Introduction
Stem-cell therapy is a therapeutic field with the potential for healing a myriad of diseases by transplanting healthy stem cells to restore tissues affected by illness or injury. One suggested treatment is for prepubescent boys who risk loss of fertility due to chemotherapy or radiation treatments for cancer. These patients could recover fertility if their SSCs are isolated before treatment and then transplanted back into the testes after treatment (Brinster, Reference Brinster2007). Because the number of SSCs in the testes is normally very low, a method should be developed to ensure the proliferation and survival of germ cells during culture. The proliferation and enrichment of SSCs in vitro could be a decisive strategy for helping to study SSCs in detail to improve the chance of success of in vivo SSC transplantation (Jalayeri et al., Reference Jalayeri, Pirnia, Najafabad, Varzi and Gholami2017).
Spermatogenesis is a complex process regulated by the endocrine system and, locally, by multiple interactions between developing germ cells and their surroundings through autocrine and paracrine factors (Caires et al., Reference Caires, Broady and McLean2010; Hai et al., Reference Hai, Hou, Liu, Liu, Yang, Li and He2014; Giudice et al., Reference Giudice, De Michele, Poels, Vermeulen and Wyns2017). Studies have shown that the response of cells in a three-dimensional (3D) culture system is much more similar to the behaviour of cells in vivo when compared with the 2D culture system. In a 3D culture, the cells interact with each other, with an extracellular matrix and with the microenvironment around them. These communications in 3D structures affect the proliferation, differentiation, cell morphology, and gene and protein expression as well as the cell responses to external factors (Mohammadzadeh et al., Reference Mohammadzadeh, Mirzapour, Nowroozi, Nazarian, Piryaei, Alipour, Modarres Mousavi and Ghaffari Novin2019).
Microcapsule technology is now commonly used for drug encapsulation for sustained release, enzyme immobilization, and microbioreactor cell culture. The alginate system has been a focus of attention for microcapsules (Lim and Sun, Reference Lim and Sun1980; O’Shea and Sun, Reference O’Shea and Sun1986), particularly the sodium alginate microcapsule, because of its ease of production and the efficient recovery of cells. This method has been used to cultivate different types of cells. In addition to mesenchymal stem cells (Bidarra and Barrias, Reference Bidarra and Barrias2019), human neuroblastoma cells (Lewicki et al., Reference Lewicki, Bergman, Kerins and Hermanson2019), bone marrow (Barralet et al., Reference Barralet, Wang, Lawson, Triffitt, Cooper and Shelton2005) and other SSCs have been evaluated in alginate gel. A previous investigation has identified the antioxidant and non-toxic properties of alginate hydrogel, which forms a scaffold that is applicable for 3D culture and cryopreservation of spermatogonial stem cells (Pravdyuk et al., Reference Pravdyuk, Petrenko, Fuller and Petrenko2013; Jalayeri et al., Reference Jalayeri, Pirnia, Najafabad, Varzi and Gholami2017; Pirnia et al., Reference Pirnia, Parivar, Hemadi, Yaghmaei and Gholami2017).
In reproductive biology, maintenance of SSC self-renewal is important (Song and Wilkinson, Reference Song and Wilkinson2014). If SSCs self-renew too frequently, the result will be deficiencies in spermatogenesis. Conversely, insufficient SSC self-renewal will result in progressive loss of germ cells (Meng et al., Reference Meng, Lindahl, Hyvönen, Parvinen, de Rooij, Hess, Raatikainen-Ahokas, Sainio, Rauvala, Lakso, Pichel, Westphal, Saarma and Sariola2000). Oct4, Nanog, and Sox2 are transcriptional regulators involved in the maintenance of self-renewal of embryonic stem cells (Assadollahi et al., Reference Assadollahi, Fathi, Abdi, Khadem Erfan, Soleimani and Banafshi2019a, 2019b). Bcl6b has been identified as promoting SSC self-renewal and is highly expressed in germ cells (Song and Wilkinson, Reference Song and Wilkinson2014). Plzf and Nanos2 are important genes that initiate SSC maintenance (Buaas et al., Reference Buaas, Kirsh, Sharma, McLean, Morris, Griswold, de Rooij and Braun2004; Schmidt et al., Reference Schmidt, Avarbock, Tobias and Brinster2009).
Although SSC transplantation techniques provide quantitative measurements of stem-cell performance in donor cell populations, this approach is technically challenging. It is a retrospective procedure that requires a 2–3 month span of time between transplantation and analysis (Phillips et al., Reference Phillips, Gassei and Orwig2010). In an effort to accelerate research in this critical area, several studies have suggested that the quality and quantity of a SSC culture can be measured over a shorter period of time using 3D culture in a scaffold (Yeh et al., Reference Yeh, Zhang and Nagano2007). The aim of the present study was to evaluate self-renewal gene expression and provide information about morphological and structural characterization and colony formation of SSCs encapsulated in alginate hydrogel.
Materials and methods
SSC isolation and purification
BALB/c mice were purchased from the Razi Laboratory Animal Center (Tehran, Iran). All procedures were approved by and performed in accordance with the National Research Council guidelines. The testes were extracted from 6-day-old pups and transferred to Dulbecco’s modified Eagle’s medium (DMEM; Gibco) containing penicillin (100 IU/ml) and streptomycin (100 µg/ml; Gibco). Then the epididymis and tunica albuginea were separated and the seminiferous tubules were extracted to a culture medium containing DMEM, 2 mg/ml collagenase (Gibco) and 200–500 µg/ml DNase I (Gibco). The testicular suspension was held at 37°C and centrifuged at 100 g for 5 min. The cell pellet then was resuspended in 1 ml trypsin/EDTA (Sigma) and gently pipetted to provide a testicular cell suspension, which was filtered through #60 nylon micro-mesh (Gholami et al., Reference Gholami, Hemadi, Saki, Zendedel, Khodadadi and Mohammadi-Asl2013a, 2013b, 2014).
Separation and purification with laminin and MACS
Petri dishes containing laminin were washed with phosphate buffered saline (PBS) and then incubated with bovine serum albumin (BSA) to prevent nonspecific binding. For SSC purification, the MACS method was performed using the specific marker for SSCs, Thy1 (CD90.1 MicroBeads, mouse-Miltenyi Biotec, 130-094-523), as a positive control and c-kit (CD117 MicroBeads, mouse-Miltenyi Biotec, 130-091-224) as a negative control (columns: MS, LS, XS, auto MACS columns).
In total, 107 cells were centrifuged at 300 g for 10 min and then were resuspended in 90 μl of PBS with a pH of 7.2, 0.5% BSA, and 2 mM EDTA. The MACS BSA stock solution (#130-91-376) was diluted with 1:20 auto MACS rinsing solution (#130-091-222) and 10 μl CD90.1 microbeads. The buffer was incubated for 15 min in the refrigerator and centrifuged for 10 min. The cell suspension was loaded onto a MACS column and was placed in the magnetic field of a MACS separator.
SSC encapsulation in alginate scaffold
The SSCs initially were resuspended in DMEM and alginic acid (sodium salt; Sigma-Aldrich, Germany) dissolved in 0.9% NaCl (pH 7.4) to achieve a final concentration of 1%. The SSCs then were resuspended in alginate hydrogel to achieve a final cell density of 106 cells/ml. This cell/scaffold mixture was dripped (10 µl droplets) into 135 mmol/l CaCl solutions for polymerization. The alginate beads were washed twice in sterile 0.9% NaCl (Merck, Germany).
3D cell culture
Encapsulated SSCs were cultured in DMEM containing 10% fetal bovine serum and 10 ng/ml glial cell-derived neurotrophic factor (GDNF; Sigma) for 2 months. The medium was replaced every 48 h.
Haematoxylin–eosin staining
After 2 months, the 3D culture of SSCs in alginate hydrogel microbeads was fixed in formalin. After being embedded in paraffin, the tissue sections were cut and dehydrated and then stained with haematoxylin–eosin to determine their histological structure.
Alginate depolymerization
The alginate depolymerization solution used contained 119 mmol/l trisodium citrate and 0.9% NaCl (pH 7.6) for extraction of the SSCs.
Viability
SSC viability assays were performed after staining with trypan blue (0.4%).
RNA extraction and cDNA synthesis
RNA was isolated using a Qiagen kit (Qiagene, cat. no. PP-2105) following the manufacturer’s instructions. cDNA synthesis was performed using a Bioneer kit (Bioneer, cat. no. k-2046) according to manufacturer’s instructions.
Real-time PCR
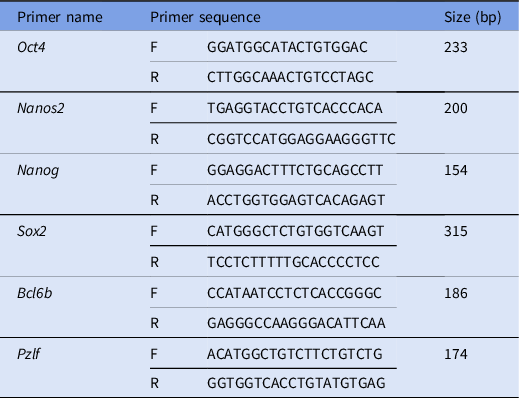
Real-time PCR was performed using a Qiagen Rotor Gene 6000 instrument. Forty-five reactions were considered. Each reaction cycle was performed for 15 s at 95°C and 45 s at 60°C (Gholami et al., Reference Gholami, Hemadi, Saki, Zendedel, Khodadadi and Mohammadi-Asl2013a). Table 1 lists the primers.
Table 1. Primers used in this study. Sequences and sizes (bp) of primers presented in the table
Scanning electron microscopy
The Santana freeze-dried method was used to prepare the alginate hydrogel microbeads containing encapsulated SSCs for SEM imaging to determine the SSC morphology after 1 month of 3D culture.
Statistical analysis
Gene expression was analyzed using REST-2009 software. Other data were presented as the mean ± standard deviation. The Mann–Whitney test was used to detect significant differences between groups. A P-value of 0.05 was considered to be statistically significant. All statistical computations were performed using SPSS 17.0 software (SPSS, USA).
Results
Viability
Figure 1 compares the viability of the 3D alginate culture as the experimental group with the freshly isolated SSC culture as the control group. The viability of the freshly isolated SSCs and 3D cultures was determined after dying with trypan blue. The mean survival rate of the SSCs encapsulated in 3D alginate hydrogel after 2 months was 77.41%, which is significantly lower than the control group at 96.90% (P = 0.011; Fig. 1).

Figure 1. Viability of SSCs encapsulated in alginate after 2 months of culture. Viability of encapsulated SSCs and Fresh SSCs was 96% and 77.4% respectively. *P = 0.011.
Gene expression
The ability of the 3D alginate hydrogel to maintain undifferentiated mouse SSCs was investigated by quantifying the expression levels of Oct4, Sox2, Nanog, Nanos2, Bcl6b and Plzf. These are self-renewing genes specific to undifferentiated SSCs. After 2 months of culture, the expression levels of Oct4, Sox2 and Nanos2 genes decreased in the alginate-encapsulated SSCs compared with the freshly isolated testicular cells (Fig. 2 and Table 2). qRT-PCR analysis was carried out on day 60 after seeding and showed that there was no significant difference between the relative expression of the Nanog, Bcl6b and Plzf genes in the 3D culture experimental group and the freshly isolated control group. The expression levels of the Nanog, Bcl6b and Plzf genes showed no change (Fig. 2 and Table 2).

Figure 2. Expression of stemness gene of 6-day-old mice SSCs that were encapsulated in alginate and were cultured for 2 months. Oct4, Sox2 and Nanos2 decreased, but Nanog, Bcl6b and Plzf did not show significant changes.
Table 2. Analysis results of expression of stemness gene. Results showed that Oct4, Sox2 and Nanos2 decreased, but Nanog, Bcl6b and Plzf did not show significant changes. The results were produced by Rest 2009 software. This software compares gene expression between samples, SSCs were encapsulated for 2 months in alginate, with control, fresh SSCs, in the presence of Gapdh (housekeeping gene known as the reference gene)

Haematoxylin–eosin assessment
The capsules were spherical in shape with a uniform margin and a homogenous distribution of the cells inside the capsule. At 60 days, the capsules had maintained their structural integrity and spherical form. The SSCs in the alginate capsules were circular, but did not bind to the surface. Haematoxylin–eosin examination showed that SSCs with pale nuclei and numerous nucleoli had formed colonies (Fig. 3a–c).
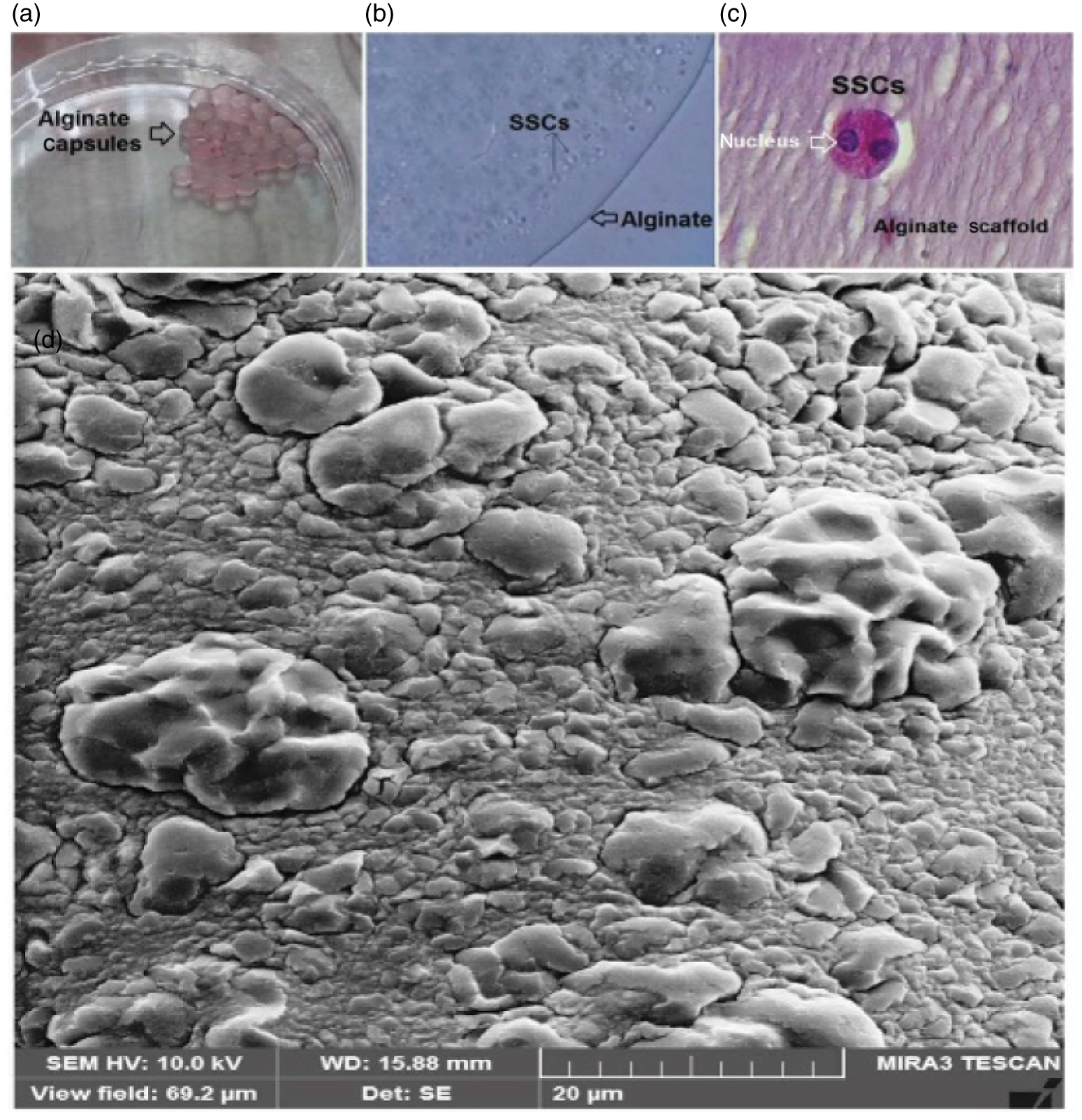
Figure 3. (a, b) SSCs encapsulated alginate microbeads, 3D culture of encapsulated. (c) Microbeads after 3D long-term culture stained with haematoxylin and eosin (H&E) dye. Stained sections show normal spermatogonia morphology. The image shows SSCs mitosis in the alginate scaffold. (d) SEM micrograph from SSCs encapsulated in alginate hydrogel. SSCs encapsulated in alginate scaffold can be seen attached to the surface of the hydrogel scaffold 2 months after culture.
SEM analysis
The SSCs encapsulated in the alginate scaffold were evaluated by SEM. The images showed that the SSCs were in a 3D structure and the formation of the stem cells into colonies was clearly visible. SEM evaluation revealed that the alginate scaffold structure had preserved the SSC morphology and density at 60 days of culture (Fig. 3d).
Discussion
SSCs are undifferentiated spermatogonia that can maintain their capacity for self-renewal and retain their ability to differentiate into dedicated germ cells (Kanatsu-Shinohara and Shinohara, Reference Kanatsu-Shinohara and Shinohara2013). To maintain the features of SSCs, external factors secreted from surrounding somatic cells and internal factors, such as specific gene expression, play important roles (Orwig et al., Reference Orwig, Ryu, Master, Phillips, Mack, Avarbock, Chodosh and Brinster2008). SSCs are an appealing model for the identification of the molecular pathways involved in their regulation of self-renewal and differentiation.
Because of the significant difference in the SSC population in juvenile and adult mice (Aponte et al., Reference Aponte, Van Bragt, De Rooij and Van Pelt2005; McLean et al., Reference McLean, Friel, Johnston and Griswold2003), the testes of 6-day-old mice were used in this study. Evidence also suggested improved spermatogonial viability and differential capacity in immature mice (Nagano et al., Reference Nagano, Ryu, Brinster, Avarbock and Brinster2003). In general, because of the limited number of SSCs and their lack of unique cell-surface markers, it is extremely difficult to isolate a purified population of SSCs (Khajavi et al., Reference Khajavi, Akbari, Abdolsamadi, Abolhassani, Dehpour, Koruji and Habibi Roudkenar2014). The MACS method is the most appropriate technique and causes minimal stress to the SSCs during isolation (Oatley et al., Reference Oatley, Avarbock and Brinster2007). A cell-surface marker that is expressed exclusively on undifferentiated SSCs produced successful MACS isolation (Sofikitis et al., Reference Sofikitis, Pappas, Kawatani, Baltogiannis, Loutradis, Kanakas, Giannakis, Dimitriadis, Tsoukanelis, Georgiou, Makrydimas, Mio, Tarlatzis, Melekos and Miyagawa2005). Thy1 (or CD90) has been identified in rodents, non-human primates and cattle as a surface marker of SSCs (Oatley et al., Reference Oatley, Kaucher, Avarbock and Brinster2010). Thy1+ testis cell fraction isolation has resulted in the enrichment of SSCs. The cultivation of Thy1+ mouse germ cells under conditions supported by GDNF showed increased SSC numbers over prolonged periods of time (Kubota et al., Reference Kubota, Avarbock and Brinster2004). In the current study, purified populations of Thy1+ SSCs were isolated using the MACS method.
The ability of SSCs to maintain spermatogenesis homeostasis is necessary for treatment of infertility by in vivo transplantation (Kanatsu-Shinohara and Shinohara, Reference Kanatsu-Shinohara and Shinohara2013). However, because of the limited number of SSCs (Khajavi et al., Reference Khajavi, Akbari, Abdolsamadi, Abolhassani, Dehpour, Koruji and Habibi Roudkenar2014), the development of efficient culture systems capable of maintaining SSC self-renewal and promoting their differentiation has attracted attention in the stem-cell research community (Ziloochi Kashani et al., Reference Ziloochi Kashani, Bagher, Asgari, Najafi, Koruji and Mehraein2020). In the current investigation, after isolating mouse neonatal testicular cells by enzymatic digestion (Jalayeri et al., Reference Jalayeri, Pirnia, Najafabad, Varzi and Gholami2017; Pirnia et al., Reference Pirnia, Parivar, Hemadi, Yaghmaei and Gholami2017) to evaluate the effect of alginate hydrogel on SSC proliferation and self-renewal, isolated mouse testicular cells were cultured in the presence of GDNF.
GDNF is considered to be the most important factor in the proliferation (Hofmann, Reference Hofmann2008), survival (Oatley et al., Reference Oatley, Kaucher, Avarbock and Brinster2010) and self-renewal (Oatley et al., Reference Oatley, Avarbock, Telaranta, Fearon and Brinster2006) of SSCs. It is produced by Sertoli cells after birth and is currently the only recognized paracrine factor in the testes that is directly responsible for in vivo and in vitro maintenance and self-renewal of SSCs (Hofmann, Reference Hofmann2008). In the present study, cell viability analysis was determined after staining with trypan blue and revealed significant differences between freshly isolated SSCs and those in the 3D alginate culture system. Although the percentage of viable cells in the 3D alginate culture was lower than in the freshly isolated SSCs, the mean survival rate of SSCs in the alginate hydrogel after 2 months was high. This result is similar to those of Jalayeri and colleagues (Jalayeri et al., Reference Jalayeri, Pirnia, Najafabad, Varzi and Gholami2017). They showed that, after 60 days of encapsulation of SSCs, the viability of these cells remained high at c. 74%. Wang and co-workers also showed that embryonic stem cells in alginate encapsulation had survival rates of c. 72% after 10 days (Wang et al., Reference Wang, Adams, Buttery, Falcone and Stolnik2009).
Alginate hydrogels are non-adhesive, highly hydrophobic and hydrated 3D networks. They are similar to extracellular matrices, cross-linking processes that do not damage the cells and are highly porous structures. Moreover, alginate hydrogel does not require toxic activators or a temperature change to promote cellular activity and metabolism. The antioxidant properties of the alginate that enhance cell survival and facilitate the exchange of ions and other nutrients from cells to the culture medium are important properties of alginate hydrogel that make this scaffold a promising candidate for 3D cell cultures (Azevedo et al., Reference Azevedo, Bourbon, Vicente and Cerqueira2014; Sarker et al., Reference Sarker, Rompf, Silva, Lang, Detsch, Kaschta, Fabry and Boccaccini2015; Jalayeri et al., Reference Jalayeri, Pirnia, Najafabad, Varzi and Gholami2017). In the present study, SEM micrographs showed that the cell morphology and their spread and proliferation had been preserved after 60 days of culturing.
Oct4 is expressed in undifferentiated spermatogonia of newborn, juvenile and adult mouse testes (Pesce et al., Reference Pesce, Wang, Wolgemuth and Schöler1998) and plays a role in the self-renewal of SSCs (Ketkar and Reddy, Reference Ketkar and Reddy2012). In the present study, the results showed that expression of Oct4 was significantly lower in SSCs encapsulated in alginate scaffold after 60 days of culturing. It is possible that the cells in this group withdrew from pluripotency and were differentiated (Afsartala et al., Reference Afsartala, Rezvanfar, Hodjat, Tanha, Assadollahi, Bijangi, Abdollahi and Ghasemzadeh-Hasankolaei2016). Although the expression of Oct4 has been demonstrated by Wu and colleagues it is not necessary for in vitro maintenance of SSCs (Wu et al., Reference Wu, Oatley, Oatley, Kaucher, Avarbock and Brinster2010).
Sox2 as an important transcription factor involved in embryogenesis, stem-cell pluripotency and primordial germ cell proliferation (Assadollahi et al., Reference Assadollahi, Fathi, Abdi, Khadem Erfan, Soleimani and Banafshi2019a). Kanatsu-Shinohara and colleagues showed that Sox2, like Oct4, was already being expressed at low levels in cultured SSCs (Kanatsu-Shinohara et al., Reference Kanatsu-Shinohara, Lee, Inoue, Ogonuki, Miki, Toyokuni, Ikawa, Nakamura, Ogura and Shinohara2008) and appeared to be an important factor in their expression (de Oliveira et al., Reference de Oliveira, Tesser, Nunes and Stumpp2019). The results of the current study showed that Sox2 and Nanos2 expression, similarly to Oct4, decreased in the SSCs encapsulated in the alginate scaffold for 60 days. Nanos2 is primarily expressed in specific types of undifferentiated A-spermatogonia (Ishii et al., Reference Ishii, Kanatsu-Shinohara, Toyokuni and Shinohara2012). It has been shown that the expression level of Nanos2 is heterogeneous and that this heterogeneity at the Nanos2 expression level has practical significance.
The Nanog transcription factor homeobox plays a crucial role in embryonic development/proliferation and maintenance of SSCs by interacting with Oct4 and Sox2 (Patra et al., Reference Patra, Vemulawada, Soren, Sundaray, Panda and Barman2018), although no Nanog or Sox2 protein expression has been detected in these cells (Kanatsu-Shinohara et al., Reference Kanatsu-Shinohara, Lee, Inoue, Ogonuki, Miki, Toyokuni, Ikawa, Nakamura, Ogura and Shinohara2008). Unlike Oct4 and Sox2, the expression of the Nanog gene did not change significantly in the SSCs encapsulated in an alginate scaffold for 60 days in the present study.
Plzf (also called ZBTB16 or ZFP145) is a transcription factor that inhibits the differentiation of stem cells and maintains spermatogonia in an undifferentiated state (Jalayeri et al., Reference Jalayeri, Pirnia, Najafabad, Varzi and Gholami2017). Recent studies have shown that Bcl6b is involved in spermatogenesis and essential for the maintenance of self-renewal of SSCs in vitro (Pesce et al., Reference Pesce, Wang, Wolgemuth and Schöler1998; Afsartala et al., Reference Afsartala, Rezvanfar, Hodjat, Tanha, Assadollahi, Bijangi, Abdollahi and Ghasemzadeh-Hasankolaei2016). Even though Bcl6b overexpression has been shown to cause germ cell tumours (Pesce et al., Reference Pesce, Wang, Wolgemuth and Schöler1998) and based on the findings obtained using siRNA, spermatogenesis degeneration was observed in the Bcl6b-null testes (Oatley et al., Reference Oatley, Kaucher, Avarbock and Brinster2010). Similarly, Nanog, Plzf and Bcl6b genes did not change in expression in the encapsulated SCCs in the alginate scaffold in this study.
The comparison of differentiated cells and SSCs has shown that SSCs express genes that have different functions. Previous investigations have reported that that there are 1133 genes in the SSCs with expression levels that are more than twice that of differentiated cells. Among the 1133 genes, 900 gene sequences encode known proteins and more than 200 genes are unknown functionally. Also, 50 genes such as Nanos, Lin28, Oct4 and Plzf, in SSCs have expression levels of greater than five-fold. In contrast, 462 genes have expression levels of greater than five-fold. This evidence suggests that regulation of gene expression can play a key role in determining the properties of pluripotent cells (Yang et al., Reference Yang, Wu and Qi2013).
The male germ cells are located in the intact testes in the 3D structure of the seminiferous tubules. It has been argued that the 3D culture of SSCs could provide an environment that more closely resembles in situ conditions (Huleihel et al., Reference Huleihel, Nourashrafeddin and Plant2015). It also has been suggested that 3D systems can possibly provide niches that replicate, to some degree, the microenvironment and spatial structure of the in vivo conditions in the seminiferous tubules, in which germ cells are embedded in Sertoli cells (Sharpe, Reference Sharpe, (Knobil and Neill1994).
Cell–cell interactions are essential for clonal expansion and differentiation of germ cells, therefore 3D culture systems could provide an enhanced structural environment. In addition, these systems may enable germ cells to organize into densely packed clusters that facilitate the delivery of oxygen, nutrients, and other factors, thereby allowing their survival and proliferation to be maintained. These clusters could also provide and maintain contacts between germ cells, which are required during differentiation (Mahmoud, Reference Mahmoud2012). Despite the decrease in some SSC stemness genes in this study, additional research in the use of alginate scaffolds could find them a prospective candidate for long-term culture.
Acknowledgements
The author would like to thanks the research affairs of Ahvaz Jundishapur University of Medical Sciences which funded the project (no. 1793).
Funding
Ahvaz Jundishapur University of Medical Sciences (no. 1793).
Competing interests
The authors declare that they have no competing interests.
Data availability
Data sharing is not applicable to this article as no new data were created or analyzed in this study.







