PIP2 hydrolysis, inhibiting the synthesis of IP3 and the heparin blocks IP3 binding to the endoplasmic reticulum and resulting, in both cases, in a decrease of the release of Ca2+. These results allow us to suggest that the IP3/Ca2+ signalling pathway is required during vitellogenesis.
In concordance with the above results, when follicles were cultured with EDTA, a complete inhibition of endocytic activity was shown. This result confirms that Ca2+ is required for yolk uptake (Fig. 4C).
However, when the oocytes were cultured in a medium that contained the PLC enzyme activity inhibitor U-73122, the vitellogenin incorporation was not completely inhibited showing a remarkable decrease in the VTG endocytic uptake (Fig. 4D). The effect caused by U-73122 to directly block the active sites of the PLC shows the importance of this enzyme in the endocytic pathway of vitellogenin. These results are consistent with the results obtained with neomycin. However, caution should be exercised in the interpretation of the data using U-73122, as several other studies have suggested that this inhibitor has additional effects unrelated to the inhibition of PLC including not only the depletion of intracellular Ca2+ stores in PC12 cells (Hollywood et al., Reference Hollywood, Sergeant, Thornbury and MacHale2010) but also the increase of IP3-mediated Ca2+ release and direct stimulation of cation channels in other cells (Mogami et al., Reference Mogami, Lloyd-Mills and Gallacher1997).
The follicles incubated with CdCl2, another chemical known to block Ca2+ channel, showed a reduced vitellogenin uptake (Fig. 4E). However, some follicles showed near normal levels of endocytosis and similar effects were observed during the vitellogenesis of Oncopeltus fasciatus follicles treated with CdCl2 (Brown et al., Reference Brown, Herbert and Woodruff2010).
Nevertheless, putting together all our results we can deduce a direct participation of the IP3/Ca2+ signalling pathway during the vitellogenin incorporation process.
Rescue of VTG endocytic uptake in follicles treated with inhibitors of the IP3/Ca2+ signalling pathway
The results of the rescue experiments performed using the A-23187 ionophore are shown in Fig. 5. Three different groups of vitellogenic follicles were treated with either neomycin (10 mM), or EDTA (1 mM) or U-73122 (10 μM). When the ionophore was incorporated into the cultured medium that contained the inhibitor, the follicles presented endocytic VTG uptake and labelled yolk platelets were observed. Results showed that the ionophore is able to rescue the endocytic activity in the oocytes treated with the different inhibitors neomycin, EDTA and U-73122 (arrowheads in Fig. 5A, B and C, respectively).

Figure 5 Rescue experiments with A-23187 ionophore. Micrographs of stage IV oocyte sections revealed by biotinylated bovine serum albumin (b-BSA) detection in order to display VTG incorporation. Oocytes cultured with (A) neomycin, (B) ethylene diamine tetraacetic acid (EDTA) and (C) U-73122. After a period of incubation with the inhibitors the ionophore A-23187 was added to the three set of cultures. Observe the mark which indicates a rescued endocytic capability (arrowheads in (A), (B) and (C)). n, nucleus; pi, pigment.
These results fully corroborate the implication of the IP3/Ca2+ signalling pathway in the vitellogenin endocytosis process of X. laevis.
Discussion
During amphibian oogenesis, only vitellogenic follicles are actively involved in the endocytic process for yolk uptake from the bloodstream. In previous studies we demonstrated that functional gap junctions between oocyte and follicle epithelial cells are needed for the acquisition of endocytic competence by Xenopus oocytes (Mónaco et al., Reference Mónaco, Villecco and Sánchez2007).
Through open gap junctions a signal molecule identified as cAMP causes the beginning and maintenance of active endocytosis (Luque et al., Reference Luque, Serrano, Mónaco, Villecco and Sánchez2013). Without this appropriate signal from follicle cells, oocytes were unable to uptake the yolk precursor. Similarly, to start the vitellogenesis process in insect oocytes, a chemical signal transmitted from epithelial cells to oocytes it is needed (Brooks & Woodruff, Reference Brooks and Woodruff2004), suggesting that this mechanism is widespread between invertebrates and vertebrates. However, for insects, the molecular signal is the calcium-binding protein calmodulin (CaM) (Anderson & Woodruff, Reference Anderson and Woodruff2001) and it has been shown that, once inside the insect oocytes, CaM directly or indirectly activates a membrane-bound PLC, stimulating as a consequence, a release of Ca2+ from internal stores (Brown et al., Reference Brown, Herbert and Woodruff2010).
In previous findings we have demonstrated that, as well as in insects, CaM has a regulatory role during Xenopus vitellogenesis (Luque et al., Reference Luque, Serrano, Mónaco, Villecco and Sánchez2013), however its participation is downstream of the cAMP molecule, while the signalling steps that might link the cAMP function with the CaM regulation role during the Xenopus vitellogenin uptake process remains unknown.
Bearing this factor in mind, the main goal pursued in this work was to gain an insight about how, once inside the oocyte, does the cAMP to triggers the vitellogenic process. In this sense, we identified the expression of Xepac through the different stages of the oogenetic process, indicating that it could play a key role in it. Merging together the critical role of the cAMP during Xenopus vitellogenesis and the persistent presence of Xepac in that stage, we wondered whether Xepac is the next step followed by cAMP. Attempting to answer this question, we performed a rescue experiment with a validation reagent of cAMP signalling, 8-CPT (Grandoch et al., Reference Grandoch, Roscioni and Schmidt2010), demonstrating that cAMP might binds Xepac to trigger several signalling cascades that might allow the vitellogenin uptake. Consistently with this situation, it has been shown in ascidian oocytes that cAMP acts to activate an Epac molecule and its consequent signalling pathways, allowing the progression of the oogenesis (Lambert, Reference Lambert2011).
Despite these findings, additional experiments are necessary to elucidate the whole regulation process, including further approaches to test a possible synergism or antagonism between both canonical (PKA) and non-canonical (Xepac) cAMP pathways (Xiaodong et al., Reference Xiaodong, Zhenyu, Tamara and Fang2008).
Our next task was to comprehend the way in which Xepac allows vitellogenesis to take place. To gain a better understanding of this matter we performed several inhibition/rescue experiments to prove whether the PLC pathway and its consequent Ca2+ release are the following steps of cAMP-activated Xepac. Our experimental approach aimed to test this pathway at different levels to ensure the obtained results, to prove not only the inner cellular mechanism for Ca2+ release, but also the mechanism that allows the Ca2+ entrance from the outside media.
The inhibition experiments performed with neomycin, heparin and EDTA showed a complete inhibition of vitellogenin uptake, evidencing the participation of the IP3/Ca2+ pathway in this process. However, when we attempted to specifically inhibit the PLC action with U-73122 or the whole set of the calcium channels with CdCl2, we observed a decrease but not a complete blockage of the endocytic activity. We attributed these results to the nature of the reagents, due to the non-specific PLC isoform inhibition of the U-73122 and to the high toxicity of the ClCd2. Supporting this situation, our results of the PLC/IP3/Ca2+ participation during the vitellogenic process in Xenopus oocytes, were fully confirmed with the re-established endocytic capability by the oocyte after the addition of the A-23187 ionophore.
Interestingly, our data are coincident not only with findings in insects in which the IP3/Ca2+ pathway has been implicated in the vitellogenic process (Brown et al., Reference Brown, Herbert and Woodruff2010), but also with the recent discovery of a new PLC isoform, the PLCε, that might be the link between the cAMP-activated Xepac and the triggered IP3/Ca2+ pathway (Bunney et al., Reference Bunney, Baxendale and Katan2009).
All our results suggest us that. finally, Xenopus vitellogenesis is the result of a well regulated increase of cytoplasmic calcium as a consequence of the cAMP-activated Xepac which might trigger the IP3/Ca2+ pathway. Moreover, this is congruent with our previous findings in which we proved a regulatory effect of the CaM-Ca2+ complex in a downstream level regarding the cAMP (Luque et al., Reference Luque, Serrano, Mónaco, Villecco and Sánchez2013), due to the formation of this complex is dependent on the cytoplasmic calcium level.
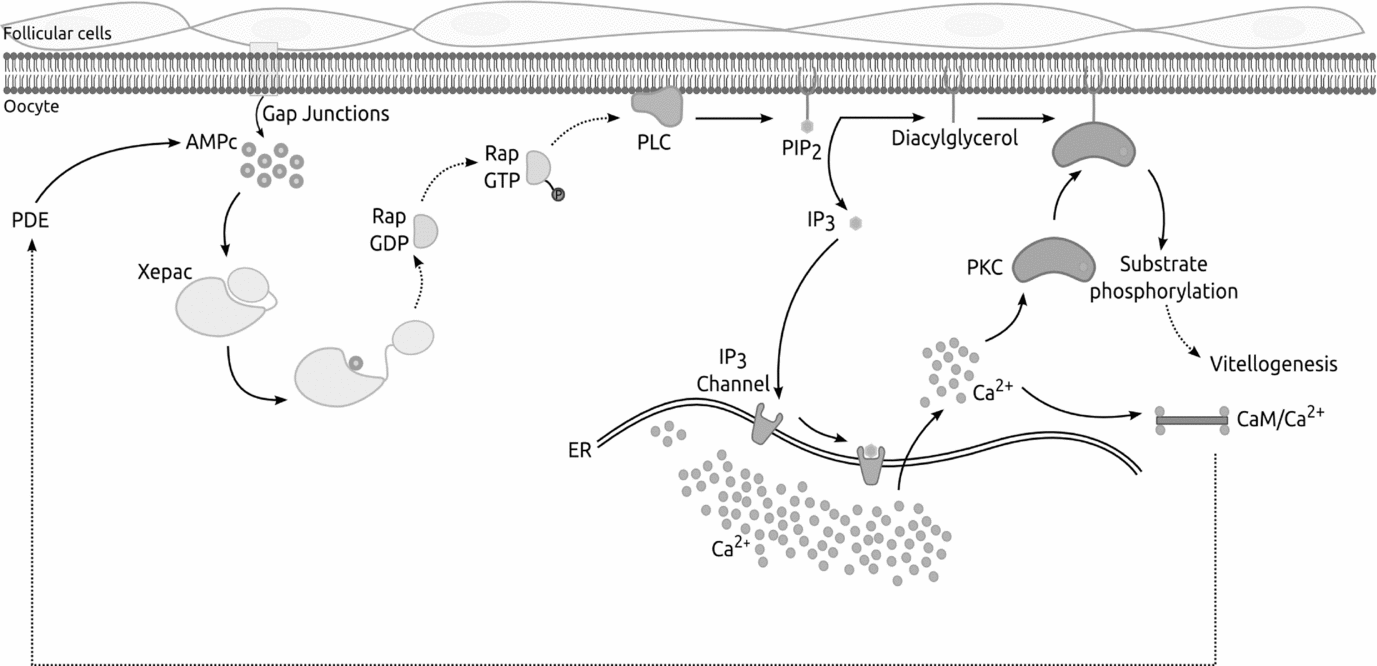
Combining together our results we are able to obtain a first draft of the signalling pathways implicated in the beginning and maintenance of X. laevis vitellogenesis (Fig. 6).

Figure 6 Schematic representation of the signalling pathway suggested for Xenopus laevis vitellogenesis. Combining together our results, we propose a signalling mechanism for X. laevis vitellogenesis, in which cAMP passes through gap junctions from follicle cells to the oocyte and binds Xepac protein. Xepac might act by activating a specific phospholipase C (PLC) triggering, as a consequence, a cytoplasmic Ca2+ release allowing cellular events that could maintain and regulate the whole process. Continuous line, established process; dotted line, unproven interaction mechanism.
Acknowledgements
The authors acknowledge Dr Cecilia Mundiña-Weilenmann, Dr Marta I. Bühler and Dr Graciela Sánchez Toranzo for providing specific reagents and Mr Stephen G. Monaghan for proofreading.
This research was supported by Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Agencia Nacional de Promoción Científica y Técnológica (ANPCyT) and Consejo de Investigaciones de la Universidad Nacional de Tucumán (CIUNT) grants to S.S.S.
Declaration of interests
The authors have declared that no conflict of interest exists.