Introduction
The blastula stage in the sea urchin Lytechinus pictus begins at the 128-cell stage, when the embryo is comprised of a single layer of epithelial cells. The vegetal pole of the blastula contains descendants of large and small micromeres that emerged early on after the 5th division. Small micromeres are believed to contribute to the germline in the sea urchin (Juliano et al., Reference Juliano, Voronina, Stack, Aldrich, Cameron and Wessel2006; Yajima & Wessel, Reference Yajima and Wessel2011), while large micromeres give rise to primary mesenchyme cells (PMC). As gastrulation begins, the cells of the vegetal pole thicken to form a vegetal plate, while the PMCs ingress, a stage at which a decrease in cell-to-cell adhesion plays a key role (Gustafson & Wolpert, Reference Gustafson and Wolpert1963).
The sea urchin gut rudiment (archenteron) forms and elongates by a thickening and inward bending of the vegetal plate. At this initial stage the first secondary mesenchyme cells (SMC) become visible at the tip of the gut rudiment and continue growing towards the animal pole (Kominami & Takata, Reference Kominami and Takata2004). The second stage of archenteron elongation depends on shortening of thin filopodia that connect the apical plate to the tip of the archenteron, effectively pulling the two together as the archenteron elongates (Hardin, Reference Hardin1988; Hardin & McClay, Reference Hardin and McClay1990; Miller et al., Reference Miller, Fraser and McClay1995). Through this process of extension, the archenteron reaches the roof of the blastula where the mouth of the animal will ultimately form.
It has been shown previously that glycan sugars such as d-mannose may play important roles in organizing and anchoring the archenteron during gastrulation (Latham et al., Reference Latham, Tully and Oppenheimer1999; Khurrum et al., Reference Khurrum, Hernandez, Esklaei, Badali, Coyle-Thompson and Oppenheimer2004; Idoni et al., Reference Idoni, Ghazarian, Metzenberg, Hutchins-Carroll, Oppenheimer and Carroll2010). In this study we extend our previous work by investigating the types of mannoside linkages that are required for gastrulation and cellular adhesion. We find that α-mannosidase from Jack bean (Canavalia ensiformis) strongly interferes with normal sea urchin gastrulation. As this enzyme cleaves only terminal mannose residues and lacks endoglycosidase activity, our findings suggest that cleavage of high-mannose glycans interferes with gastrulation. While expression of high-mannose glycans is a notable feature of cancer cells (de Leoz et al., 2011), it is assumed that these represent an aberrant lack of completion of complex glycan formation. A requirement for terminal mannosides during sea urchin gastrulation suggests that high-mannose glycans may have important roles in normal cells as well.
Materials and methods
Extraction of enzyme from ammonium sulfate suspension
α-Mannosidase (EC 3.2.1.24) from Canavalia ensiformis (Jack bean)/(JBαM) was purchased as an ammonium sulfate suspension from Sigma-Aldrich (St. Louis, MO, USA) (CAS 9025-42-7; SKU-Pack size: M7257-25MG, Lot 081M7403V; SLBC4303V). The product is reported by the manufacturer to have less than 0.05% contaminating activities of α-galactosidase, β-galactosidase, α-l-fucosidase, β-N-acetyl-glucosaminidase. The enzyme in ammonium sulfate suspension was collected by centrifugation at 15,000 g for 10 min, and dissolved in deionized water. The resulting solution was dialyzed (3500 MWCO Mini Dialysis Unit, Pierce) against ice-cold deionized water for 6 h to remove any remaining ammonium sulfate, and assayed for enzymatic activity before use. According to Li (Reference Li1967), α-mannosidase is stable at 25°C for 17 h within a 6.0 to 8.5 pH range. The denatured control sample of α-mannosidase was prepared by incubation at 100ºC for 12 h, followed by incubation at room temperature for another 12 h.
Enzymatic assay of α-mannosidase
An enzymatic assay for α-mannosidase was performed following the manufacturer's recommendations. A 0.5 ml aliquot of 100 mM citrate buffer (pH 4.5) (Sigma-Aldrich, No. C-7129) was mixed with the colorimetric substrate; 0.5 ml of 10 mM p-nitrophenyl α-d-mannoside (Sigma-Aldrich, No. N-2127), and equilibrated to 25°C. A fresh dilution of 0.1 ml of 0.1 unit/ml α-mannosidase was added to the equilibrated solution, which was then incubated at 25°C for 5.0 min. Finally, the reaction was stopped by addition of 2.0 ml 200 mM sodium borate (Sigma-Aldrich, No. B-0252) (pH 9.8) to the reaction mix and the optical absorbance at 405 nm was determined. One unit of α-mannosidase activity is defined as the amount of enzyme required for release of 1 µmole of p-nitrophenol (extinction coefficient 18.5 mM–1 cm–1) per minute, under these reaction conditions.
Enzymatic assay of α-mannosidase with added urchin material
The α-mannosidase assay with p-nitrophenyl α-d-mannoside was also conducted with freshly lysed sea urchin embryos, to determine whether there were inhibitors in the urchin material that might interfere with enzyme activity in live embryos. Sea urchin embryos were lysed by sonication at early gastrula stage (three 30 s courses of sonication, separated by 1 min pauses). The resulting lysate was centrifuged at 700 g for 10 min, and the enzyme assay was conducted in the supernatant as described earlier.
Enzyme characterization by polyacrylamide gel electrophoresis
A Bio-Rad (Hercules, CA) Mini Protean III gel apparatus with 0.75 mm spacers was used for sodium dodecyl sulfate polyacrylamide gel electrophoresis (Laemmli, Reference Laemmli1970). A 10% polyacrylamide separating gel was poured, with an 8% polyacrylamide stacking gel, using materials provided in the kit Sigma-Aldrich (product #08091; lot# BCBC3644) except that a 5× loading buffer containing 50 mM dithiothreitol was added to the sample before denaturation. Molecular weight markers (Dual Color Precision Plus Protein™ Prestained Standards) were purchased from Bio-Rad (#161-0374). The standards along with the enzyme samples were subjected to electrophoresis at 180 V, 20–25 mA for 1 h, and stained with 0.1% Coomassie Blue (Bio-Rad #161-0406), and photographed.
Azocoll protease activity assay for α-mannosidase
Azocoll (Sigma-Aldrich #A4341) was used as a colorimetric substrate to detect contaminating proteases in the α-mannosidase enzyme. An amount of 25 mg of Azo Dye Impregnated Collagen (Azocoll) was incubated with 1.0–1.2 units of α-mannosidase or denatured α-mannosidase, in 5 ml of 100 mM potassium phosphate (Sigma-Aldrich #P5379), at pH 7.8 for a total period of 0 to 12 h with absorbance measurements at 520 nm taken at every 3 h. A standard curve was prepared using a Bacillus licheniformis protease (Sigma-Aldrich #P5380).
Sea urchin fertilization
Sea urchins (Lytechinus pictus) were obtained from South Coast Bio-Marine (San Pedro, CA, USA) and maintained in refrigerated aquaria at 10°C. Gametes were collected by intracoelomic injection of 0.55 M KCl. Artificial seawater (ASW) was prepared (Woods Hole, MA, USA; Bidwell & Spotte, Reference Bidwell and Spotte1985) to obtain a final solution containing 423 mM NaCl, 9 mM KCl, 9.3 mM CaCl2•2H2O, 22.9 mM MgCl2•6H2O, 25.5 mM MgSO4•7H2O, 2.1 mM NaHCO3, pH 8.0. ASW was used to wash the eggs three times. Eggs that looked healthy and sperm that were moving under the microscope were used for fertilization in 30 inch × 20 inch glass dishes filled with 1 inch of ASW. Each glass dish contained collected eggs from one female and 100 μl of sperm from one male. Incubation was at 15–17°C until early gastrulation (approximately 24 h following fertilization).
Microplate assay with α-mannosidase and denatured α-mannosidase
Ninety-six well microplates (Sigma-Aldrich, #CLS3990) were used for assays, with approximately 75 urchin embryos (24 h post-fertilization) in each well, in a volume of 75 μl, and 25 μl of a prepared diluted enzyme solution, or ASW control, was added to each well that contained the embryos. Control treatments and treatments with enzyme dilutions were tested with an average of 40 wells per concentration tested, or 36 wells per concentration tested in the case of heat-denatured enzyme. Incubation was performed at 15–17°C until the embryos in the control treatments reached late gastrulation stage (approximately at the 48 hours post-fertilization). Ten percent formaldehyde solution was then added to all wells to a final concentration of 2.5% v/v as a fixative for the embryos, followed by scoring of morphology by microscopy. Two-tailed t-tests were utilized to analyze the significance of the results.
Microdissection assay followed by treatment with α-mannosidase
Formaldehyde-fixed (2.5% v/v) embryos at late gastrula stage were washed three times with ASW and were dissected on Sigmacote®-coated (Sigma-Aldrich, #SL2) glass slides. Fine Black Enamelled insect pins (00 gauge, Australia, BioQuip, Gardena, CA, USA) were used as instruments for dissection (Coyle-Thompson & Oppenheimer, Reference Coyle-Thompson and Oppenheimer2005). After separation of each blastocoel roof from the archenteron, physically separated embryos were treated with 0.64 U/ml α-mannosidase, or denatured enzyme, or ASW as a control. The adherence of the blastocoel roof and archenteron was scored after incubation at 22°C for 2 min, and again after 8 h, using a WESCO 500Lx light microscope (Western Scientific Co., Inc., Valencia, CA, USA) at ×100 magnification. Photographs were taken using ProgRes® MacCapturePro 2.7.6 (Jenoptic, Jena, Germany) to control camera settings and images were captured using a digital camera (Digital Microscope Camera ProgRes® C14plus, Jenoptic, Jena, Germany).
Images
All images were processed for color, contrast, brightness and scale bars using ImageJ software (developed by Wayne Rasband, Research Services Branch, National Institute of Mental Health, Bethesda, Maryland, USA; https://imagej.nih.gov/ij/docs/intro.html).
Results
Characterization of the α-mannosidase enzyme
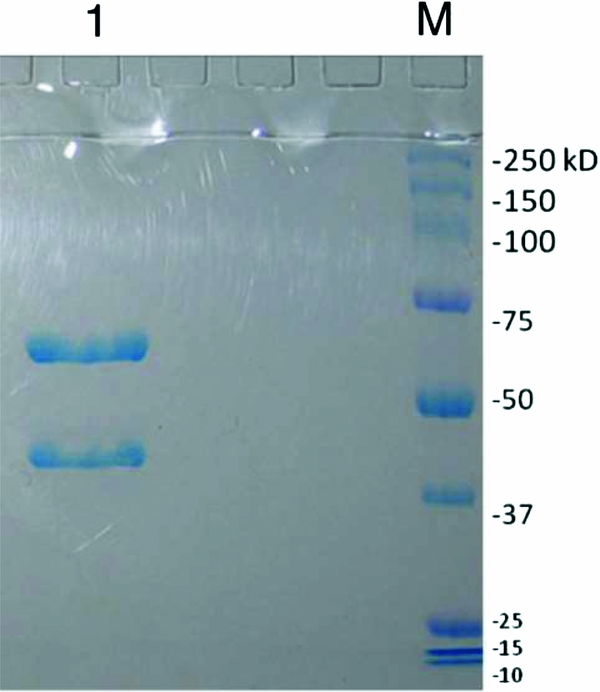
SDS–polyacrylamide gel electrophoresis was performed under reducing conditions to confirm the size and homogeneity of the α-mannosidase used in these studies. The glycosidase is tetrameric, with two subunits of 66 kDa and two of 44 kDa, and the electrophoretic results indicate two bands with the expected migrations compared with molecular weight markers (Fig. 1). There was also no evidence of degradation or contamination of the sample. Azo Dye Impregnated Collagen was used to examine possible protease contamination within a 12 h period of incubation. Protease contamination levels were found to be below the limit of detection of the assay (<1 × 10–4 unit-equivalents of the B. licheniformis protease standard per unit α-mannosidase).
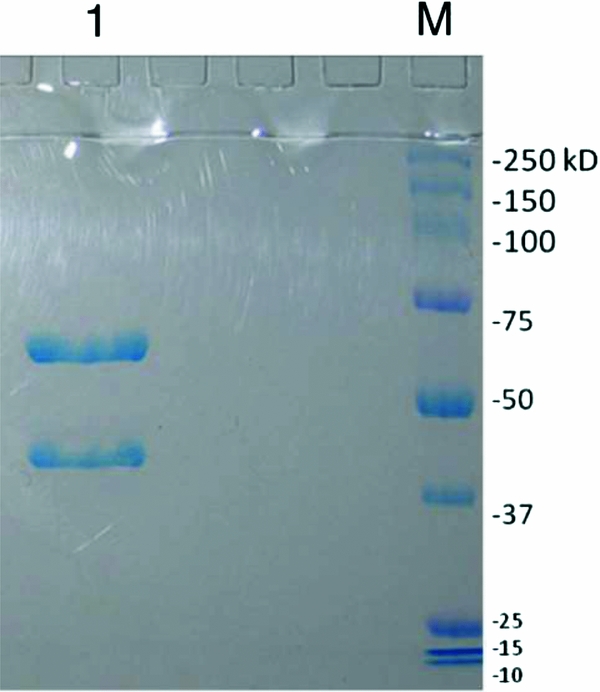
Figure 1 Characterization of α-mannosidase by SDS-PAGE. The bands at 66 kDa and 44 kDa represent the expected sizes of the subunits of α-mannosidase (lane 1). Molecular weight markers: Dual Color Precision Plus Protein™ (lane M).
The enzymatic activity of the α-mannosidase was determined each time the enzyme was employed in embryo-treatment experiments. As there was a significant decline in enzyme activity following dialysis against ice-cold distilled water to remove the ammonium sulfate shipping agent, all enzymatic activities for treatments were calculated based on the post-dialysis activity. Enzymatic activity was also reduced when urchin embryos were added to the assay mixture (Table 1), consistent with either competitive inhibition of the colorimetric substrate by embryonic glycans, or the presence of other enzymatic inhibitors in the mixed enzyme-embryo system. As expected, denatured α-mannosidase showed no measurable activity in an a homogeneous (embryo-free) assay (Table 1).
Table 1 The α-mannosidase enzyme is functional in the presence of embryos. Enzyme activities of samples were determined by cleavage of the colorimetric substrate p-nitrophenyl α-d-mannoside, as described in Materials and Methods. The analyses were conducted on the same equivalent enzymatic units of starting material

α-Mannosidase treatment interferes with archenteron development
Embryos in the early gastrula stage of development were treated with varying amounts of α-mannosidase and the effects on continued development were studied by microscopy. At the point at which control embryos reached late gastrula stage, the embryos were fixed with formaldehyde in advance of morphological analysis. In total, 6324 embryos were scored for five principal morphology types: complete archenteron (CA), incomplete archenteron (IA), non-invaginated embryos (NV), exogastrulation (EXO), or dead.
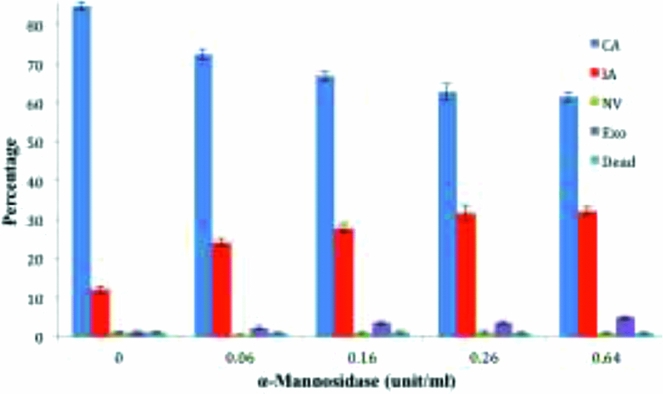
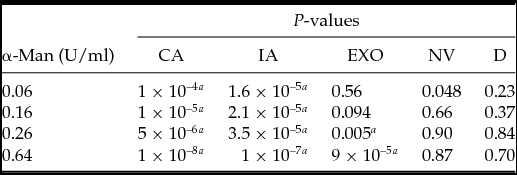
Development of the archenteron was inhibited by α-mannosidase enzyme from Canavalia ensiformis in a dose-dependent manner, with examples of the principal morphological patterns of development shown in Fig. 2 and the summary of patterns shown in Fig. 3. With no added enzyme 15% of the embryos failed to develop a complete archenteron, and there was a steady increase to 38% as the α-mannosidase concentration in the treatment was increased from 0.06 U/ml to 0.64 U/ml. This was largely due to an increase in the fraction of embryos with incomplete archenterons (12% control, to 32% with 0.64 U/ml) A two-tailed t-test was applied for comparing the α-mannosidase-treated embryos with the control embryos, and the fractions of complete, incomplete, and exogastrulated archenterons showed highly significant differences in the fraction of complete archenteron (CA) and incomplete archenteron (IA), and exogastrulation (EXO) at higher levels of α-mannosidase (Table 2).
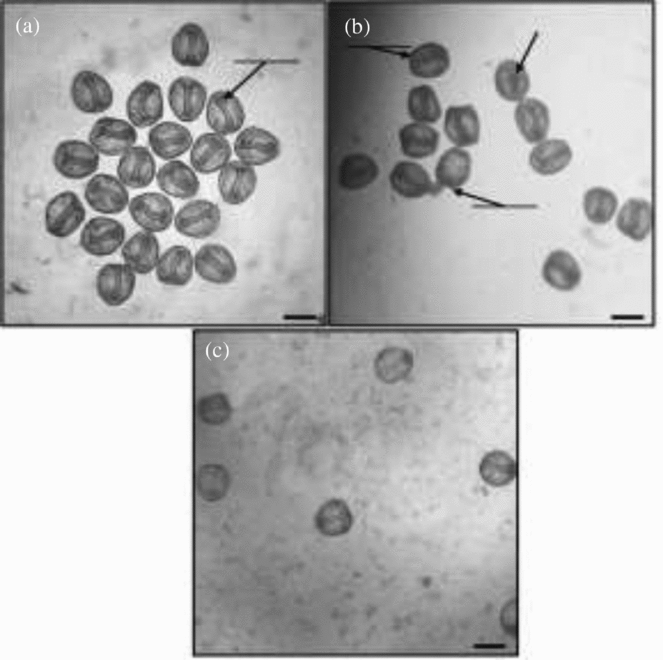
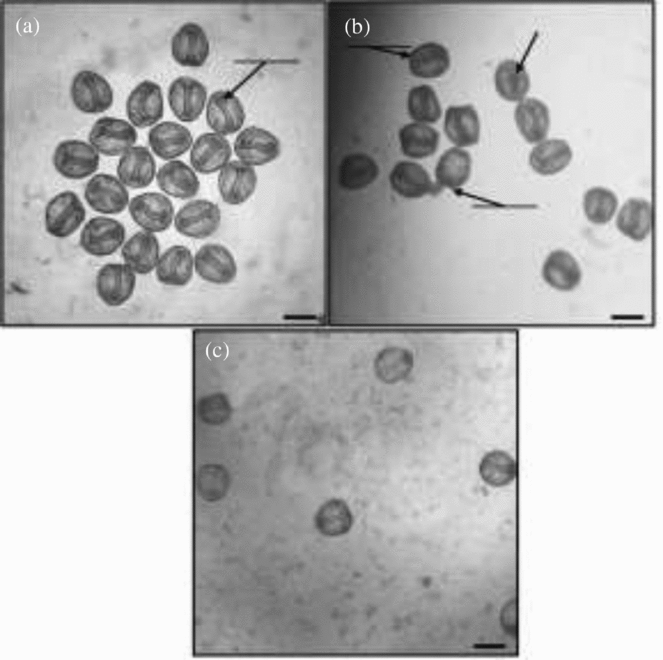
Figure 2 Light micrographs of formaldehyde-fixed L. pictus embryos at 48 hours post-fertilization, with or without treatment with α-mannosidase. (a) Completely developed, normal archenterons in control embryos. (b) Exogastrulated and incompletely developed archenterons in embryos treatment with 0.64 U/ml α-mannosidase. (c) Completely developed, normal archenterons in embryos treated with heat-denatured α-mannosidase (1/20 dilution, equivalent of 0.64 unit/ml prior to denaturation). Scale bars: 100 µm.
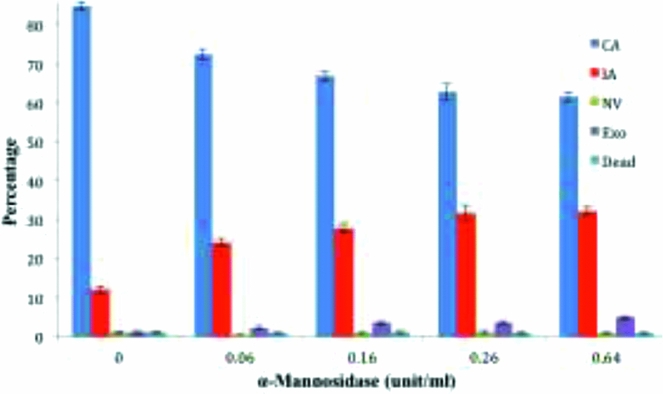
Figure 3 α-Mannosidase interferes with archenteron development. The percentage of each type of morphology is shown as a function of increasing unit/ml concentration of α-mannosidase: complete archenteron (CA), incomplete archenteron (IA), non-invaginated embryos (NV), exogastrulation (EXO), and dead. Error bars represent standard error. Two-tailed t-test, P < 0.05 for morphologies with incomplete and complete archenteron development compared to controls.
Table 2 Two-tailed t-test to compare the significance of results in the control group and α-mannosidase (α-man)-treated embryos. Each morphology type in specific treatments was compared with that in the control group: complete archenteron (CA), incomplete archenteron (IA), non-invaginated embryos (NV), exogastrulation (EXO), and dead (D)
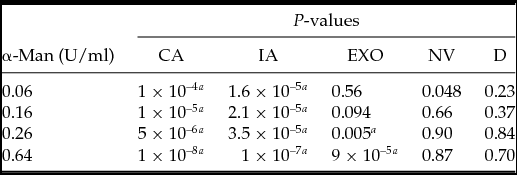
a Highly significant.
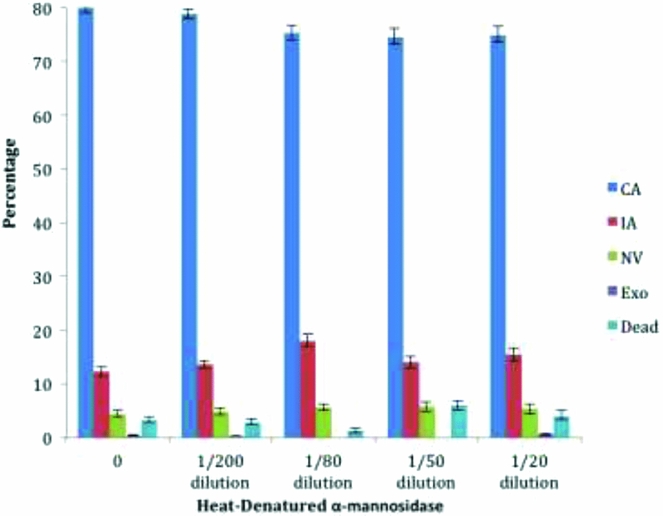
When a heat-denatured a-mannosidase was substituted for active enzyme the fraction of embryos with a complete archenteron did not fall below 75% and there were no significant differences between the treatment and control embryos by two-tailed t-test (see Fig. 4). In this experiment the sample of heat-denatured enzyme was diluted in parallel, such that the 1:20 dilution was equivalent to the 0.64 U/ml sample of native enzyme.
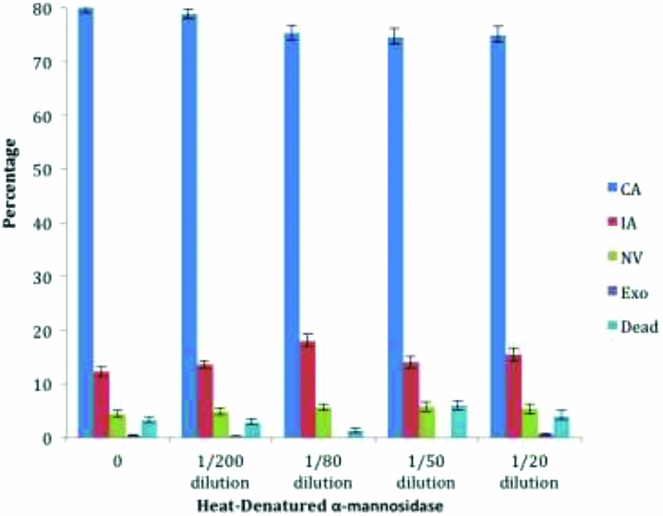
Figure 4 Heat-denatured α-mannosidase does not interfere with archenteron development. The percentage of each type of morphology is shown as a function of increasing unit/ml concentration of α-mannosidase: complete archenteron (CA), incomplete archenteron (IA), non-invaginated embryos (NV), exogastrulation (EXO), and dead. A 1/20 dilution of the heat-denatured enzyme is an amount corresponding to 0.64 U/ml prior to denaturation. Two-tailed t-test showed no significant difference between the control and the denatured enzyme results (two-sided t-test, P > 0.05, comparison between each morphology with control group). Error bars represent standard error.
Adhesive properties of fixed embryo parts is sensitive to α-mannosidase
We performed microdissection on fixed L. pictus embryos after development to the late gastrula stage, to assess whether the adhesion of the archenteron to the blastocoel roof can be eliminated by α-mannosidase treatment post-fixation. In total, 30 fixed embryos were microdissected with fine needles, with 10 treated with 0.64 U/ml a-mannosidase, 10 treated with an equivalent amount of heat-denatured enzyme, and 10 treated with ASW as a control. After incubation for 2 min, only two out of the 10 dissected embryos treated with 0.64 U/ml of α-mannosidase displayed a stable attachment of archenteron tip and blastocoel roof, when re-assembled mechanically. All 10 treated with heat-denatured enzyme and all 10 treated with ASW maintained adhesion after mechanical re-assembly. Extension of the incubation to 8 h did not alter these results.

Figure 5 Light micrographs of fixed, microdissected embryos, showing the adhesion of embryo parts. (a) Freshly microdissected embryo prior to re-assembly. (b) Adhesion of parts after mechanical re-assembly (control). (c) α-Mannosidase-treated embryo parts, with failure to adhere after attempted re-assembly. Scale bars: 100 μm. Arrows indicate: archenterons and blastocoel roofs.
Discussion
It was previously shown by confocal microscopy that mannose-specific lectins from Lens culinaris and Pisium sativum can enter sea urchin embryos, and bind to secondary mesenchyme cells involved in archenteron anchoring and development, as well as inducing morphological changes such as exogastrulation in sea urchin embryos (Latham, et al., Reference Latham, Martinez, Cazares, Hamburger, Tully and Oppenheimer1998, Reference Latham, Tully and Oppenheimer1999). Previous qualitative studies have shown that α-mannosidase causes disorganized archenteron development at very high concentrations (5 mg/ml enzyme, probably >75 U/ml), but those studies did not include an analysis of α-mannosidase activity or a test for contaminating protease activities (Khurrum et al., Reference Khurrum, Hernandez, Esklaei, Badali, Coyle-Thompson and Oppenheimer2004). The present study indicates that α-mannosidase causes detectable effects on embryo development at concentrations as low as 0.06 U/ml, which based on the specific activity indicated by the supplier would be below 5 μg/ml. At these low concentrations, and with the additional characterizations we have performed, it is unlikely that any contaminating glycosidase or protease could be responsible for the biological effects we detect. Furthermore, we have shown that the α-mannosidase enzyme retains activity in the presence of L. pictus embryos, and that it is therefore plausible that the enzyme is not inhibited from cleaving terminal mannose groups from glycans in the embryo.
The present studies suggest that removal of terminal mannose residues in glycans using α-mannosidase interferes with sea urchin development. Such terminal mannose residues could be present in high-mannose glycans associated with membrane proteins, or they could be part of the extracellular matrix (ECM). Their roles could include cell-cell recognition, control of cell shape or migration, or cellular signaling factors or receptors. High-mannose glycans, a form of protein modification against which α-mannosidase would be active, are intermediate steps in the elaboration of more complex glycosides on proteins. It is sometimes suggested that these are aberrant, incompletely processed glycosylations, perhaps the output of an endomembrane system that is not sufficiently efficient to convert every glycan to a complex one. In cases of viral infection this may be the case, as the output of glycosylated viral envelope proteins may overwhelm the cellular machinery. However, the development of L. pictus embryos is a ‘normal’ process and it seems unlikely that terminal mannose residues could be both critical for development and aberrant.
It is interesting to note that treatment of S. purpuratus embryos with deoxynojirimycin (DNJ), which inhibits the initial glucosidic trimming of glycans in the endomembrane system, or deoxymannojirimycin (DMJ), which inhibits subsequent mannosidase trimming, inhibits spiculogenesis but has no effect on gastrulation (Kabakoff and Lennarz, Reference Kabakoff and Lennarz1990). Both of these drugs inhibit the expression of complex glycans on proteins, but not high-mannose ones. Conversely, tunicamycin prevents all N-linked glycosylation (complex and high-mannose) and also prevents development beyond the early gastrula stage in sea urchins (Schneider et al., Reference Schneider, Nguyen and Lennarz1978). Put together, these observations suggest that high-mannose (but not complex) glycans are required for sea urchin gastrulation (Kabakoff & Lennarz, Reference Kabakoff and Lennarz1990), consistent with our observations of incomplete archenteron development following treatment with α-mannosidase. A similar finding that the complex glycans are only required later in development, but that non-complex glycans are both necessary and sufficient at earlier stages, has been seen in mice (Metzler et al., Reference Metzler, Gertz, Sarkar, Schachter, Schrader and Marth1994; Marek et al., Reference Marek, Vijay and Marth1999).
Previously, this laboratory has studied several glycan effects during sea urchin gastrulation using similar assays (Latham et al., Reference Latham, Martinez, Cazares, Hamburger, Tully and Oppenheimer1998, Reference Latham, Tully and Oppenheimer1999; Khurrum et al., Reference Khurrum, Hernandez, Esklaei, Badali, Coyle-Thompson and Oppenheimer2004; Coyle-Thompson & Oppenheimer Reference Coyle-Thompson and Oppenheimer2005; Razinia et al. Reference Razinia, Carroll and Oppenheimer2007; Singh et al., Reference Singh, Karabidian, Kandel, Metzenberg, Carroll and Oppenheimer2014; Liang et al., Reference Liang, Aleksanyan, Metzenberg and Oppenheimer2015). In the present study α-mannosidase enzyme inhibited proper organization and attachment of the Lytechinus pictus archenteron suggesting the presence of one or all types of α1–2-, α1–3-, or α1–6-linkages. The results with microdissected embryos treated with enzyme suggest that there is an adhesion between the embryo parts that is sensitive to α-mannosidase. This adhesiveness is present after formaldehyde fixation, and may play a role in the normal interaction between archenteron and blastocoel roof. The surface properties of fixed and live cells as measured by lectin bead binding and binding to other derivatized beads are similar suggesting that formaldehyde-fixed cells display authentic surface properties (Navarro et al., Reference Navarro, Walker, Badali, Abundis, Ngo, Weerasinghe, Barajas, Zem and Oppenheimer2002).
Gastrulation is a dynamic process that involves cell-cell communication through signaling molecules and cell-surface receptors, and these are also possible targets of α-mannosidase in developing embryos. The Wnt signaling pathway, for example, is known to direct the cell shape changes and migrations that lead to invagination (Chisholm, Reference Chisholm2006), and some Wnt family members have both complex and high-mannose glycans (Yamamoto, et al., Reference Yamamoto, Awada, Hanaki, Sakane, Tsujimoto, Takahashi, Takao and Kikuchi2013). If high-mannose glycans in Wnt are required for function, then their digestion by α-mannosidase in these experiments could have interfered with archenteron development. Regulation of signaling molecules by differential glycosylation is well established in the Notch/Delta system, where the glycosyltransferase Fringe controls Notch activity through O-fucosylation (Brückner et al., Reference Brückner, Perez, Clausen and Cohen2000). Notch is required for SMC specification in sea urchin (Sherwood & McClay, Reference Sherwood and McClay1999).
Structural proteins in the extracellular matrix (ECM) and cell surface may also be affected by α-mannosidase. Endo16 is a secreted adhesive protein that is expressed in the endomesoderm and required for sea urchin gastrulation (Soltysik-Española et al., Reference Soltysik-Española, Klinzing, Pfarr, Burke and Ernst1994; Romano & Wray, Reference Romano and Wray2006). It is a multidomain protein, first expressed in the vegetal plate just prior to gastrulation (Nocente-McGrath et al., Reference Nocente-McGrath, Brenner and Ernst1989). Endo16 is induced by micromeres (Ransick & Davidson, Reference Ransick and Davidson1995), mediated by the signaling molecule ActivinB (Sethi et al., Reference Sethi, Angerer and Angerer2009), which is likely the molecular explanation for classic experiments involving micromere transplantation (Hörstadius, Reference Hörstadius1935). There are multiple putative N-glycosylation sites in Endo16, and to the extent that these may express high-mannose glycans in early stages of gastrulation, this adhesive protein is a possible target of the α-mannosidase in these experiments.
Financial support
This work was directly or indirectly supported by a National Science Foundation USA (NSF) Presidential Award (0731633), and Sydney Stern Memorial Trust, United States National Institutes of Health Score (S06 48680), RISE (Resilience, Innovation, Service, Empowerment), NIH MARC, the Joseph Drown Foundation, California State University Northridge, USA and Julie Gorchynski MD Scholarship awards.