Introduction
In the reproductive system, oocyte maturation is a critical process. The follicle’s mature oocytes are inhibited at the germinal vesicle (GV) phase and can continue meiosis only after being released from the follicle. After germinal vesicle breakdown (GVBD), the oocyte enters the metaphase I (MI) stage, starting spindle migration and first polar body extrusion. Then, the oocyte enters the metaphase II (MII) stage and remains in this stage until fertilization. The maturation of oocytes directly determines fertilization and subsequent embryo development. Studies have found that oxidative stress is one of the reasons for the decline in oocyte maturation capability (Zhang et al., Reference Zhang, Han, Zhu, Tang, Cui, Kim and Sun2016; Jia et al., Reference Jia, Zhang, Zhou, Xu and Feng2019). Reactive oxygen species (ROS) are necessary for nuclear maturation under physiological conditions. However, an imbalance between ROS production and antioxidant capacity can lead to DNA damage and apoptosis (Roth, Reference Roth2018). Oxidative phosphorylation in the mitochondria is the primary ROS source, destroying cellular proteins, lipids, and nucleotides (Holmström and Finkel, Reference Holmström and Finkel2014; Luddi et al., Reference Luddi, Capaldo, Focarelli, Gori, Morgante, Piomboni and De Leo2016; Perkins et al., Reference Perkins, Das, Panzera and Bickel2016). Oxidative stress can also induce a decrease in the mitochondrial membrane potential (ΔΨm) (Roth, Reference Roth2018).
With the development of agricultural economies, the emergence of pesticides has increased convenience in people’s lives. However, the extensive use of pesticides poses a potential threat to human and animal living environments. Methomyl (S-methyl N-(methyl-carbamoyl)) is a broad-spectrum carbamate insecticide researched and developed by the Dupont Company in the United States in 1966 and was registered and listed in the United States in 1968 (Van Scoy et al., Reference Van Scoy, Yue, Deng and Tjeerdema2013). Dupont first used the name ’Lannate’ to promote the use of methomyl, which has a history of over 50 years. However, in daily practice, people have found that the widespread use of methomyl can cause residues to accumulate in the water, accompanied by certain toxic effects, and endanger the health of livestock and humans. In 1997, the World Wildlife Fund listed methomyl as an environmental oestrogen (Meng et al., Reference Meng, Qiu, Hu, Fan, Song, Zheng, Wu, Qu, Li, Chen and Xu2016). Methomyl is a white crystalline solid in its pure state and is stable and non-corrosive when stored in an aqueous solution. As an exogenous pollutant, methomyl is toxic to the reproductive system of animals. It has been reported that methomyl can reduce sperm count and motility in mice. Saenphet and co-workers used 2 mg/kg methomyl to continuously gavage male rats for 60 days and found that the male rats’ reproductive organs had pathological changes; the weight of the male rats’ testicles and seminal vesicles decreased significantly, while the epididymal sperm concentration also decreased (Farag et al., Reference Farag, Eweidah and El-Okazy2000). Shanthalatha and colleagues fed female rats with 5 mg/kg of methomyl for 3 months and found that the relative weights of the fallopian tubes, uteri, and ovaries were significantly decreased (Shanthalatha et al., Reference Shanthalatha, Madhuranath and Yajurvedi2012). Because methomyl was shown to affect the reproductive systems of mammals, we speculated that methomyl might have toxic effects on oocyte maturation. Moreover, methomyl can cause oxidative stress in rat red blood cells and tissues (Mansour et al., Reference Mansour, Mossa and Heikal2009; Djeffal et al., Reference Djeffal, Messarah, Boumendjel, Kadeche and Feki2015). Although methomyl has been extensively studied, the effect of methomyl exposure on the maturation of mammalian oocytes remains unclear.
In this study, we investigated whether methomyl exposure affects oocyte maturation. We used mice as a model to determine the effect of methomyl on the polar body extrusion rate, ROS level, ΔΨm, abnormal spindle morphology rate, and mRNA expression of apoptosis-related genes in mouse oocytes. The results of our research demonstrated the potential damage of methomyl to oocyte maturation.
Materials and methods
All chemicals and reagents used in this study were purchased from Sigma-Aldrich (St. Louis, MO, USA) unless otherwise stated.
Mice
All animal experiments were performed following the guidelines of the Animal Research Committee of Yanbian University, China. Female Kunming mice, 6–8 weeks of age, were purchased from the Experimental Animal Production Center of Yanbian University, China. Mice were kept in temperature-controlled rooms and provided with sufficient food and water at a constant temperature (22 ± 2°C). Female mice were reared for 7 days to adapt their physiological cycle to the environment for experimental use.
Oocyte collection and culture
Female Kunming mice were injected with 10 IU PMSG intraperitoneally, and germinal vesicle-intact oocytes were harvested from both sides of mouse ovaries after a 48 h treatment. Methomyl was dissolved in deionized water to prepare a concentrated stock solution. In the treatment group, GV-stage oocytes were treated with different methomyl concentrations (10, 30, 60, 100, or 200 μM) diluted in M16 medium for 14 h in a cell incubator (at 37°C in a humidified atmosphere with 5% CO2). Oocytes from the control group were cultured in M16 medium without treatment.
Immunofluorescence
MI oocytes were fixed with 3.7% paraformaldehyde dissolved in phosphate-buffered saline (PBS) for 30 min and permeabilized in 0.5% Triton X-100 for 20 min. After 1 h incubation in blocking buffer (1% BSA in PBS), the oocytes were incubated overnight at 4°C with FITC-conjugated anti-α-tubulin antibody, followed by incubation with Hoechst 33342 stain (1:1000, in PBS), which was used for DNA counterstaining for 15 min at 37°C. The oocytes were then mounted onto glass slides and analyzed, and imaged with a confocal microscope (Zeiss LSM 710 META, Oberkochen, Germany).
Measurement of ROS level in MII oocytes
For ROS level determination, MII oocytes were stained with 10 μmol/l of a 2′,7′-dichlorodihydrofluorescein diacetate probe (H2DCFDA; Thermo Fisher Scientific, Waltham, MA, USA) by incubation in the dark at 37° C for 30 min and then washed three times with 1% BSA in PBS, followed by observation using a fluorescence microscope (Nikon, Tokyo, Japan). The fluorescence intensity of the oocytes was analyzed using ImageJ software.
Quantification of ΔΨm
To assess ΔΨm, MII oocytes were incubated with 2 μM JC-1 (Invitrogen, Carlsbad, CA, USA) at 37°C in the dark for 30 min and then washed three times with 1% BSA in PBS. ΔΨm was calculated as the ratio of red fluorescence (activated mitochondria/J-aggregates) to green fluorescence (less activated mitochondria/J-monomers). The fluorescence intensity of the resulting MII oocytes was analyzed using ImageJ software.
Real-time quantitative polymerase chain reaction (RT-qPCR)
Total mRNA was extracted from oocytes after they were cultured with methomyl for 14 h starting from the MII stage using a DynaBeads® mRNA DIRECT™ Kit (Invitrogen Dynal AS, Oslo, Norway), according to the manufacturer’s instructions. Then cDNA strands were synthesized using the QuantiNova® Reverse Transcription Kit (50) (Qiagen GmbH, Germany). RT-qPCR was performed using the KAPA SYBR® FAST kit (Kapa Biosystems, Cape Town, South Africa). The 20 μl reaction system comprised 10 μl SYBR, 0.5 μl forward primer, 0.5 μl reverse primer, 2 μl cDNA, 6.6 μl H2O, 0.2 μl KSF ROXHigh, and 0.2 μl KSF ROXLow. cDNA amplification was conducted in a thermocycler using the following program: 95°C for 3 min, 40 cycles at 95°C for 3 s, 60°C for 30 s, and a final extension at 72°C for 20 s. The target genes were Bax, Caspase-3, and Bcl-2. The gene encoding glyceraldehyde-3-phosphate (GAPDH) was used as a reference. The primers used to amplify each gene are shown in Table 1. mRNA quantification data were analyzed using the 2 −ΔΔCT method (Rao et al., Reference Rao, Huang, Zhou and Lin2013).
Table 1. Primer sequences used for RT-qPCR
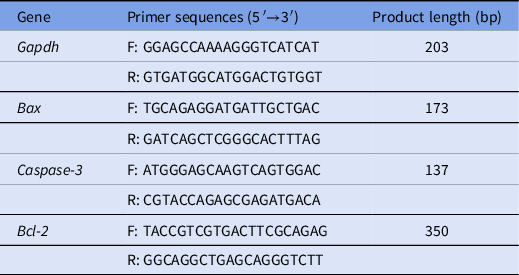
F: forward primer; R: reverse primer.
Note: The annealing temperature for all reactions was 60°C.
Statistical analysis
Results are presented as mean ± standard deviation (SD). Statistical analysis was performed using SPSS software (version 19.0; SPSS, Chicago, IL, USA). Figures were generated using the GraphPad Prism software package (v.6.01; GraphPad, La Jolla, CA, USA). Total numbers (n) of oocytes used in each group and replicates (R) of each experiment are shown in the data column and each figure legends, respectively. Data obtained from only two groups were compared using an independent sample t-test. Data with three or more means were analyzed using one-way analysis of variance.
Results
Methomyl treatment inhibits the polar body extrusion in mouse oocytes
To investigate methomyl’s effect on the maturation of mouse oocytes, polar body I (PB I) extrusion rate was evaluated. As shown in Fig. 1A, mouse oocytes at the GV stage were collected and cultured in M16 medium containing 10, 30, 60, 100, or 200 μM methomyl for 14 h to observe the extrusion of PB Ι. The results showed that, compared with that in the control group, 60 and 100 μM methomyl significantly reduced the rate of PB I extrusion (62.48 ± 2.14% vs. 37.80 ± 5.72% vs. 23.03 ± 7.83%, respectively, P < 0.05; Fig. 1B). All oocytes in the 200 μM methomyl group died. In our subsequent study, 60 μM methomyl was used because this concentration not only caused abnormal polar body extrusion but also allowed a certain proportion of oocytes to develop into the MII stage for subsequent research.
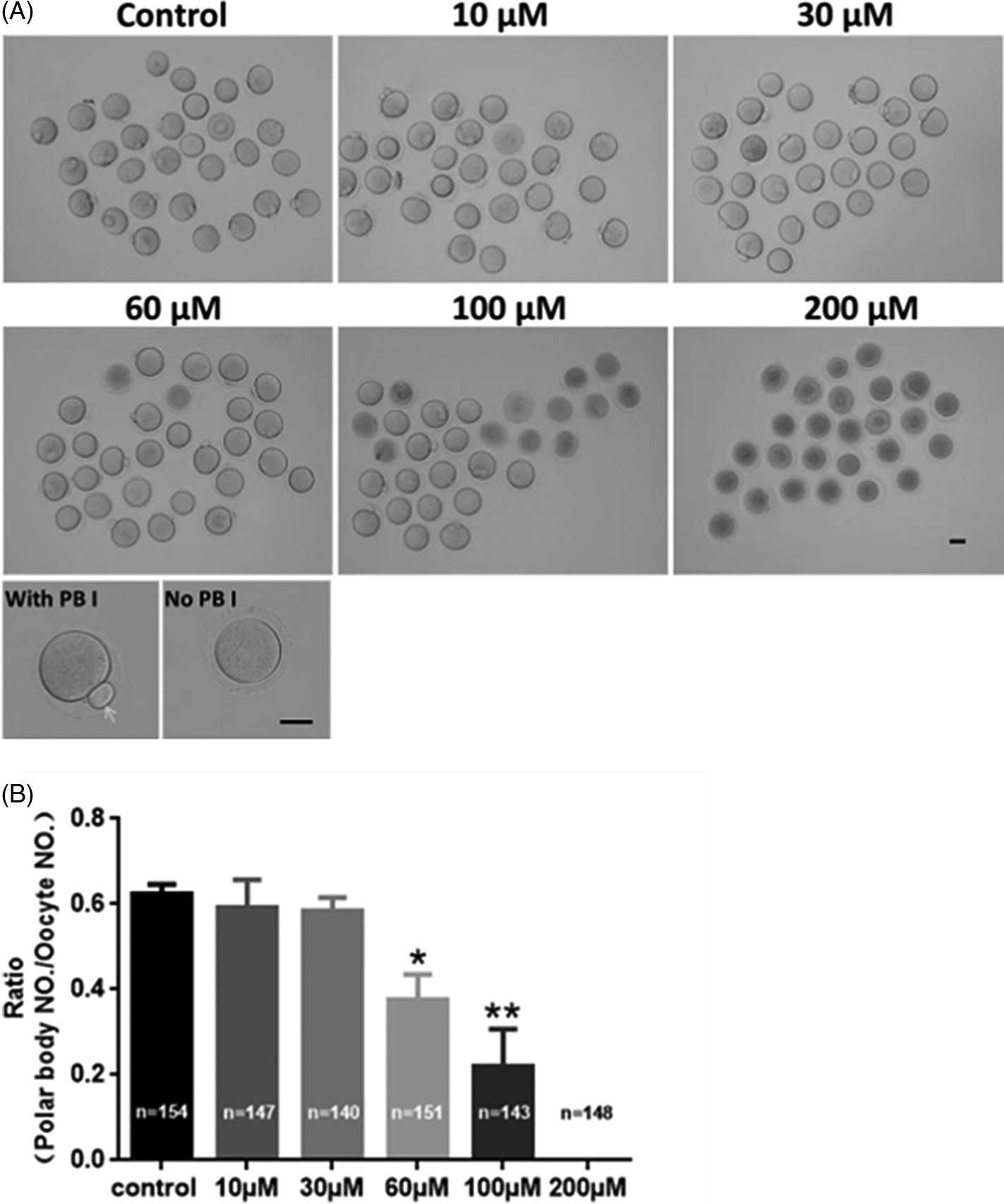
Figure 1. Methomyl exposure affects mouse oocyte PB I extrusion. (A) Polar body extrusion of GV-stage oocytes treated with different methomyl concentrations (10, 30, 60, 100, and 200 μM) for 14 h. Scale bars: 100 μm. (B) After methomyl treatment, the polar body extrusion ratio was significantly reduced, R = 5. *P < 0.05, **P < 0.01.
Methomyl treatment increases ROS levels in mouse oocytes
We observed that some oocytes treated with methomyl were unable to reach the MII stage. As shown in Fig. 2A, ROS levels were measured to understand how methomyl affected mouse oocyte maturation. Fig. 2B shows that, compared with the control group, the oocyte ROS level in the methomyl group was significantly increased (1.00 ± 0.23 vs. 1.54 ± 0.29; P < 0.05), indicating that methomyl induced oxidative stress in mouse oocytes.
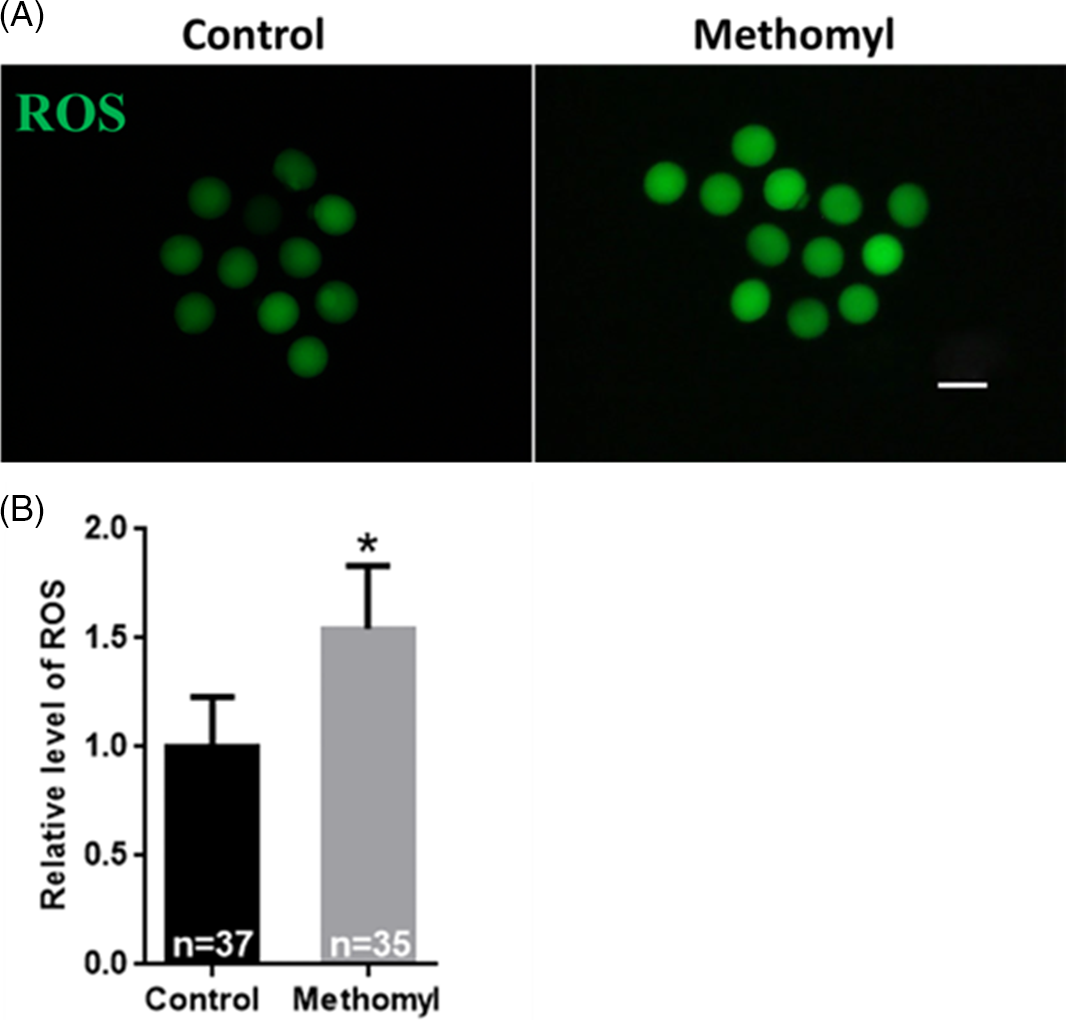
Figure 2. Effect of methomyl on the level of ROS in oocytes. (A) ROS level was significantly increased after methomyl exposure. ROS: green. Scale bar: 200 μm. (B) Shows the fluorescence intensity analysis of ROS level, R = 3. *P < 0.05.
Methomyl treatment reduces the level of ΔΨm in mouse oocytes
As mitochondria are important indicators of oocyte maturation, the present study used JC-1 to demonstrate changes in ΔΨm in mouse oocytes. As shown in Fig. 3A, the control group oocytes showed a higher ΔΨm, while the methomyl group oocytes showed reduced red fluorescence and increased green fluorescence. Fig. 3B shows the red/green fluorescence ratio of JC-1, an indicator of ΔΨm; the methomyl group had a significantly lower ratio compared with the control group (1.00 ± 0.16 vs. 0.63 ± 0.04; P < 0.05). Therefore, methomyl interferes with mitochondrial function in mouse oocytes.
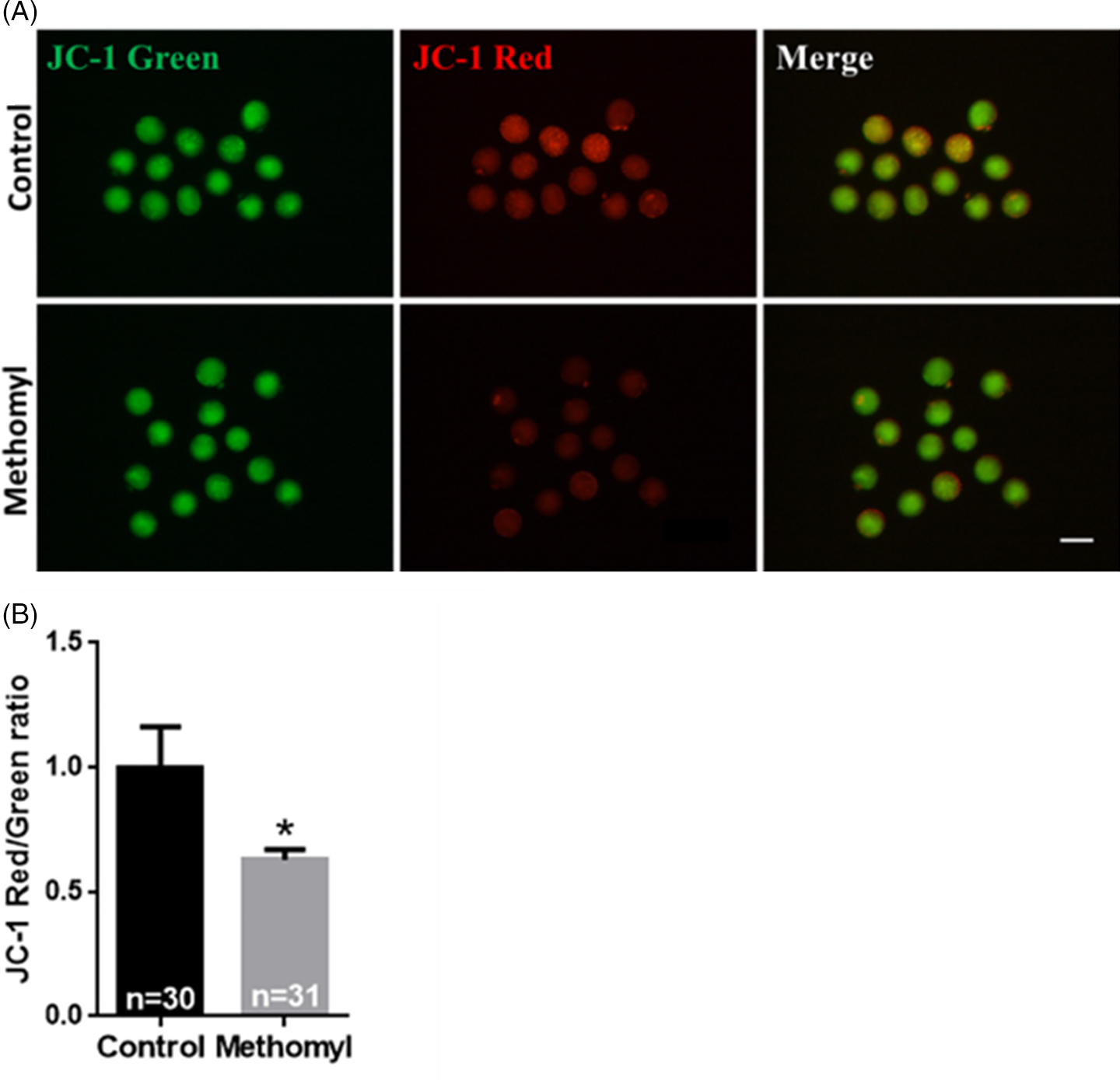
Figure 3. Effect of methomyl on the mitochondrial membrane potential of oocytes (ΔΨm). (A) Average ΔΨm fluorescence intensity in mouse oocytes after methomyl exposure. JC-1: green, JC-1: red. Scale bar: 200 μm. (B) After JC-1 staining, the oocyte ΔΨm was analyzed, R = 3. *P < 0.05.
Methomyl treatment induces abnormal morphology of mouse oocyte spindle
To investigate the presence of spindle morphology defects in methomyl-treated oocytes, α-tubulin antibody was used to detect the cytoskeleton kinetics of mouse oocytes when they reached MI stage after 8.5 h of culture. As shown in Fig. 4A, the spindles of oocytes in the control group were mostly barrel shaped and regular in shape, while the spindle morphology in the methomyl treatment group was abnormal in shape. As shown in Fig. 4B, the abnormal rate of spindle assembly in the methomyl treatment group was significantly higher than that in the control group (15.76 ± 2.74 vs. 39.34 ± 1.25; P < 0.05). These results suggested that methomyl can disrupt spindle morphology during MI oocytes.
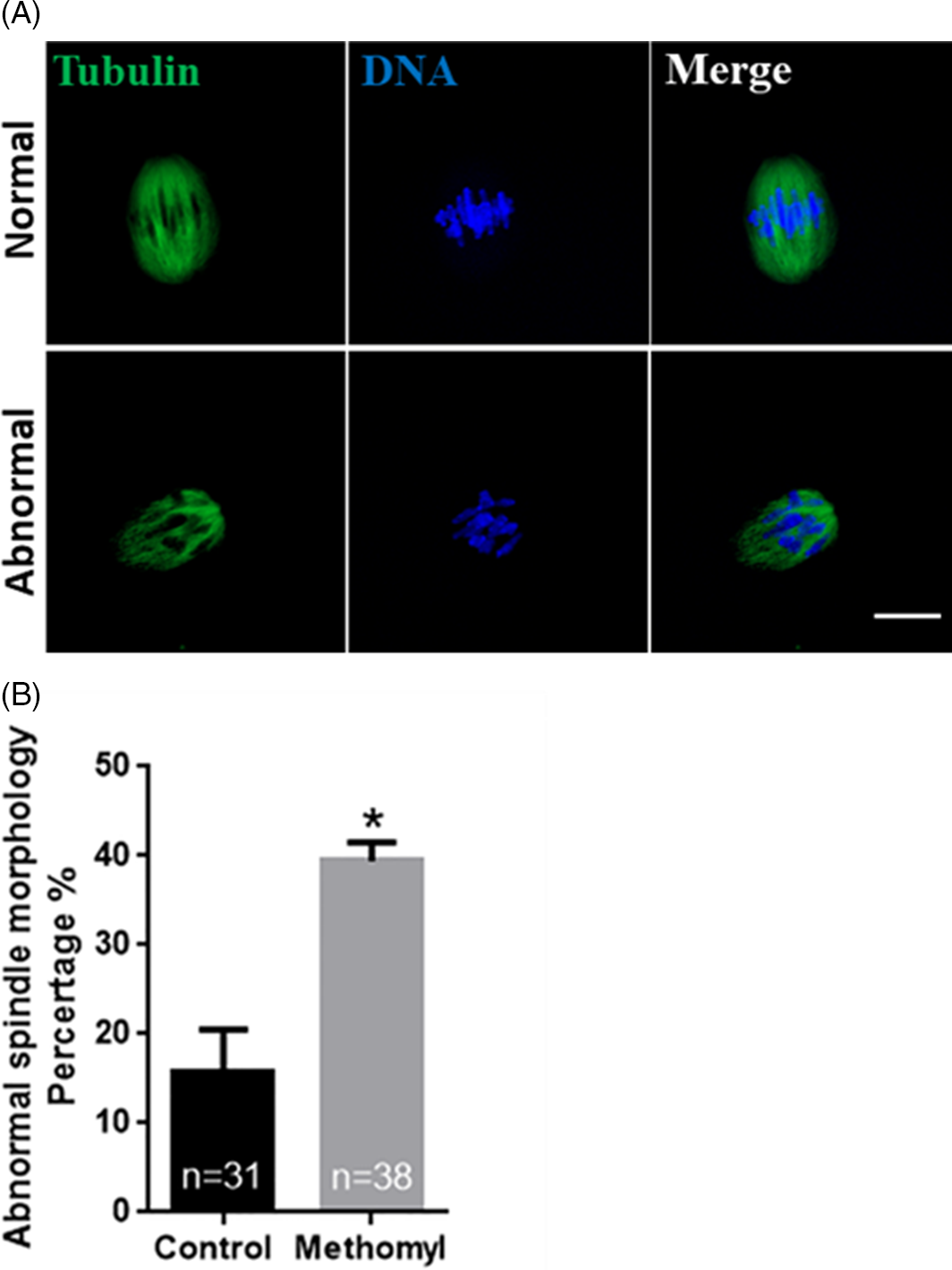
Figure 4. Effect of methomyl on the spindle morphology in oocytes. (A) Representative picture of abnormal spindle morphology in oocytes after methomyl treatment. α-tubulin: green, DNA: blue. Scale bar: 10 μm. (B) After methomyl treatment, the ratio of abnormal spindle morphology in oocytes was analyzed, R = 3. *P < 0.05.
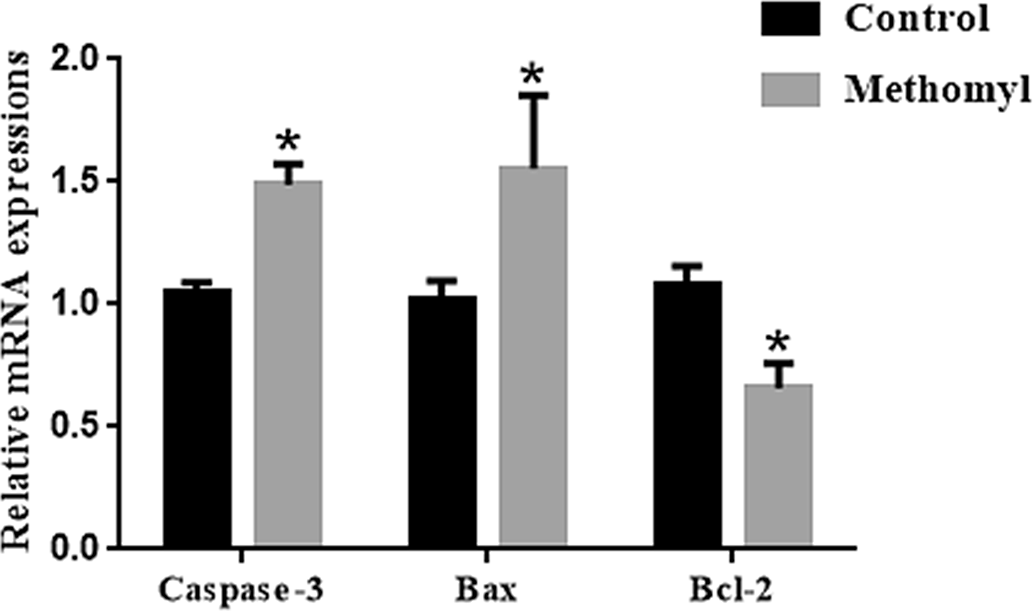
Methomyl treatment affects the expression of apoptotic genes in mouse oocytes
The mRNA transcription levels of apoptosis-related genes in oocytes were examined to further explore methomyl’s effect in mouse oocytes. As shown in Fig. 5, the mRNA transcription levels of pro-apoptotic genes Caspase-3 and Bax in the methomyl group were significantly higher than those in the control group, and the expression of anti-apoptotic gene Bcl-2 was significantly reduced (P < 0.05).
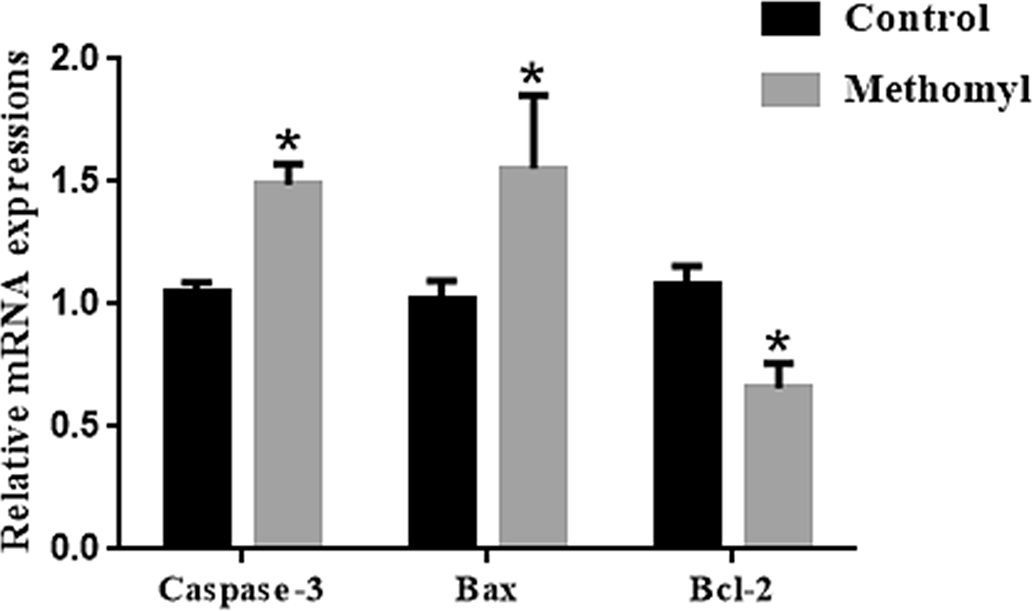
Figure 5. Effect of methomyl on apoptotic genes (Caspase-3, Bax, and Bcl-2) in oocytes. The mRNA levels of Caspase-3 and Bax in the methomyl group’s oocytes were significantly higher than those of the control group in M16, R = 3. *P < 0.05.
Discussion
This study examined the effects of methomyl on mouse oocyte polar body extrusion, ROS level, ΔΨm, abnormal spindle morphology rate, and the expression of apoptosis-related genes were determined. Results showed that methomyl affects the first polar body extrusion of mouse oocytes, ROS level, ΔΨm, spindle morphology, and apoptosis-related gene expression. These results suggest a potential mechanism by which methomyl affects oocyte maturation in mice.
Oocyte maturation plays a crucial role in determining early embryonic development. Methomyl dosing experiments were performed with concentrations ranging from 10 μM to 200 μM; 60 μM and 100 μM methomyl was found to have significant toxic effects on mouse oocytes at the cellular level and reduce the rate of oocyte first polar body extrusion. Moreover, the effect of methomyl on mouse oocyte maturation rate was demonstrated for the first time. These results are similar to those from previous studies on the effects of other environmental estrogens, zearalenone and bisphenol S, on porcine and mouse oocyte function in vitro (Sambuu et al., Reference Sambuu, Takagi, Namula, Otoi, Shiga, Rodrigues dos Santos and Fink-Gremmels2011; Hou et al., Reference Hou, Zhu, Xu, Cui, Kim and Sun2015; Žalmanová et al., Reference Žalmanová, Hošková, Nevoral, Adámková, Kott, Šulc, Kotíková, Prokešová, Jílek, Králíčková and Petr2017), indicating that environmental estrogens affect mammalian eggs. Environmental estrogens have a general toxic effect on the maturation of oocytes, which will affect the development of oocytes.
Mammalian oocytes and embryos are very sensitive to oxidative stress, and slightly higher oxidative stress levels can interfere with oocyte maturation and embryo development. When oocytes are subjected to various harmful stimuli, ROS are excessively produced, and the oxidative and antioxidant systems become unbalanced, resulting in oocyte damage (Jia et al., Reference Jia, Zhang, Zhou, Xu and Feng2019; Zhang et al., Reference Zhang, Miao, Chen, Cai, Dong, Dai, Lu, Zhou, Cui and Xiong2018; Zhu et al., Reference Zhu, Liu, Zhang, Liu, Cao, Huang, Yuan, Bian and Liu2018). ROS are oxygen-containing molecules divided into three types: free radicals, hydroxyl radicals, and non-radicals (Tsutsumi, Reference Tsutsumi2005). We investigated whether mouse oocytes undergo oxidative stress after methomyl treatment and determined ROS levels. Results indicated that methomyl-treated oocytes had a higher fluorescence intensity of ROS compared with the control group, implying that methomyl caused ROS overproduction, which led to a decrease in oocyte maturation. Our results are similar to those of previous studies, in which exposure of porcine oocytes and mice to the environmental oestrogen atrazine increased ROS production (Gely-Pernot et al., Reference Gely-Pernot, Saci, Kernanec, Hao, Giton, Kervarrec, Tevosian, Mazaud-Guittot and Smagulova2017; Yuan et al., Reference Yuan, Liang, Jin, Zhang, Zhang and Kim2017). The present study suggested that methomyl plays a role in oxidative stress during mammalian oocyte maturation in vitro. It is well known that ROS accumulation can cause oxidative damage, which can cause mitochondrial dysfunction and DNA damage. In this case, ROS, a cell-signalling molecule, can also induce autophagy and apoptosis (Zhang et al., Reference Zhang, Kong, Kang, Su, Li, Zhong and Sun2009; Chen et al., Reference Chen, Azad and Gibson2009) and reduce oxidative damage and ROS levels by removing protein aggregates and damaged organelles such as mitochondria (Ureshino et al., Reference Ureshino, Rocha, Lopes, Bincoletto and Smaili2014).
During the development of mouse oocytes, mitochondria have important functions, including programmed cell death, apoptosis and ATP production. ΔΨm is one of the most commonly used indicators for evaluating mitochondrial activity. The change in the threshold is associated with the degree of oxidative phosphorylation. The higher the ΔΨm, the higher is the efficiency of mitochondrial ATP production (Bernardi et al., Reference Bernardi, Di Lisa, Fogolari and Lippe2015). To observe the level of ΔΨm in oocytes, Van Blerkom and colleagues performed JC-1 staining and found that high levels of oocyte mitochondrial polarity played a vital role in the process of fertilization (Van Blerkom and Davis, Reference Van Blerkom and Davis2007). Therefore, in this study, we determined ΔΨm levels in mouse oocytes after methomyl treatment. Results indicated that the level of ΔΨm in the methomyl treatment group decreased, which was consistent with the finding that environmental oestrogen BPA can reduce the ΔΨm of pig oocytes (Park et al., Reference Park, Park, Kim, Yang, Kim, Jegal, Kim, Choo and Koo2018).
The cytoskeleton plays an important supporting role in the maturation of oocytes. The morphological changes of the spindle can intuitively express the meiotic ability and subsequent maturation of the oocyte, and the integrity of the spindle assembly determines its subsequent correct separation (Shen et al., Reference Shen, Wang, Shen, Zheng, Zhang, Feng, Hu and Lei2015). Abnormal spindle morphology can cause failure of meiosis. This study found that spindle morphology in MI oocytes after methomyl treatment was abnormal. This indicated that methomyl will lead to failure of oocyte spindle assembly and inhibit oocyte maturation.
Apoptosis has been reported to impair mammalian oocyte developmental capacity (Cui et al., Reference Cui, Jeong, Lee, Cheon and Kim2004; Balboula et al., Reference Balboula, Yamanaka, Sakatani, Kawahara, Hegab, Zaabel and Takahashi2013) and is associated with embryonic stagnation and early embryo fragmentation (Yu et al., Reference Yu, Qiu, Li, Zeng, Chen, Li and Quan2011). Caspase-3 is a member of the caspase family, and its activation plays a central role in cell apoptosis (Yu et al., Reference Yu, Huang, Zhou, Tang and Yu2017; Yao et al., Reference Yao, Jiang, Liang, Shen, Gao, Xu and Kim2018). Caspase-3 is a frequently activated death protease that can catalyze the specific cleavage of many key cellular proteins (Porter and Jänicke, Reference Porter and Jänicke1999). Studies have shown that caspase-3 activity is related to oocyte quality (Nicholas et al., Reference Nicholas, Alberio, Fouladi-Nashta and Webb2005). Bcl-2 prevents cell apoptosis by blocking the leakage of cytochrome c through mitochondrial membrane pores (Yang et al., Reference Yang, Hsieh, Tsai and Hsu2002). The high transcription level of Bax gene is related to oocytes with reduced development speed and poor morphological quality (Jiang et al., Reference Jiang, Yao, Zhao, Gao, Jin, Li, Yan and Xu2019). In this study, mRNA transcription levels of Caspase-3 and Bax in methomyl-treated oocytes were higher than those of the control group. In addition, the expression of apoptosis-suppressing gene Bcl-2 in methomyl-treated oocytes was lower than that of the control group. Apoptosis frequently occurs during oocyte development and has a significant effect on oocyte development. Our findings indicated that methomyl induces oocyte apoptosis, reducing oocyte quality in turn. However, the mechanism of apoptosis in the oocyte treated by methomyl remains exclusive and further work is needed to investigate this.
In conclusion, we showed that methomyl exposure induces oxidative stress in mouse oocytes, causes apoptosis, and disrupts mitochondrial function and spindle morphology. These data could expand our knowledge on the mechanisms of action of methomyl on the reproductive system. As we innovatively revealed the cytotoxicity of methomyl in the oocytes, future work should be concentrated on the concrete effects of methomyl in vivo.
Financial support
Supported by the Conservation and Development and Utilization of Yanbian Yellow Cattle Germplasm Resources by Jilin Provincial Development and Reform Commission (2020C037-4), Research Fund of Engineering Research Center of North-East Cold Region Beef Cattle Science & Technology Innovation, Ministry of Education, China and the 111 Project (D20034).
Conflict of interest
None.
Ethical standards
All mouse care and protocols were used in accordance with guidelines of the Animal Research Committee of Yanbian University, China (SYXK2020–0009).